Abstract
Genistein in soy activates estrogen receptor (ER)-α and ERβ and acts as an estradiol in multiple target tissues. Because estrogens increase breast cancer risk and genistein promotes the growth of ER-positive human breast cancer cells, it has remained unclear whether this isoflavone or soy is safe. Results reviewed here suggest that women consuming moderate amounts of soy throughout their life have lower breast cancer risk than women who do not consume soy; however, this protective effect may originate from soy intake early in life. We also review the literature regarding potential risks genistein poses for breast cancer survivors. Findings obtained in 2 recent human studies show that a moderate consumption of diet containing this isoflavone does not increase the risk of breast cancer recurrence in Western women, and Asian breast cancer survivors exhibit better prognosis if they continue consuming a soy diet. The mechanisms explaining the breast cancer risk-reducing effect of early soy intake or the protective effect in Asian breast cancer survivors remain to be established. We propose that the reduction in risk involves epigenetic changes that result in alterations in the expression of genes that regulate mammary epithelial cell fate, i.e. cell proliferation and differentiation. Lifetime soy consumption at a moderate level may prevent breast cancer recurrence through mechanisms that change the biology of tumors; e.g. women who consumed soy during childhood develop breast cancers that express significantly reduced Human epidermal growth factor receptor 2 levels. More research is needed to understand why soy intake during early life may both reduce breast cancer risk and risk of recurrence.
Breast cancer is strongly associated with estrogens. Ovarian estrogens, adipose-derived estrogens, and estrogenic compounds, e.g. in the diet, promote the growth of estrogen receptor (ER)7 positive (ER+) breast cancer cells in vitro and in vivo in athymic mice (1). In addition, estrogens and estrogenic compounds promote the growth of rodent ER+ mammary tumors (2). In humans, lifetime exposure to high estrogen levels is associated with increased breast cancer risk (3). For these reasons, estrogens present in our food and environment may pose a risk to humans.
Isoflavones are known to accumulate in many plant species at various concentrations. Dietary legumes such as black beans, lentils, lima beans, Mung beans, and soybeans are sources of a variety of isoflavones, but only the soybeans are a primary source of genistein in human diet (4, 5). A diverse array of soybean foods and derivates is widely available in Western diets, such as oil, meal, flour, protein isolates, and dairy and meat substitutes, including milk, yogurt, ice cream, cheese, sausages, and “veggie” burgers. Many of these contain several-fold more isoflavones than traditional Asian soy products (6). Despite the vast variety of soy foods in the Western markets, soy food consumption is still low, except in cases where dietary habits can lead to exceptionally high exposure to soy and isoflavones (7). For example, consumption is significantly different even in Chinese Americans (4 g/d) compared with their counterparts from China (36 g/d) (8). Most of the rise in popularity of soy products, and lately of dietary supplements containing soy isoflavones, has come from their portrayal as the panacea for a variety of health ailments from relieving postmenopausal symptoms (9, 10) to the prevention of cardiovascular disease (11, 12) and osteoporosis (13, 14). The evidence for such health claims has been partially supportive in some cases (15–17) and is addressed in detail in the other articles in this issue.
The idea that soy protects against breast cancer originates from the observation that Asian women who consume soy as a part of their stable diet have a 3- to 5-fold lower breast cancer risk than Caucasian women who do not regularly consume soy (18). However, other lifestyle factors, such as degree of physical activity, could be important contributors to explain a population’s disparities of breast cancer incidence (19). When Asian women immigrate to the West, their daughters who were born in the West have higher risk than the mothers and granddaughters’ risk is similar to that of Caucasian women (20). Because Asian American women consume significantly less soy foods than Asian women, it is reasonable to assume that soy protects Asians from developing breast cancer. This idea has been challenged by the apparent estrogenicity of soy isoflavones, particularly genistein.
Estrogenicity of soy foods and genistein
Soybeans and soy foods contain several compounds with the putative ability to inhibit carcinogenesis, including protease inhibitors (21), phytates (22, 23), and isoflavones (24–28). Genistein has been investigated for its biological activity related to breast cancer (29) and its estrogenic activity is well documented.
Genistein has a very similar chemical structure to the most potent ovarian estrogen, estradiol (E2), and consequently it binds and activates both the ERα and ERβ. The difference in function between the 2 ER remains to be established, but it is generally assumed that ERα mediates the proliferative actions of estrogens and ERβ binds to ERα and inhibits its action (30–32). Although genistein binds more strongly to ERβ than to ERα (33), physiological doses of genistein activate ERα equally well as E2 does (34). Only at very low genistein concentrations (5–8 nmol/L; in comparison, genistein concentrations in Asian women are ∼1000 nmol/L) does this isoflavone potentially act through ERβ rather than ERα (34). The actions of genistein through the 2 ER also depend on the developmental stage of the breast: ERβ is expressed at a significantly higher level than ERα during early development and in normal adult breast, while in the breast tumor, ERα expression is higher than ERβ expression (35). Additional evidence for genistein binding and activating ERα in breast tumors is that it activates a number of estrogen-responsive genes, including pS2 and c-fos, in ER+ breast cancer cell lines in culture (36–38).
Animal data in rodents.
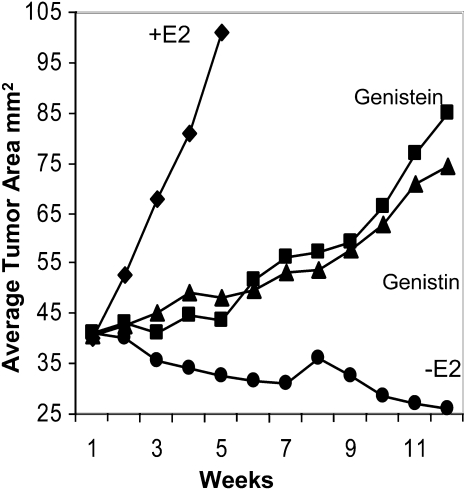
Findings obtained in animal studies have further demonstrated the estrogenicity of genistein. Ovariectomized rats consuming genistein at 750 mg/kg diet had higher uterine wet and dry weights and more rapid mammary gland growth than the controls. In addition, the expression of c-fos and pS2 in the tumors increased (37). In a similar model, dietary genistein at 125–1000 mg/kg diet (genistein serum levels of 0.39–3.36 μmol/L) promoted the growth of estrogen-dependent human breast cancer cells in a dose-dependent manner (39, 40). These doses parallel those observed in Asians. Using a 750-mg/kg diet dose, Allred et al. (41) showed that genistin or its aglycone form, genistein, stimulated the growth of human breast cancer cells in a preclinical, postmenopausal breast cancer model (Fig. 1). Withdrawal of either isoflavone form resulted in tumor regression. If mice were fed <125 mg/kg diet of genistein or genistein was administered via subcutaneous injections, the stimulatory effect was not seen (26, 42, 43). Thus, genistein elicits different effects in vivo depending on the dose and route of administration.
FIGURE 1.
Equivalent amounts of dietary genistin and genistein (750 μg/g) stimulate growth of estrogen-dependent breast cancer tumors MCF-7 in athymic mice. Cells (1.5 × 105 MCF-7 cells/site) were injected subcutaneously into dorsal flanks of ovariectomized athymic mice. After tumor establishment (40 mm2), E2 pellets were removed and mice were randomly assigned to 4 treatment groups. Positive control (+E2) mice were reimplanted with a new E2 pellet and along with negative controls (−E2) were fed an AIN 93G diet alone. For phytoestrogen groups, mice consumed either dietary genistin (1200 μg/g) or genistein (750 μg/g) mixed with AIN 93G. Tumors were monitored weekly and tumor size was calculated and is expressed as mean cross sectional area (mm2) of all tumors in each treatment group ± SEM. Data from wk 11 of treatment is presented. Figure reproduced with permission from (41).
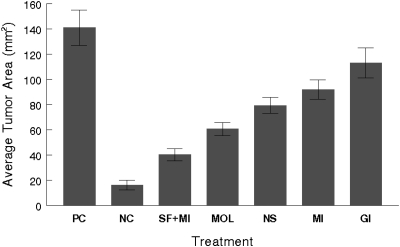
Estrogenicity of genistein may also be modified by the matrix it is given. Allred et al. (43) reported that consumption of equivalent amounts of genistein from soy products at different processing stages differently affects the proliferation of MCF-7 human breast cancer cells in ovariectomized athymic mice (Fig. 2). The diet containing soy flour did not promote tumor growth or tumor regression, but the diet containing genistein only did. When the effects of soy consumption on intact mouse mammary gland were explored, it was found that genistein in the context of whole soy acts as an estrogen. Specifically, Penttinen-Damdimopoulou et al. (44) reported that consumption of whole soy activated estrogen response element (ERE) in the mammary glands of luciferase transgenic mice. In this study, ovariectomized mice received 160 μg/g genistein through a soy diet for 2 d, after which there was a 6-fold increase in ERE induction in their mammary glands compared with control-diet fed mice. Thus, dietary intake of whole soy activates estrogenic pathways in a mouse mammary gland. However, this study only determined whether ERE was activated; it did not assess changes in mammary cell proliferation or any other functional endpoints.
FIGURE 2.
Food matrix modulates the estrogenic effects of equivalent amounts of genistein aglycone (750 μg/g) on estrogen-dependent MCF-7 tumor growth in athymic mice. Cells were injected subcutaneously into dorsal flanks of ovariectomized athymic mice. After tumor establishment, E2 pellets were removed and mice were randomly assigned to treatment groups. Positive control (PC) mice were reimplanted with a new E2 pellet. Both the negative control (NC) and PC mice were fed a low-phytoestrogen control diet (AIN-93G). Treatment groups also included an AIN-93G diet with genistein (GI), mixed isoflavones (MI), Novasoy (NS), molasses (MOL), and soy flour + mixed isoflavones (SF + MI). Tumors were monitored weekly and tumor size was calculated and is expressed as mean cross sectional area (mm2) of all tumors in each treatment group ± SEM. Data from wk 11 of treatment is presented. Figure reproduced with permission from (43).
Animal data in nonhuman primates.
Dietary exposure during adulthood to isoflavones does not have estrogenic effects on nonhuman primate models. The use of a nonhuman primate animal model is critical in reproductive biology, because the endometrial and ovarian physiologies of monkeys are almost identical to that of women (45). In these studies, intact or ovariectomized cynomolgus monkeys were fed a control diet or a diet containing soy or isoflavones (46, 47). Uterine weight, endometrial thickness, glandular area, and epithelial proliferation in the uterus were not significantly altered. In addition, no evidence of estrogenic effects on the mammary gland were observed, indicating that dietary soy isoflavones do not have estrogenic effects on the uterus or mammary gland. These investigators also observed that the combination of high exogenous E2 and high dietary isoflavones reduced circulating (47) and intra-breast (48) E2 concentrations in monkeys. Furthermore, a decrease in breast epithelial proliferation and thickness of the endometrium was noted (48). However, when the exposure occurred during early development, blood testosterone levels and development of the testis were affected (49). These findings suggest that although adult exposure to genistein does not elicit functional changes in the reproductive system or mammary gland of nonhuman primates, early-life exposure may have adverse effects.
Human data.
In humans, the estrogenic effects of isoflavones alone or in soy foods have been investigated in numerous studies with conflicting outcomes. Although the data by Lu et al. (50) suggested that soy intake reduces circulating estrogen levels, several subsequent studies have not found any marked differences (51, 52). The effects of soy intake or exposure to isoflavones on mammary epithelial cell proliferation have also been studied. Some of these studies indicate that soy protein isolate increases the volume of breast fluid (marker of increased breast cancer risk), presence of hyperplastic epithelial cells, and cell proliferation (53, 54). One study found that soy increased expression of pS2, suggesting an estrogenic effect on the breast (55). Studies that have explored the effect of consumption of isoflavones alone or in soy foods on high mammographic density, a strong marker of increased breast cancer risk (56), have found no effect (57–59). None of these studies, even those from the same authors, used similar amounts of isoflavones or foods. In the study by Maskarinec et al. (57), the nutritional intervention consisted of a choice of 2 daily servings of soy. This serving was defined as the amount of food that contained ~25 mg of isoflavones and corresponded to 180 g soymilk, 126 g tofu, a 58-g soy protein bar, 31 g soy protein powder, or 23 g roasted soy nuts. In contrast, another study by Maskarinec et al. (58) used purified forms of isoflavones, where soy germ was the main source. This is a significant change, because glycitein highly accumulates in soy germ, providing a unique isoflavone profile that no other traditional or Westernized soy food has. Thus, these studies used food sources that greatly differ in the amount and profile of isoflavones and other bioactive component factors that dictate final bioaccesibility and bioavailability of isoflavones.
In summary, human studies that have investigated changes in circulating hormone levels or mammographic density in pre- or postmenopausal women by diets high in isoflavones from dietary supplements or soy foods have found no significant effects, suggesting that they do not alter breast cancer risk. However, these studies have used moderate doses of isoflavones, reflective of Asian soy food consumption, and it is possible that higher doses could yield different results.
Epidemiological studies on soy intake and breast cancer risk
Two meta-analyses have been performed that investigated the effect of soy on breast cancer risk. The first was published in 2006 by Trock et al. (60) and included 18 studies. The findings of this study indicated that soy intake was associated with reduced breast cancer risk among women living in the West. The reduction in risk was strongest among premenopausal women. However, among Asian women living in Asia, high soy consumption did not significantly reduce the risk of developing breast cancer [odds ratio (OR) = 0.89; 95% CI 0.71–1.12]. A meta-analysis published 2 y later by Wu et al. (61) concluded the opposite, i.e. that no protective effect was seen in Western women who consumed either no or low levels of soy products, most of which are not traditional soy foods, but a significant reduction was seen in Asian women and Asian American women [OR = 0.71; 95% CI 0.60–0.85]. The differing conclusions reached by these 2 meta-analyses are most likely caused by inclusion of Asian American women in the Western category by Trock et al. (60) and in the Asian category by Wu et al. (61).
Together, these findings suggest that a protective effect is seen among Asian American women who continue consuming moderate levels of soy on a daily basis compared with Asian American women who adopt Western dietary habits. Thus, these women are able to maintain the low breast cancer risk of Asian countries if they continue consuming soy, but if their diet becomes Westernized, these women exhibit the same high risk as Caucasian women do. Another possible explanation is that soy intake is closely related to some other dietary habit or lifestyle that provides protection against breast cancer. For example, Asian consume more rice, fish, vegetables, and tea and less fat than Caucasians. The reason why a protective effect is not seen in Asian countries may be related to the fact that everybody consumes soy and even the lowest level of intake, which corresponds to high intake in Western countries, is enough soy to reduce the risk. Additionally, the reduction in risk may be seen only if soy is consumed throughout life or during periods before or during extensive development of the breast, i.e. puberty and pregnancy.
Most studies conducted using adult animals do not support an association between an exposure to genistein and mammary tumorigenesis (62, 63). These studies exposed animals to genistein by injection or feeding them genistein, isoflavones, or soy isolate. However, animal studies show that soy/genistein intake before puberty onset provides strong protection (64).
Soy intake during childhood and adolescence and later breast cancer risk.
Five case-control studies have investigated whether soy intake during childhood or adolescence affects later breast cancer risk. A study by Korde et al. (65) assessed (separately during childhood, adolescence, and adulthood) soy intake in 966 controls and 597 cases who were 20–55 y old at the time of the interview, and found a significantly reduced risk among those women who consumed the highest level of soy either during childhood (5–11 y) or adult life (over 20 y) (Table 1) [OR = 0.42 (95% CI: 0.20–0.90); P < 0.02]. Three other case-control studies found a significantly reduced risk among those who consumed the highest quartile of soy between ages 12 and 19 y compared with those consuming the lowest quartile (Table 1). In 2 of these studies, both conducted in Asian women living either in Asia (66) or the US (67), the risk was one-half of that in the comparison group (lowest soy intake quartile). Soy intake in these studies is reflective of what is commonly consumed in Asia or the US among Asians, i.e. 1–2 servings/d of traditional Asian soy products. Western soy products prepared using isoflavone supplements can contain several-fold higher levels of, e.g., genistein and therefore may have estrogenic effects. The only study involving Caucasian women showed the lowest, but still significant, reduction in risk (68). One study conducted in Asian women did not find any protective effect by adolescent soy intake and breast cancer risk (69). However, this study showed that women who consumed soy both during early life and adulthood had a significantly reduced risk of breast cancer (Table 2).
TABLE 1.
OR and 95% CI of breast cancer risk in women consuming different levels of soy during childhood/adolescence and adult life1
| Soy intake |
||||
| Authors | Childhood | Adolescence | Adult | Ethnicity |
| Shu et al. 2001 (66) | 0.51 (0.40–0.65) | Asian | ||
| Wu et al. 2002 (67) | 0.51 (0.31–0.84) | 0.51 (0.33–0.78) | Asian American | |
| Thanos et al. 2006 (68)2 | 0.81 (0.71–0.94) | Caucasian | ||
| Korde et al. 2009 (65) | 0.42 (0.20–0.90) | 0.77 (0.57–1.04) | 0.71 (0.53–0.95) | Asian American |
| Lee et al. 2009 (69) | 0.97 (0.76–1.27) | 0.89 (0.66–1.15) | Asian | |
Breast cancer risk was lowest in women who consumed high levels of soy both early in life and adulthood.
Isoflavone intake was measured in this study.
TABLE 2.
OR and 95% CI of breast cancer risk in women consuming different levels of soy during childhood/adolescence and adult life1
| Childhood/adolescence intake |
Population ethnicity | |||||
| Authors | Cases vs. controls | Adult intake | Low | Medium | High | |
| Wu et al. 2002 (67) | 501 cases vs. 594 controls | Low | 1 | 0.77 (0.51–1.16) | Asian American | |
| High | 0.93 (0.58–1.48) | 0.53 (0.36–0.78) | ||||
| Korde et al. 2009 (65) | 597 cases vs. 966 controls | Low/medium | 1 | 0.44 (0.18–1.03) | 0.36 (0.15–0.86) | Asian American |
| High | 1.52 (0.30–7.62) | 0.51 (0.18–1.46) | 0.60 (0.20–1.78) | |||
| Lee et al. 2009 (69) | 592 cases vs. 72,223 total | Low | 1 | 0.96 (0.62–1.49) | 0.62 (0.33–1.19) | Asian |
| Medium | 0.57 (0.32–0.99) | 0.56 (0.33–0.94) | 0.67 (0.39–1.15) | |||
| High | 0.57 (0.30–1.08) | 0.59 (0.34–1.03) | 0.41 (0.22–0.75) | |||
Breast cancer risk was lowest in women who consumed high levels of soy both early in life and adulthood.
Why childhood soy consumption reduces later breast cancer risk
Because genistein acts through activation of ERα and ERβ, it is not surprising that prepubertal genistein exposure causes a long-lasting change in the expression of the ER. In rats and mice, pubertal exposure to genistein causes a persistent upregulation of both ERα and ERβ, whereas E2 downregulates ERα and upregulates ERβ in the mammary gland (70, 71). Similar findings have been reported in humans; women consuming soy early in life had elevated estrogen and progesterone receptor expression in benign mammary tissue (72).
High expression of ERα in normal mammary tissue does not necessarily mean increased breast cancer risk. This is because proliferating mammary cells do not express ERα (73); only proliferating mammary tumor cells do (74). The increase in E2-induced proliferation requires that ER activates amphiregulin, which then binds to epidermal growth factor receptor (EGFR); activation of EGFR by amphiregulin leads to mammary epithelial proliferation (75). Amphiregulin is a tyrosine kinase, as are EGF, transforming growth factors, and others, which in addition to EGFR bind to other members of tyrosine kinase receptor family, such as human epidermal growth factor receptor 2 (Her2). Genistein is a potent tyrosine kinase inhibitor (76) and therefore may prevent E2-induced cell proliferation. This is supported by findings in numerous studies showing that exposure to genistein during the prepubertal period inhibits mammary epithelial cell proliferation (77, 78). We have recently found that prepubertal dietary exposure to genistein, at the level Asians consume it, silences the expression of amphiregulin in the mouse mammary gland (S. de Assis, A. Warri, L. Hilakivi-Clarke, unpublished data). In addition, we (S. de Assis, A. Warri, L. Hilakivi-Clarke, unpublished data) and others (72) have shown in mice and humans that prepubertal genistein exposure (mice) and soy intake (humans) reduces Her2 expression in mammary tumors. Genistein also has been reported to downregulate Her2 in human breast cancer cells (79). Together, these findings suggest that although genistein/soy upregulates ERα, it inhibits mammary cell proliferation, possibly by silencing amphiregulin and preventing EGFR activation.
In addition to changes in ER, genistein affects the expression of many transcription factors that are directly or indirectly regulated by ER, including tumor suppressor genes BRCA1 (70, 80) and PTEN (81), both in vitro and vivo at physiological concentrations of this isoflavone. Our unpublished data indicate that pubertal genistein exposure causes a persistent upregulation of caveolin-1, also a tumor suppressor regulated by estrogens (82). These tumor suppressor genes have multiple functions, including inhibiting the PI3K/Akt signaling pathway, repairing DNA damage, and inducing epithelial differentiation (83–87). PTEN (88) and caveolin-1 (89) both regulate Wnt/β-catenin signaling, which in turn has been linked to several cancers, including breast cancer (90). Further, hyperactive Wnt/β-catenin signaling leads to dysregulation of mammary stem cell behavior (91), which may be the cornerstone of cancer susceptibility and growth.
Several microarray analyses have studied genes modified by genistein, most of which were done using human breast cancer cells, such as MCF-7 cells. Lavigne et al. (92) found that physiological doses of genistein (1–5 μmol/L) elicited an expression pattern indicative of increased mitogenic activity, while a pharmacological dose (25 μmol/L) generated a pattern related to a high level of apoptosis and decreased cell proliferation in these cells. These findings are consistent with the high breast cancer cell proliferation seen with physiological genistein doses and inhibition of growth at pharmacological doses (1).
Su et al. (93) isolated mammary epithelial cells from the glands of rats fed a diet containing no isoflavones, soy protein isolate, or genistein throughout the fetal period until killing on postnatal d 50 and performed gene microarrays. They found that genistein affected Wnt and Notch signaling, indicating an effect on stem cell behavior, i.e. proliferation and differentiation. In a subsequent study, Su et al. (94) investigated the effects of lifetime genistein exposure on adhesion molecule E-cadherin. Results from this study indicated that E-cadherin was upregulated in the adult mammary gland by genistein; this might lead to inhibition of tumor progression.
Although genistein has been reported to upregulate several tumor suppressor genes and downregulate oncogenes, it is not known whether there is a causal relationship between these transcriptional changes and reduced tumorigenesis. Our recent data generated in heterozygous Brca1+/− knockout mice indicate that upregulation of this tumor suppressor is required for the mammary cancer-reducing effects of prepubertal dietary genistein exposure (S. de Assis, A. Warri, L. Hilakivi-Clarke, unpublished data).
Genistein modulates gene expression by epigenetic means
The mechanisms responsible for persistent changes in gene expression in the mammary glands of individuals exposed to genistein or other (estrogenic) compounds before onset of puberty may involve epigenetic modifications. Epigenetic regulation of gene expression has been identified as the key process allowing the environment during embryonic development to interact with the genotype, resulting in the observed phenotype (95, 96). These epigenetic mechanisms may control gene expression, which influences the differentiation process of the mammary epithelium. For example, DNA hypomethylation induced by treatment with 5-aza-2′ deoxycytidine prevents epithelial differentiation, indicating that DNA methylation is essential for this process (97).
We and others have shown that prepubertal genistein exposure increases the differentiation of the mammary epithelial tree (64). Because differentiation of mammary epithelial structures is closely related to breast cancer risk (98), it is possible that the protective effect of childhood and adolescent soy consumption on mammary gland morphology is epigenetically induced. For example, Wnt/β-catenin signaling regulates progenitor cells; overactivity of this pathway leads to excessive mammary outgrowth (90, 91). Chromatin remodeling factor Pygo2 epigenetically regulates transcriptional activation of β-catenin downstream of Wnt signaling (99). As discussed above, Wnt/β-catenin is one of the pathways affected by genistein/soy (93, 94) and, therefore, early-life exposure to soy may affect later breast cancer risk by altering gene expression epigenetically in a manner that affects mammary epithelial cell proliferation and differentiation.
Interestingly, genistein seems to induce both gene promoter methylation (100) and demethylation (101). When genistein is administered in utero, an increase in methylation takes place (100). Specifically, maternal exposure to genistein increases methylation of 6 cytosine-guanine sites in a retrotransposon upstream of the transcription start site of the Agouti gene at the viable yellow Agouti mice (100), increasing the number of Agouti offspring that exhibit the pseudo agouti phenotype linked to lowered cancer risk. Further, maternal genistein exposure negates the DNA hypomethylating effect of an endocrine disruptor, bisphenol A (102). However, genistein also can reactivate genes by promoter demethylation and active histone modification (103). It is not known whether prepubertal genistein exposure might increase or reduce methylation patterns.
Safety of soy intake after breast cancer diagnosis
Because of the apparent estrogenicity of genistein is soy, women diagnosed with breast cancer have been advised to avoid it. The concern about soy’s ability to promote breast cancer growth is heightened by our findings (40, 104) obtained in MCF-7 human breast cancer cells in vitro and in vivo. We have shown that doses of genistein mimicking human exposure levels increase proliferation of these cancer cells similarly to E2. Even more concerning is that genistein prevents the action of tamoxifen to inhibit the growth of the MCF-7 breast cancer cells in vitro and in vivo (105).
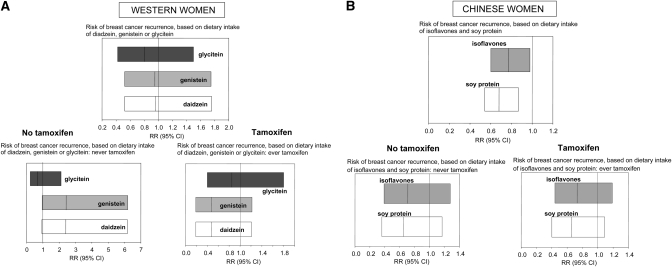
In 2009, 2 human studies were published that investigated the recurrence and metastasis of breast cancer in relation to soy intake or circulating isoflavone levels after diagnosis. A study done in 1954 female breast cancer survivors in the US determined their genistein, daidzein, and glycitein intake using the Fred Hutchinson Cancer Research Center FFQ and asking survivors about their use of herbal supplements and herbs (106). The mean follow-up time for these women was 6.31 y. No evidence of an association between risk of recurrence and intake of genistein [HR = 0.95 (95% CI 0.52–1.75)] or daidzein [HR = 0.96 (95% CI 0.52–1.76)] was found, but high glycitein intake was nonsignificantly associated with reduced risk (P-trend < 0.10) (Fig. 3). The study also addressed whether soy intake affected the risk of recurrence among women using tamoxifen, because animal studies have indicated that genistein prevents tamoxifen action in MCF-7 human breast cancer cells in vitro or in vivo (106). Tamoxifen users who consumed the highest level of soy products exhibited no increase in the risk of recurrence, but nonusers did. Women who had never taken tamoxifen but were estimated to have the highest intake of daidzein [HR = 2.40 (95% CI 0.93–6.18)] or genistein [HR = 2.42 (95% CI 0.95–6.21)] exhibited an almost significant increase in risk of recurrence. Because tamoxifen is not commonly given to women who exhibit ER-negative breast cancer, which is more aggressive than ER-positive tumors, the results are not surprising.
FIGURE 3.
Risk of recurrence of breast cancer by soy intake, assessed using FFQ, among 1954 Western breast cancer survivors (101) (A) and 5042 Chinese breast cancer survivors (102) (B). Relative risk (RR) and 95% CI are shown for glycitein, genistein, and daidzein intake in A and for isoflavone and soy protein intake in B. The study done in Western women showed no protective effect and an almost significantly increased risk of recurrence among those not taking tamoxifen and having the highest intake of genistein and daidzein. In contrast, the study done in Chinese women found a significantly reduced risk of recurrence in women having the highest level of isoflavones and soy protein in their diet. Reproduced with permission.
The study by Shu et al. (107) included 5042 Chinese breast cancer survivors diagnosed between 2002 and 2006 and followed for 3.9 y on average. Most of these women had radical mastectomy. Soy protein and isoflavone intakes were assessed from FFQ and by interview. The data indicated that women who continued to consume soy after diagnoses and treatment had significantly lower risk of recurrence than women in the lowest soy intake category [soy protein HR = 0.68 (95% CI 0.54–0.87); soy isoflavone HR = 0.77 (95% CI 0.60–0.98)]. The protective effect occurred in both women diagnosed with ER+ or ER− breast tumors and in tamoxifen users and nonusers. Although soy intake may not have affected their risk of developing breast cancer (60), these results provide powerful evidence in support of soy reducing the risk of recurrence among women who have consumed soy throughout their life.
It is puzzling why the data obtained using well-established breast cancer models that are used widely among investigators and findings in the 2 recent human studies are in stark contrast with each other. One likely explanation for the survival benefit in Chinese women consuming high levels of soy is that they probably consumed soy throughout the life. Although they developed breast cancer despite soy intake, the tumors may differ among the high and low soy consumers. Further, soy intake might inhibit the action of factors that are associated with breast cancer recurrence, such as angiogenesis or genes that promote the spread of cancer (108). Maskarinec et al. (72) found that breast tumors in women who consumed soy early in life expressed lower levels of HER2/neu protein and had reduced PCNA staining compared with tumors in women consuming low levels of soy. We have obtained similar data in mice fed a genistein-containing diet before puberty (S. de Assis, A. Warri, L. Hilakivi-Clarke, unpublished data).
Another factor that might play a role in explaining the differences in the results obtained in vitro and in vivo studies that used MCF-7 human breast cancer cells and recent human studies is that studies in MCF-7 cells do not take into account epithelial-stromal interactions, which are increasingly recognized as being critical for breast cancer initiation and growth (109, 110). As discussed above, activation of the ERα leads to cell proliferation through stromal EGFR (75). Further, normal stroma has been shown to prevent progression of transformed epithelial cells, whereas if the stroma is altered, it supports malignant growth and spread of (epithelial) breast cancer (111).
In conclusion, the breast cancer studies discussed in this review mostly have been done using genistein. Only a few studies have used whole soy and therefore it is not clear whether the effects of, e.g., genistein alone, genistein in isoflavone mix, genistein in soy protein isolate, or genistein in soy beans have similar biological effects. It is important to consider the potential interactions between phytoestrogens and other bioactive components in the food matrix that could either enhance or reduce their ultimate effect on health. Westernized soy products are quite different from those consumed in the traditional Asian diet. Most Asian soy products use whole soybeans with or without fermentation. Soy products or second generation soy foods in the US are mostly based on soy protein at different levels of purification or extraction such as texturized vegetable protein (~45% protein), soy protein concentrate (~70% protein), or isolates (~90% protein), each with a different profile of nutrient and non-nutrient compounds, including isoflavones and saponins (112, 113). It is likely that processing of soy foods modulates the profile of isoflavones and modifies their bioaccesibility and bioavailability, but how these differences affect breast cancer risk and risk of recurrence need to be investigated.
It can be concluded that consumption of soy foods or intake of soy isoflavones remains in the spotlight as a possible “treatment” to reduce menopausal symptoms and prevent bone loss. The beneficial effects of soy are more convincing if soy has been consumed throughout life rather than if the intake starts at menopause (114, 115). In the breast, soy intake during childhood and adolescence might provide lifelong protection against breast cancer (65–69) and sensitize for the protective effects of adult soy intake (65, 67). Although 2 recent human studies in breast cancer survivors did not indicate adverse effects (106, 107) and suggest a reduction in the risk of recurrence in Asian women consuming soy regularly, more studies are needed to determine whether Western soy products or isoflavone supplements are safe for women diagnosed with breast cancer.
Acknowledgments
L.H-C., J.E.A., and W.H. wrote the paper and L.H-C. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Published in a supplement to The Journal of Nutrition. Presented at the conference, “Soy Summit: Exploration of the Nutrition and Health Effects of Whole Soy,” held in New York, NY, September 21–22, 2009. The conference was organized by the Institute of Human Nutrition, Columbia University, through an unrestricted educational grant from Pharmavite, LLC. The supplement coordinators for this supplement are Sharon R. Akabas, Columbia University, and Connie M. Weaver, Purdue University. Supplement Coordinator disclosure: S. Akabas and C. Weaver received travel costs and an honorarium from a nonrestricted educational grant provided by Pharmavite LLC to Columbia University for the Soy Summit, which served as the basis for this supplement. The grant provided funding for the summit and also covered the cost of this journal supplement. Connie M. Weaver serves on Pharmavite’s Advisory Board. The Guest Editor for this supplement is Neil Shay. Guest Editor Disclosure: Neil Shay declares no conflict of interest. The supplement is the responsibility of the guest editors to whom the Editor of The Journal of Nutrition has delegated supervision of both technical conformity to the published regulations of The Journal of Nutrition and general oversight of the scientific merit of each article. Publication costs for this supplement were defrayed in part by the payment of page charges. This publication must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of The Journal of Nutrition.
Supported by the National Cancer Institute (U54 CA000970 to L.H-C.), the National Institute on Aging (P01 AG024387 to W.G.H.), the National Institute for Complementary and Alternative Medicine, the Office of Dietary Supplements, and the Women’s Health Initiative.
Abbreviations used: E2, estradiol; EGFR, epidermal growth factor receptor; ER, estrogen receptor; ERE, estrogen response element; Her2, human epidermal growth factor receptor 2; OR, odds ratio.
Literature Cited
- 1.Helferich WG, Andrade JE, Hoagland MS. Phytoestrogens and breast cancer: a complex story. Inflammopharmacology. 2008;16:219–26 [DOI] [PubMed] [Google Scholar]
- 2.Medina D. Mammary developmental fate and breast cancer risk. Endocr Relat Cancer. 2005;12:483–95 [DOI] [PubMed] [Google Scholar]
- 3.Henderson BE, Bernstein L. Endogenous and exogenous hormonal factors. : Harris JR, Lippman ME, Morrow M, Hellman S, Diseases of the breast. Philadelphia: Lippincott; 1996. p. 185–200 [Google Scholar]
- 4.Franke AA, Custer LJ. High-performance liquid chromatographic assay of isoflavonoids and coumestrol from human urine. J Chromatogr B Biomed Appl. 1994;662:47–60 [DOI] [PubMed] [Google Scholar]
- 5.Mazur W, Fotsis T, Wahala K, Ojala S, Salakka A, Adlercreutz H. Isotope dilution gas chromatographic-mass spectrometric method for the determination of isoflavonoids, coumestrol, and lignans in food samples. Anal Biochem. 1996;233:169–80 [DOI] [PubMed] [Google Scholar]
- 6.Erdman JW, Jr, Badger TM, Lampe JW, Setchell KD, Messina M. Not all soy products are created equal: caution needed in interpretation of research results. J Nutr. 2004;134:S1229–33 [DOI] [PubMed] [Google Scholar]
- 7.Chandrareddy A, Muneyyirci-Delale O, McFarlane SI, Murad OM. Adverse effects of phytoestrogens on reproductive health: a report of three cases. Complement Ther Clin Pract. 2008;14:132–5 [DOI] [PubMed] [Google Scholar]
- 8.Wu AH, Ziegler RG, Nomura AMY, West DW, Kolonel LN, Horn-Ross PL, Hoover RN, Pike MC. Soy intake and risk of breast cancer in Asians and Asian Americans. Am J Clin Nutr. 1998;68:S1437–43 [DOI] [PubMed] [Google Scholar]
- 9.Anderson JJ, Anthony MS, Cline JM, Washburn SA, Garner SC. Health potential of soy isoflavones for menopausal women. Public Health Nutr. 1999;2:489–504 [DOI] [PubMed] [Google Scholar]
- 10.Vincent A, Fitzpatrick LA. Soy isoflavones: are they useful in menopause? Mayo Clin Proc. 2000;75:1174–84 [DOI] [PubMed] [Google Scholar]
- 11.Anderson JW, Smith BM, Washnock CS. Cardiovascular and renal benefits of dry bean and soybean intake. Am J Clin Nutr. 1999;70:S464–74 [DOI] [PubMed] [Google Scholar]
- 12.Lichtenstein AH. Soy protein, isoflavones and cardiovascular disease risk. J Nutr. 1998;128:1589–92 [DOI] [PubMed] [Google Scholar]
- 13.Branca F. Dietary phyto-oestrogens and bone health. Proc Nutr Soc. 2003;62:877–87 [DOI] [PubMed] [Google Scholar]
- 14.Cotter A, Cashman KD. Genistein appears to prevent early postmenopausal bone loss as effectively as hormone replacement therapy. Nutr Rev. 2003;61:346–51 [DOI] [PubMed] [Google Scholar]
- 15.Fitzpatrick LA. Soy isoflavones: hope or hype? Maturitas. 2003;44Suppl 1:S21–9 [DOI] [PubMed] [Google Scholar]
- 16.Sirtori CR, Arnoldi A, Johnson SK. Phytoestrogens: end of a tale? Ann Med. 2005;37:423–38 [DOI] [PubMed] [Google Scholar]
- 17.Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113:1034–44 [DOI] [PubMed] [Google Scholar]
- 18.Ferlay J, Bray F, Pisani P. Cancer incidence, mortality and prevalence worldwide. IARC Cancer Base no. 5. Lyon: IARC Press; 2004.; [Google Scholar]
- 19.Ganry O. Phytoestrogen and breast cancer prevention. Eur J Cancer Prev. 2002;11:519–22 [DOI] [PubMed] [Google Scholar]
- 20.Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AMY, West DW, Wu-Williams AH, Kolonel LN, Horn-Ross PL, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819–27 [DOI] [PubMed] [Google Scholar]
- 21.Kennedy AR, Manzone H. Effects of protease inhibitors on levels of proteolytic activity in normal and premalignant cells and tissues. J Cell Biochem Suppl. 1995;22:188–94 [DOI] [PubMed] [Google Scholar]
- 22.Shamsuddin AM. Inositol phosphates have novel anticancer function. J Nutr. 1995;125:S725–32 [DOI] [PubMed] [Google Scholar]
- 23.Shamsuddin AM, Yang GY. Inositol hexaphosphate inhibits growth and induces differentiation of PC-3 human prostate cancer cells. Carcinogenesis. 1995;16:1975–9 [DOI] [PubMed] [Google Scholar]
- 24.Hedlund TE, van Bokhoven A, Johannes WU, Nordeen SK, Ogden LG. Prostatic fluid concentrations of isoflavonoids in soy consumers are sufficient to inhibit growth of benign and malignant prostatic epithelial cells in vitro. Prostate. 2006;66:557–66 [DOI] [PubMed] [Google Scholar]
- 25.Sasamura H, Takahashi A, Yuan J, Kitamura H, Masumori N, Miyao N, Itoh N, Tsukamoto T. Antiproliferative and antiangiogenic activities of genistein in human renal cell carcinoma. Urology. 2004;64:389–93 [DOI] [PubMed] [Google Scholar]
- 26.Shao ZM, Wu J, Shen ZZ, Barsky SH. Dietary genistein exerts estrogenic effects on human breast carcinoma cells. Cancer Res. 1998;58:4851–7 [PubMed] [Google Scholar]
- 27.Shen J, Tai YC, Zhou J, Stephen Wong CH, Cheang PT, Fred Wong WS, Xie Z, Khan M, Han JH, et al. Synergistic antileukemia effect of genistein and chemotherapy in mouse xenograft model and potential mechanism through MAPK signaling. Exp Hematol. 2007;35:75–83 [DOI] [PubMed] [Google Scholar]
- 28.Yanagihara K, Ito A, Toge T, Numoto M. Antiproliferative effects of isoflavones on human cancer cell lines established from the gastrointestinal tract. Cancer Res. 1993;53:5815–21 [PubMed] [Google Scholar]
- 29.This P, De La RA, Clough K, Fourquet A, Magdelenat H. Phytoestrogens after breast cancer. Endocr Relat Cancer. 2001;8:129–34 [DOI] [PubMed] [Google Scholar]
- 30.Paech K, Webb P, Kuiper GG, Gustafsson JA, Kushner PJ, Scanlan TS. Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science. 1997;277:1508–10 [DOI] [PubMed] [Google Scholar]
- 31.Sotoca AM, Ratman D, van der Saag P, Strom A, Gustafsson JA, Vervoort J, Rietjens IM, Murk AJ. Phytoestrogen-mediated inhibition of proliferation of the human T47D breast cancer cells depends on the ERα/ERβ ratio. J Steroid Biochem Mol Biol. 2008;112:171–8 [DOI] [PubMed] [Google Scholar]
- 32.Rajah TT, Du N, Drews N, Cohn R. Genistein in the presence of 17β-estradiol inhibits proliferation of ERβ breast cancer cells. Pharmacology. 2009;84:68–73 [DOI] [PubMed] [Google Scholar]
- 33.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139:4252–63 [DOI] [PubMed] [Google Scholar]
- 34.Chang EC, Charn TH, Park SH, Helferich WG, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen Receptors α and β as determinants of gene expression: influence of ligand, dose, and chromatin binding. Mol Endocrinol. 2008;22:1032–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Speirs V, Walker RA. New perspectives into the biological and clinical relevance of oestrogen receptors in the human breast. J Pathol. 2007;211:499–506 [DOI] [PubMed] [Google Scholar]
- 36.Liu G, Schwartz JA, Brooks SC. p53 Down-regulates ER-responsive genes by interfering with the binding of ER to ERE. Biochem Biophys Res Commun. 1999;264:359–64 [DOI] [PubMed] [Google Scholar]
- 37.Santell RC, Chang YC, Nair MG, Helferich WG. Dietary genistein exerts estrogenic effects upon the uterus, mammary gland and the hypothalamic/pituitary axis in rats. J Nutr. 1997;127:263–9 [DOI] [PubMed] [Google Scholar]
- 38.Strauss L, Makela S, Joshi S, Huhtaniemi I, Santti R. Genistein exerts estrogen-like effects in male mouse reproductive tract. Mol Cell Endocrinol. 1998;144:83–93 [DOI] [PubMed] [Google Scholar]
- 39.Hsieh CY, Santell RC, Haslam SZ, Helferich WG. Estrogenic effects of genistein on the growth of estrogen receptor -positive human breast cancer (MCF-7) cells in vitro and in vivo. Cancer Res. 1998;58:3833–8 [PubMed] [Google Scholar]
- 40.Ju YH, Allred CD, Allred KF, Karko KL, Doerge DR, Helferich WG. Physiological concentrations of dietary genistein dose-dependently stimulate growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in athymic nude mice. J Nutr. 2001;131:2957–62 [DOI] [PubMed] [Google Scholar]
- 41.Allred CD, Ju YH, Allred KF, Chang J, Helferich WG. Dietary genistin stimulates growth of estrogen-dependent breast cancer tumors similar to that observed with genistein. Carcinogenesis. 2001;22:1667–73 [DOI] [PubMed] [Google Scholar]
- 42.Gallo D, Ferlini C, Fabrizi M, Prislei S, Scambia G. Lack of stimulatory activity of a phytoestrogen-containing soy extract on the growth of breast cancer tumors in mice. Carcinogenesis. 2006;27:1404–9 [DOI] [PubMed] [Google Scholar]
- 43.Allred CD, Allred KF, Ju YH, Goeppinger TS, Doerge DR, Helferich WG. Soy processing influences growth of estrogen-dependent breast cancer tumors. Carcinogenesis. 2004;25:1649–57 [DOI] [PubMed] [Google Scholar]
- 44.Penttinen-Damdimopoulou PE, Power KA, Hurmerinta TT, Nurmi T, van der Saag PT, Makela SI. Dietary sources of lignans and isoflavones modulate responses to estradiol in estrogen reporter mice. Mol Nutr Food Res. 2009;53:996–1006 [DOI] [PubMed] [Google Scholar]
- 45.Cline JM, Wood CE. Estrogen/isoflavone interactions in cynomolgus macaques (Macaca fascicularis). Am J Primatol. 2009;71:722–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wood CE, Appt SE, Clarkson TB, Franke AA, Lees CJ, Doerge DR, Cline JM. Effects of high-dose soy isoflavones and equol on reproductive tissues in female cynomolgus monkeys. Biol Reprod. 2006;75:477–86 [DOI] [PubMed] [Google Scholar]
- 47.Wood CE, Kaplan JR, Stute P, Cline JM. Effects of soy on the mammary glands of premenopausal female monkeys. Fertil Steril. 2006;85Suppl 1:1179–86 [DOI] [PubMed] [Google Scholar]
- 48.Wood CE, Register TC, Cline JM. Soy isoflavonoid effects on endogenous estrogen metabolism in postmenopausal female monkeys. Carcinogenesis. 2007;28:801–8 [DOI] [PubMed] [Google Scholar]
- 49.Sharpe RM, Martin B, Morris K, Greig I, McKinnell C, McNeilly AS, Walker M. Infant feeding with soy formula milk: effects on the testis and on blood testosterone levels in marmoset monkeys during the period of neonatal testicular activity. Hum Reprod. 2002;17:1692–703 [DOI] [PubMed] [Google Scholar]
- 50.Lu L-JW, Anderson KE, Grady JJ, Nagamani M. Effects of soya consumption for one month on steroid hormones in premenopausal women: implications for breast cancer risk reduction. Cancer Epidemiol Biomarkers Prev. 1996;5:63–70 [PubMed] [Google Scholar]
- 51.Kurzer MS. Hormonal effects of soy isoflavones: studies in premenopausal and postmenopausal women. J Nutr. 2000;130:S660–1 [DOI] [PubMed] [Google Scholar]
- 52.Hooper L, Ryder JJ, Kurzer MS, Lampe JW, Messina MJ, Phipps WR, Cassidy A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update. 2009;15:423–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petrakis NL, Barnes S, King EB, Lowenstein J, Wiencke J, Lee MM, Miike R, Kirk M, Coward L. Stimulatory influence of soy protein isolate on breast secretion in pre- and postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1996;5:785–94 [PubMed] [Google Scholar]
- 54.McMichael-Phillips DF, Harding C, Morton M, Robert SA, Howell A, Potten CS, Bundred NJ. Effects of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. Am J Clin Nutr. 1998;68:S1431–6 [DOI] [PubMed] [Google Scholar]
- 55.Hargreaves DF, Potten CS, Harding C. Two-week soy supplementation has an estrogenic effect on normal premenopausal breast. J Clin Endocrinol Metab. 1999;84:4017–24 [DOI] [PubMed] [Google Scholar]
- 56.Boyd NF, Martin LJ, Stone J, Greenberg C, Minkin S, Yaffe MJ. Mammographic densities as a marker of human breast cancer risk and their use in chemoprevention. Curr Oncol Rep. 2001;3:314–21 [DOI] [PubMed] [Google Scholar]
- 57.Maskarinec G, Takata Y, Franke AA, Williams AE, Murphy SP. A 2-year soy intervention in premenopausal women does not change mammographic densities. J Nutr. 2004;134:3089–94 [DOI] [PubMed] [Google Scholar]
- 58.Maskarinec G, Verheus M, Steinberg FM, Amato P, Cramer MK, Lewis RD, Murray MJ, Young RL, Wong WW. Various doses of soy isoflavones do not modify mammographic density in postmenopausal women. J Nutr. 2009;139:981–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verheus M, van Gils CH, Kreijkamp-Kaspers S, Kok L, Peeters PH, Grobbee DE, van der Schouw YT. Soy protein containing isoflavones and mammographic density in a randomized controlled trial in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:2632–8 [DOI] [PubMed] [Google Scholar]
- 60.Trock B, Butler LW, Clarke R, Hilakivi-Clarke L. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst. 2006;98:459–71 [DOI] [PubMed] [Google Scholar]
- 61.Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br J Cancer. 2008;98:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen LA, Zhao Z, Pittman BSJA. Effect of intact and isoflavone-depleted soy protein on NMU-induced rat mammary tumorigenesis. Carcinogenesis. 2000;21:929–35 [DOI] [PubMed] [Google Scholar]
- 63.Kim H, Hall P, Smith M, Kirk M, Prasain JK, Barnes S, Grubbs C. Chemoprevention by grape seed extract and genistein in carcinogen-induced mammary cancer in rats is diet dependent. J Nutr. 2004;134:S3445–52 [DOI] [PubMed] [Google Scholar]
- 64.Warri A, Saarinen NM, Makela SI, Hilakivi-Clarke L. The role of early life genistein exposures in modifying breast cancer risk. Br J Cancer 2008;98:1485–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Korde LA, Wu AH, Fear T. Childhood soy intake and breast cancer risk in Asian-American women. Cancer Epidemiol Biomarkers Prev. 2009;18:1050–9 [DOI] [PubMed] [Google Scholar]
- 66.Shu XO, Jin F, Dai Q, Wen W, Potter JD, Kushi LH, Ruan Z, Gao YT, Zheng W. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol Biomarkers Prev. 2001;10:483–8 [PubMed] [Google Scholar]
- 67.Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, Pike MC. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis. 2002;23:1491–6 [DOI] [PubMed] [Google Scholar]
- 68.Thanos J, Cotterchio M, Boucher BA, Kreiger N, Thompson LU. Adolescent dietary phytoestrogen intake and breast cancer risk (Canada). Cancer Causes Control. 2006;17:1253–61 [DOI] [PubMed] [Google Scholar]
- 69.Lee SA, Shu XO, Li H, Yang G, Cai H, Wen W, Ji BT, Gao J, Gao YT, et al. Adolescent and adult soy food intake and breast cancer risk: results from the Shanghai Women's Health Study. Am J Clin Nutr. 2009;89:1920–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cabanes A, Wang M, Olivo S, de Assis S, Gustafsson JA, Khan G, Hilakivi-Clarke L. Prepubertal estradiol and genistein exposures up-regulate BRCA1 mRNA and reduce mammary tumorigenesis. Carcinogenesis. 2004;25:741–8 [DOI] [PubMed] [Google Scholar]
- 71.Su Y, Eason RR, Geng Y, Till SR, Badger TM, Simmen RC. In utero exposure to maternal diets containing soy protein isolate, but not genistein alone, protects young adult rat offspring from NMU-induced mammary tumorigenesis. Carcinogenesis. 2007;28:1046–51 [DOI] [PubMed] [Google Scholar]
- 72.Maskarinec G, Erber E, Verheus M, Hernandez BY, Killeen J, Cashin S, Cline JM. Soy consumption and histopathologic markers in breast tissue using tissue microarrays. Nutr Cancer. 2009;61:708–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clarke RB, Howell A, Potten CS, Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57:4987–91 [PubMed] [Google Scholar]
- 74.Shoker BS, Jarvis C, Clarke RB, Anderson E, Hewlett J, Davies MP, Sibson DR, Sloane JP. Estrogen receptor positive proliferating cells in the normal and precancerous breast. Am J Pathol. 1999;155:1811–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor α function in mammary gland development. Proc Natl Acad Sci USA. 2007;104:5455–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peterson G. Evaluation of the biochemical targets of genistein in tumor cells. J Nutr. 1995;125:S784–9 [DOI] [PubMed] [Google Scholar]
- 77.Kim HA, Jeong KS, Park DH, Lee JA, Jeong WI, Kim YK. Heavy water labeling method for measuring the effect of genistein on mammary gland carcinogenesis. Mol Cell Biochem. 2007;301:201–8 [DOI] [PubMed] [Google Scholar]
- 78.Pei RJ, Sato M, Yuri T, Danbara N, Nikaido Y, Tsubura A. Effect of prenatal and prepubertal genistein exposure on N-methyl-N-nitrosourea-induced mammary tumorigenesis in female Sprague-Dawley rats. In Vivo. 2003;17:349–57 [PubMed] [Google Scholar]
- 79.Sakla MS, Shenouda NS, Ansell PJ, MacDonald RS, Lubahn DB. Genistein affects HER2 protein concentration, activation, and promoter regulation in BT-474 human breast cancer cells. Endocrine. 2007;32:69–78 [DOI] [PubMed] [Google Scholar]
- 80.Fan S, Meng Q, Auborn K, Carter T, Rosen EM. BRCA1 and BRCA2 as molecular targets for phytochemicals indole-3-carbinol and genistein in breast and prostate cancer cells. Br J Cancer. 2006;94:407–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dave B, Eason RR, Till SR, Geng Y, Velarde MC, Badger TM, Simmen RC. The soy isoflavone genistein promotes apoptosis in mammary epithelial cells by inducing the tumor suppressor PTEN. Carcinogenesis. 2005;26:1793–803 [DOI] [PubMed] [Google Scholar]
- 82.Shajahan A, Goel S, de Assis S, Yu B, Clarke R, Hilakivi-Clarke L. Changes in mammary caveolin-1 signaling pathways are associated with breast cancer risk in rats exposed to estradiol in utero or during prepuberty. Hormone Molecular Biology and Clinical Investigation. In press2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blanco-Aparicio C, Renner O, Leal JF, Carnero A. PTEN, more than the AKT pathway. Carcinogenesis. 2007;28:1379–86 [DOI] [PubMed] [Google Scholar]
- 84.Stiles B, Groszer M, Wang S, Jiao J, Wu H. PTENless means more. Dev Biol. 2004;273:175–84 [DOI] [PubMed] [Google Scholar]
- 85.Ashworth A. Refocusing on BRCA1. Nat Cell Biol. 2004;6:916–7 [DOI] [PubMed] [Google Scholar]
- 86.Dupont J, Renou JP, Shani M, Hennighausen L, LeRoith D. PTEN overexpression suppresses proliferation and differentiation and enhances apoptosis of the mouse mammary epithelium. J Clin Invest. 2002;110:815–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005;288:C494–506 [DOI] [PubMed] [Google Scholar]
- 88.Li Y, Podsypanina K, Liu X, Crane A, Tan LK, Parsons R, Varmus HE. Deficiency of Pten accelerates mammary oncogenesis in MMTV-Wnt-1 transgenic mice. BMC Mol Biol. 2001;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sotgia F, Williams TM, Cohen AW, Minetti C, Pestell RG, Lisanti MP. Caveolin-1-deficient mice have an increased mammary stem cell population with upregulation of Wnt/β-catenin signaling. Cell Cycle. 2005;4:1808–16 [DOI] [PubMed] [Google Scholar]
- 90.Bocchinfuso WP, Hively WP, Couse JF, Varmus HE, Korach KS. A mouse mammary tumor virus-Wnt-1 transgene induces mammary gland hyperplasia and tumorigenesis in mice lacking estrogen receptor-α. Cancer Res. 1999;59:1869–76 [PubMed] [Google Scholar]
- 91.Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci USA. 2003;100:15853–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lavigne JA, Takahashi Y, Chandramouli GV, Liu H, Perkins SN, Hursting SD, Wang TT. Concentration-dependent effects of genistein on global gene expression in MCF-7 breast cancer cells: an oligo microarray study. Breast Cancer Res Treat. 2008;110:85–98 [DOI] [PubMed] [Google Scholar]
- 93.Su Y, Simmen FA, Xiao R, Simmen RC. Expression profiling of rat mammary epithelial cells reveals candidate signaling pathways in dietary protection from mammary tumors. Physiol Genomics. 2007;30:8–16 [DOI] [PubMed] [Google Scholar]
- 94.Su Y, Simmen RC. Soy isoflavone genistein upregulates epithelial adhesion molecule E-cadherin expression and attenuates β-catenin signaling in mammary epithelial cells. Carcinogenesis. 2009;30:331–9 [DOI] [PubMed] [Google Scholar]
- 95.Shi L, Wu J. Epigenetic regulation in mammalian preimplantation embryo development. Reprod Biol Endocrinol. 2009;7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Plachot C, Lelievre SA. DNA methylation control of tissue polarity and cellular differentiation in the mammary epithelium. Exp Cell Res. 2004;298:122–32 [DOI] [PubMed] [Google Scholar]
- 98.Russo J, Mailo D, Hu YF, Balogh G, Sheriff F, Russo IH. Breast differentiation and its implication in cancer prevention. Clin Cancer Res. 2005;11:S931–6 [PubMed] [Google Scholar]
- 99.Gu B, Sun P, Yuan Y, Moraes RC, Li A, Teng A, Agrawal A, Rheaume C, Bilanchone V, et al. Pygo2 expands mammary progenitor cells by facilitating histone H3 K4 methylation. J Cell Biol. 2009;185:811–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Day JK, Bauer AM, DesBordes C, Zhuang Y, Kim BE, Newton LG, Nehra V, Forsee KM, MacDonald RS, et al. Genistein alters methylation patterns in mice. J Nutr. 2002;132:S2419–23 [DOI] [PubMed] [Google Scholar]
- 102.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA. 2007;104:13056–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Majid S, Dar AA, Shahryari V, Hirata H, Ahmad A, Saini S, Tanaka Y, Dahiya AV, Dahiya R. Genistein reverses hypermethylation and induces active histone modifications in tumor suppressor gene B-cell translocation gene 3 in prostate cancer. Cancer. 2010;116:66–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Allred CD, Allred KF, Ju YH, Virant SM, Helferich WG. Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner. Cancer Res. 2001;61:5045–50 [PubMed] [Google Scholar]
- 105.Ju YH, Doerge DR, Allred KF, Allred CD, Helferich WG. Dietary genistein negates the inhibitory effect of tamoxifen on growth of estrogen-dependent human breast cancer (MCF-7) cells implanted in athymic mice. Cancer Res. 2002;62:2474–7 [PubMed] [Google Scholar]
- 106.Guha N, Kwan ML, Quesenberry CP, Jr, Weltzien EK, Castillo AL, Caan BJ. Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: the Life After Cancer Epidemiology study. Breast Cancer Res Treat. 2009;118:395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shu XO, Zheng Y, Cai H, Gu K, Chen Z, Zheng W, Lu W. Soy food intake and breast cancer survival. JAMA. 2009;302:2437–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Messina M, McCaskill-Stevens W, Lampe JW. Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. J Natl Cancer Inst. 2006;98:1275–84 [DOI] [PubMed] [Google Scholar]
- 109.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–601 [DOI] [PubMed] [Google Scholar]
- 110.Kass L, Erler JT, Dembo M, Weaver VM. Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis. Int J Biochem Cell Biol. 2007;39:1987–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arendt LM, Rudnick JA, Keller PJ, Kuperwasser C. Stroma in breast development and disease. Semin Cell Dev Biol. 2010;21:11–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Setchell KD, Cole SJ. Variations in isoflavone levels in soy foods and soy protein isolates and issues related to isoflavone databases and food labeling. J Agric Food Chem. 2003;51:4146–55 [DOI] [PubMed] [Google Scholar]
- 113.Fang N, Yu S, Badger TM. Comprehensive phytochemical profile of soy protein isolate. J Agric Food Chem. 2004;52:4012–20 [DOI] [PubMed] [Google Scholar]
- 114.Mardon J, Mathey J, Kati-Coulibaly S, Puel C, Davicco MJ, Lebecque P, Horcajada MN, Coxam V. Influence of lifelong soy isoflavones consumption on bone mass in the rat. Exp Biol Med (Maywood). 2008;233:229–37 [DOI] [PubMed] [Google Scholar]
- 115.Piekarz AV, Ward WE. Effect of neonatal exposure to genistein on bone metabolism in mice at adulthood. Pediatr Res. 2007;61:48–53 [DOI] [PubMed] [Google Scholar]