Abstract
Aims
Results from investigations in one area of the world may not translate to another if patient characteristics and practices differ. We examine differences in the presentation and management of emergency department (ED) patients with dyspnoea from acute heart failure syndromes (AHFS) between the USA, Western Europe, and Eastern Europe.
Methods and results
The URGENT Dyspnoea study was a multinational prospective observational study of dyspnoeic ED patients with AHFS from 18 countries. Acute heart failure syndrome patients from the USA and Western and Eastern Europe underwent dyspnoea assessments within 1 h of the first physician evaluation. Patient characteristics, evaluation, and treatments were compared between geographical regions using analysis of variance and χ2 tests. Four hundred and ninety-three patients with AHFS met the inclusion criteria. Participants in the USA were more frequently non-white, younger, on chronic beta-blocker therapy, and with an ejection fraction ≤40% when compared with Eastern and Western Europe. Patients from Eastern Europe were more likely to present with de novo heart failure and have ischaemic electrocardiogram changes. Pulmonary oedema was more common on chest radiograph in Western Europe, but natriuretic peptide levels were elevated in all three regions. Diuretic use was similar across all the regions. Intravenous nitroglycerin was used more frequently in Eastern (32.8%) and Western Europe (24.4%) compared with the USA (2.5%).
Conclusion
International differences in AHFS presentations and management between regions suggest results from clinical trials in one region may not translate directly to another. These differences should be considered when designing trials and interpreting the results from clinical investigations.
Keywords: Heart failure, Emergency, Geographical differences
Introduction
Differences between countries and regions in demographics, clinical characteristics, treatment patterns, and outcomes have been described for hospitalized patients with AHFS.1–5 However, one commonality across patients with acute heart failure syndromes (AHFS) is frequent presentation to the emergency department (ED) for initial evaluation and treatment. This initial phase of management has typically been overlooked in prior AHFS study design, resulting in limited understanding of early therapeutic management and its downstream effects. Whether geography further highlights variation during the ED phase of management has not been well-delineated. Although clinical trials often recruit patients globally, there is a paucity of information to help caregivers generalize results of clinical investigations conducted outside their region in order to improve the emergent evaluation and treatment of AHFS.
Recent clinical trials have suggested that standard therapy rapidly improves dyspnoea in a majority of patients.6–8 However, the speed and magnitude of improvement as a result of standard therapy has not been prospectively studied, and how findings from one trial can be extrapolated to the care of individual patients' remains unclear. The goal of the Ularitide Global Evaluation in Acute Decompensated Heart Failure (URGENT) Dyspnoea Study was to better understand how acute standard therapy impacts dyspnoea in AHFS.9 Owing to its multinational nature, information from this study may provide an opportunity to determine differences in AHFS presentation and management between various regions of the world. We conducted this secondary analysis to describe the international variations in the presentation and management of ED patients with dyspnoea secondary to AHFS.
Methods
Study design and setting
URGENT Dyspnoea has been described previously.9,10 Briefly, this was a multinational, observational, prospective cohort study, conducted at tertiary care, and community hospitals in 18 countries, which evaluated the effect of conventional AHFS therapy on self-assessed dyspnoea during the earliest hospital phase of AHFS care. The majority of the North American hospitals were tertiary care, urban, and academic medical centres. All of the hospitals in North America were associated with residency training programmes and all of the EDs in these hospitals had emergency medicine residents participating in patient care as well as enrolling subjects in the study. The majority of enrolling hospitals in Western Europe and Eastern Europe were university-affiliated institutions. The entire list of hospitals and investigators has been previously acknowledged.9 The study was originally designed to precede the phase 3 programme with ularitide. This investigational agent was not used at any time during this study. Agents used to treat AHFS were at the discretion of the treating physician, however, investigational therapeutic agents were not allowed. Patients were enrolled by either attending physicians or physicians in training within 1 h of physician evaluation in an ED or equivalent acute care setting.
Patients
Inclusion and exclusion criteria were sufficiently broad to include patients in whom AHFS was initially suspected. AHFS was then confirmed or refuted by the investigator 6 h after enrolment, utilizing all available data. Eligible patients were 18 years or older, able to give written informed consent, with signs and symptoms of AHFS and able to self-assess dyspnoea within 1 h of initial physician evaluation. Patients were included in this secondary analysis if they were enrolled in one of 18 countries in Western Europe or Eastern Europe, or in the USA, and had a final diagnosis of AHFS as determined by the investigator at 6 h after enrolment. The study complied with the Declaration of Helsinki, and institutional review board and/or ethics committee approval was obtained from each centre.
Study protocol and data collection
Dyspnoea was assessed with three instruments:
a 5-point Likert scale done at the time of enrolment (baseline) and 6 h later: (a) not short of breath, (b) mildly short of breath, (c) moderately short of breath, (d) severely short of breath, (e) very severely short of breath;
a 7-point Likert scale administered at 6 h after baseline to determine the change from baseline: (a) markedly worse, (b) moderately worse, c) minimally worse, (d) no change, (e) minimally improved, (f) moderately improved, (g) markedly improved;
a 10 cm visual analogue scale (VAS) score done at baseline and 6 h later. A priori, this line was divided into 10 equal 1cm increments, 0–10. If patients marked anywhere within a centimetre increment, it was counted as that centimetre (i.e. 26 mm = 3 cm, 21 mm = 3 cm).
Scales were translated into local languages as needed. If the subject was intubated at the time of the second dyspnoea test, this was indicated on the data collection form and the second dyspnoea test was not administered. It was recommended that the same physician make the assessment at baseline and 6 h.
Other data collected prospectively included demographics, medical history, patient reported signs and symptoms, physical examination, and electrocardiogram (ECG) findings as documented by the treating physician. Vital signs were obtained within 1 h of each dyspnoea test. Medications administered and use of non-invasive ventilation in the ED and prior to arrival were recorded. Laboratory tests and chest radiography (CXR) findings were obtained by medical record review. The radiology reading of the CXR was taken as the criterion standard. All patient evaluation and treatment was at the treating physician's discretion. There were no pre-specified criteria required to make an AHFS diagnosis; the final diagnosis of AHFS was determined by the care provider based on all available data present at the 6 h assessment. Available data included, for example, physical examination, CXR, B-type natriuretic peptide (BNP) or NT-proBNP, troponin, medications administered, and medical records. The physician's impression as to factors precipitating AHFS as well as patient disposition at the end of their acute presentation was also prospectively documented.
Statistical analysis
Data are described using medians and ranges or means and standard deviations for continuous variables, and frequencies and percentages for categorical variables. Variables from different geographical regions were compared between the three different regions: USA, Western Europe, and Eastern Europe. Analysis of variance (ANOVA) was used to analyse variables with continuous outcome measures, and χ2 tests were used to analyse variables with proportional outcome measures. The critical P-value was set to 0.05; post hoc two-group comparisons used a Bonferroni adjusted P-value. Owing to the multiple comparisons, a highly conservative critical P-value of 0.008 can be used to maintain the overall type 1 error rate at 5%. Analyses were done with SPSS version 17 (SPSS Inc., Chicago, IL, USA).
Results
International origin of study subjects
From 1 January 2007 to 31 Aug 2007, 776 patients with suspected AHFS from 18 countries were enrolled. Of these, 524 (68%) had AHFS diagnosed at 6 h and 79 (10%) did not. For the remaining 173 (22%) patients, the diagnosis was indeterminate or was left unmarked on the case report form at the 6 h time-point. There were 31 of 524 AHFS patients from South America excluded from this analysis, leaving 493 patients with AHFS from 28 sites eligible for inclusion: 122 (24.7%) from the USA, 293 (59.4%) from Eastern Europe, and 78 (15.8%) from Western Europe (Table 1).
Table 1.
Distribution of study subjects by country
| Country | ED study sites | Subjects, n (%) |
|---|---|---|
| Belgium | 2 | 8 (1.6) |
| Bulgaria | 1 | 52 (10.5) |
| Croatia | 1 | 14 (2.8) |
| Czech Republic | 1 | 98 (19.9) |
| Finland | 1 | 1 (0.2) |
| France | 6 | 30 (6.1) |
| Germany | 3 | 17 (3.4) |
| Poland | 1 | 6 (1.2) |
| Romania | 1 | 55 (11.2) |
| Slovakia | 1 | 59 (12.0) |
| Spain | 1 | 22 (4.5) |
| USA | 8 | 122 (24.7) |
| Ukraine | 1 | 9 (1.8) |
| Total | 28 | 493 (100) |
Similarities and differences in patient characteristics, precipitating factors, signs, and symptoms
Patients from all regions were similar with respect to gender, and outpatient use of ACE inhibitors, diuretics, angiotensin II receptor blockers, and statins. Emergency department patients with AHFS from the USA were significantly more likely to be non-white, younger, prescribed chronic beta-blocker therapy as outpatients, and have an ejection fraction ≤40%; they were less likely to have valvular disease (Table 2). Patients from the USA were less likely to present with an arrhythmia, but more likely to have non-adherence as the precipitating factor of their AHFS. Patients from Eastern Europe were more likely to present with de novo heart failure. Those from Western Europe were less likely to have uncontrolled hypertension as a precipitating factor of their AHFS.
Table 2.
Baseline clinical characteristics of emergency department patients with acute heart failure syndromes by international region
| Clinical characteristic | USA, n = 122 | Western Europe, n = 78 | Eastern Europe, n = 293 | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Mean age (years, s.d.) | 63 (16) | 78 (11) | 68.8 (12) | <0.001 |
| Gender (male) | 67 (54.9%) | 38 (48.7%) | 172 (58.7%) | 0.272 |
| Race (white) | 45 (36.8%) | 74 (94.9%) | 293 (100%) | <0.001 |
| Co-morbidities | ||||
| Prior HF history | 96 (78.7%) | 53 (68.0) | 151 (51.4%) | <0.001 |
| Prior myocardial infarction | 32 (26.2%) | 15 (19.2%) | 79 (27.0%) | 0.373 |
| Valvular disease | 12 (9.8%) | 21 (26.9%) | 45 (15.4%) | 0.005 |
| LVEF < 40% | 51 (57.3%) | 7 (24.1%) | 56 (45.5%) | 0.007 |
| Asthma/COPD | 37 (30.3%) | 18 (23.1%) | 32 (10.9%) | <0.001 |
| Prior coronary bypass grafts | 21 (17.2%) | 10 (12.8%) | 9 (3.0%) | <0.001 |
| Diabetes (insulin dependent) | 37 (30.3%) | 21 (26.9%) | 26 (8.9%) | 0.032 |
| Renal insufficiency* | 38 (31.1%) | 25 (32.1%) | 70 (23.9%) | 0.173 |
| Current medications | ||||
| Beta-blocker | 84 (68.9%) | 39 (50.0%) | 159 (54.3%) | <0.001 |
| ACE inhibitor | 57 (46.7%) | 31 (39.7%) | 144 (49.1%) | 0.334 |
| Diuretic | 89 (73.0%) | 47 (60.3%) | 185 (63.1%) | 0.1 |
| Angiotensin II receptor blocker | 14 (11.5%) | 12 (15.4%) | 35 (12.0%) | 0.673 |
| Statins | 42 (34.4%) | 23 (29.5%) | 72 (24.6%) | 0.116 |
| Aldosterone antagonist | 6 (4.92%) | 6 (7.70%) | 41 (14.0%) | 0.016 |
| Digoxin | 11 (9.02%) | 8 (10.3%) | 77 (26.3%) | <0.001 |
| Coumadin | 23 (18.9%) | 15 (19.2%) | 43 (14.7%) | 0.444 |
| Pacemaker | 19 (15.6%) | 4 (5.1%) | 10 (3.4%) | <0.001 |
| Precipitating factors | ||||
| Acute coronary syndrome | 22 (18.0%) | 12 (15.4%) | 46 (15.7%) | 0.821 |
| Arrythmia | 10 (8.2%) | 15 (19.2%) | 58 (19.8%) | 0.013 |
| Medication non-adherence | 22 (18.0%) | 5 (6.4%) | 40 (13.7%) | 0.065 |
| Dietary non-adherence | 18 (14.8%) | 3 (3.9%) | 30 (10.2%) | 0.047 |
| Hypertension | 32 (26.2%) | 14 (18.0%) | 97 (33.1%) | 0.024 |
| Physical examination findings | ||||
| Jugular Venous distension | 35 (28.7%) | 33 (42.3%) | 140 (47.8%) | 0.002 |
| Rales | 61 (50.0%) | 69 (88.4%) | 197 (67.2%) | <0.001 |
| Peripheral oedema | 79 (64.7%) | 50 (64.1%) | 136 (46.4%) | <0.001 |
| Vital signs | ||||
| Heart rate (per minute, s.d.) | 85 (19) | 92 (24) | 92 (26) | 0.021 |
| Respiratory rate (per minute, s.d.) | 22 (6) | 26 (8) | 21 (6) | <0.0001 |
| Systolic blood pressure (mm Hg, s.d.) | 143 (36) | 149 (30) | 143 (34) | 0.3 |
| Temperature (°F, s.d.) | 97.6 (0.9) | 97.9 (1.3) | 97.9 (0.6) | 0.002 |
Data are presented as medians and ranges or proportions and percentages unless otherwise indicated.
*Renal insufficiency as documented in the medical record or per patient report.
HF, heart failure; LVEF, left ventricular ejection; COPD, chronic obstructive pulmonary disease.
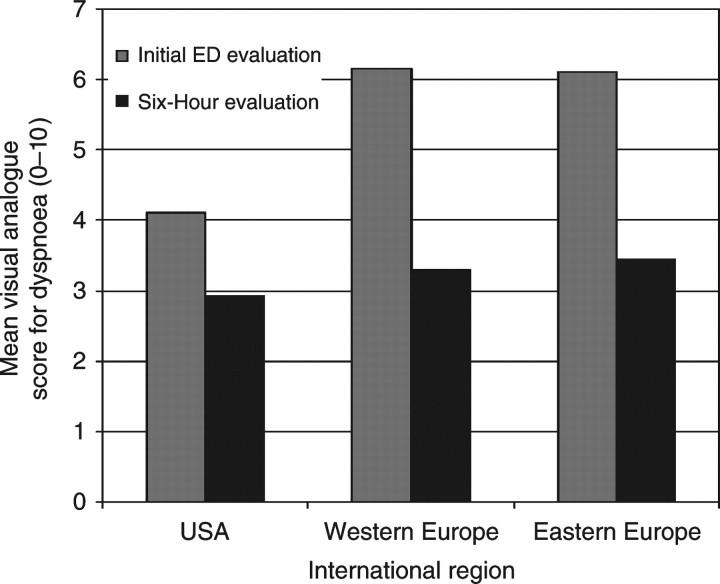
Vital signs tended to be similar between regions, although the respiratory rate was slightly higher in Western Europe. Physical signs of congestion were present in the majority of patients, but patients from Eastern Europe were more likely to have jugular venous distension and less likely to have peripheral oedema, while those from Western Europe were more likely to have rales. At the time of initial ED evaluation, patient self-perceived dyspnoea was higher in Eastern and Western Europe compared with the USA (P < 0.001; Figure 1). Although dyspnoea levels were not significantly different between the three cohorts at the 6 h evaluation, the magnitude of change was significantly greater in Eastern and Western Europe compared with the USA (P < 0.001).
Figure 1.
Visual analogue scale scores for dyspnoea at time of initial ED presentation and 6 h by international region.
International variations in diagnostic characteristics
Congestion on CXR was common across all three regions, but the proportion of CXRs interpreted as having pulmonary oedema was greater in patients from Western Europe than those in the USA and Eastern Europe (42.4% vs. 21.8% and 19.7%, respectively; Table 3) Ischaemic changes on ECG were seen more often in AHFS patients in Eastern Europe than those in the USA and Western Europe (29.6% vs. 9.2% and 9.1%, respectively). Similarly, atrial fibrillation or atrial flutter was more common in Eastern and Western Europe than in the USA (26.0% and 28.9% vs. 10.9%). Creatinine values were higher in the USA, blood urea nitrogen (BUN) values were higher in Western Europe, and there was a trend toward lower serum sodium values in Eastern and Western Europe. B-type natriuretic peptide was utilized in a greater proportion of patients in the USA, while NT-proBNP was utilized more in Eastern and Western Europe. Natriuretic peptides were significantly elevated in patients from all three regions.
Table 3.
Diagnostic characteristics of emergency department patients with acute heart failure syndromes by international region
| Diagnostic Characteristic | USA (%) | Western Europe (%) | Eastern Europe (%) | P-value |
|---|---|---|---|---|
| Chest radiograph | n = 119 | n = 66 | n = 147 | |
| Normal | 10 (8.2%) | 3 (3.85%) | 0 (0.0%) | 0.002 |
| Cardiomegaly | 18 (14.8%) | 22 (28.2%) | 71 (48.2%) | 0.046 |
| Cephalization | 27 (22.2%) | 1 (18.2%) | 37 (25.2%) | 0.039 |
| Interstitial oedema | 72 (60.5%) | 42 (63.6%) | 103 (70.1%) | <0.001 |
| Pulmonary oedema | 26 (21.8%) | 28 (42.4%) | 29 (19.7%) | <0.001 |
| Pleural effusion | 21 (17.6%) | 22 (33.3%) | 56 (38.1%) | 0.135 |
| Electrocardiogram | n = 119 | n = 77 | n = 294 | |
| Normal | 11 (9.2%) | 16 (20.8%) | 12 (4.1%) | <0.001 |
| Sinus rhythm | 54 (45.4%) | 36 (46.8%) | 182 (61.9%) | 0.001 |
| LBBB | 10 (8.4%) | 15 (19.5%) | 42 (14.3%) | 0.072 |
| RBBB | 8 (6.7%) | 4 (5.2%) | 24 (8.2%) | 0.852 |
| Paced rhythm | 20 (16.8%) | 4 (5.2%) | 12 (4.1%) | <0.001 |
| Atrial fibrillation or flutter | 13 (10.9%) | 20 (26.0%) | 85 (28.9%) | <0.001 |
| Ischaemic changes | 11 (9.2%) | 7 (9.1%) | 87 (29.6%) | <0.001 |
| Laboratory findings | ||||
| BNP done | 117 (95.9%) | 17 (21.8%) | 4 (1.4%) | |
| NT-proBNP done | 0 | 26 (33.3%) | 64 (21.8%) | |
| BNP (pg/mL) | 1258 (1263) | 1126 (1031) | 2850 (1215) | 0.037 |
| NT-proBNP (pg/mL) | 0 (0) | 7832 (7090) | 11,274 (10,192) | 0.120 |
| Sodium (mmol/L) | 138 (3) | 133 (27) | 133 (26) | 0.937 |
| BUN (mg/dL) | 27.9 (20.6) | 40.5 (43.7) | 30.3 (30.6) | <0.001 |
| Creatinine (mg/dL) | 1.8 (2.1) | 1.4 (0.8) | 1.5 (1.1) | 0.041 |
Data are presented as medians and ranges or proportions and percentages.
ECG, electrocardiogram; A fib/flutter, atrial fibrillation or atrial flutter; LBBB, left bundle branch block; RBBB, right bundle branch block; BNP, B-type natriuretic peptide; BUN, blood urea nitrogen.
International variations in acute therapy and disposition
Intravenous loop diuretics were administered to the majority of patients in all three international regions (Table 4). Although intravenous vasodilators were used significantly more often in Eastern and Western Europe, topical nitrates were used more commonly in the USA than Eastern and Western Europe. Inotropic agent and vasopressor use was infrequent in all regions but their use was proportionally greater in Eastern Europe. There was a significantly greater proportion of patients admitted to a monitored setting in the USA (56.7%) than in Western Europe (4.5%) or Eastern Europe (3.6%). Further, there was a significantly greater proportion of patients admitted to the intensive care unit (ICU) in Eastern Europe (60.6% vs. 23.3% and 22.3% for the USA and Western Europe, respectively). Unmonitored floor bed utilization was the greatest in Western Europe (64.2% vs. 14.2% and 30.5% for the USA and Eastern Europe, respectively; Table 4).
Table 4.
Treatment and disposition characteristics of emergency department patients with acute heart failure syndromes by international region
| Treatment | USA, n = 122 | Western Europe, n = 78 | Eastern Europe, n = 293 | P-value |
|---|---|---|---|---|
| CPAP/BiPAP | 7 (5.7%) | 8 (10.3%) | 5 (1.71%) | 0.006 |
| Intravenous diuretics | ||||
| Furosemide | 94 (77.1%) | 62 (79.5%) | 237 (80.9%) | 0.674 |
| Bumetanide | 0 (0.0%) | 6 (7.7%) | 0 (0.0%) | <0.001 |
| Torasemide | 0 (0.0%) | 1 (1.3%) | 1 (0.3%) | 0.366 |
| Intravenous vasodilators | ||||
| Nitroglycerin | 3 (2.5%) | 19 (24.4%) | 96 (32.8%) | <0.001 |
| Nitroprusside | 1 (0.8%) | 1 (1.3%) | 0 (0.0%) | 0.203 |
| Nesiritide | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1.000 |
| Others | ||||
| Nitroglycerin SL | 26 (21.3%) | 1 (1.3%) | 19 (6.5%) | <0.001 |
| Nitroglycerin Topical | 27 (22.1%) | 6 (7.7%) | 1 (0.3%) | <0.001 |
| Sublingual ACEI | 1 (0.8%) | 1 (1.3%) | 19 (6.5%) | 0.012 |
| Inotropes/Vasopressors | ||||
| Dobutamine | 1 (0.8%) | 1 (1.3%) | 16 (5.5%) | 0.034 |
| Dopamine | 0 (0.0%) | 1 (1.3%) | 16 (5.5%) | 0.011 |
| Disposition | <0.001 | |||
| Home | 7 (5.8%) | 6 (9.0%) | 5 (1.8%) | |
| Unmonitored floor | 17 (14.2%) | 43 (64.2%) | 86 (30.5%) | |
| Monitored floor | 68 (56.7%) | 3 (4.5%) | 10 (3.6) | |
| Intensive care unit | 28 (23.3%) | 15 (22.3%) | 171 (60.6%) | |
| Died | 0 (0%) | 0 (0%) | 10 (3.6%) | |
Data are presented as proportions and percentages.
CPAP, continuous positive airway pressure; BiPAP, bilevel positive airway pressure; SL, sublingual; ACEI, angiotensin converting enzyme inhibitor.
Discussion
Our study suggests there are differences in characteristics and management of AHFS patients presenting to EDs in the USA, Western Europe, and Eastern Europe. These findings are consistent with similarly highlighted geographical differences in other heart failure studies that have focused on inpatient and post-discharge characteristics.1,11,12 In aggregate, these findings suggest that patient characteristics starting at ED presentation and continuing through post-discharge follow-up vary substantially based on the geographic region, and should be considered when designing clinical trials as well as interpreting their results. Since the aetiologies, precipitating factors, and co-morbidities of AHFS presentations are very heterogeneous across the three regions studied, interventions and treatments in one region might be more or less effective in the other regions. Patient-perceived dyspnoea at the initial evaluation was higher in Eastern and Western Europe than in the USA and the severity of illness at the acute presentation (dyspnoea magnitude, pulmonary oedema on CXR, ischaemic ECG changes) was significantly different across the three regions. Whereas in-hospital and near-term events were not recorded as part of this study, the significantly higher use of intravenous vasodilators and intensive care admissions in Eastern and Western Europe perhaps suggest that either disease severity, resource availability, or the approach to treatment and risk-stratification of patients with AHFS is widely divergent across regions.
When comparing our data to recent registry and survey data from the USA and Western Europe, there are a number of similarities. Compared with other data, our patients from the USA were slightly younger and had a greater proportion of black patients.13 However, male sex, medical co-morbidities, and outpatient medications were similar, with the exception of digoxin, which was used much less in our cohort. In comparison with a survey conducted in England, Wales, and Northern Ireland, our patients from Western Europe had similar proportions of males, outpatient medication use, with the exception of beta-blockers, and a similar number of patients in atrial fibrillation on their electrocardiogram.14 The rate of admission to unmonitored floor beds was also similar. Despite extensive variability in the methods for assessing dyspnoea, our data are consistent with others in demonstrating significant improvement in the first 24 h in a majority of patients. In the PROTECT pilot trial, age and elevated systolic blood pressure were associated with an increased likelihood of dyspnoea improvement.15 This is consistent with a body of evidence suggesting vasodilators may be more efficacious in patients with elevated systolic blood pressure as compared with those with relatively normal systolic blood pressure.16–19 Dyspnoea improvement has been the target of therapeutic trials using intravenous vasodilators and a proposal to standardize dyspnoea assessments in clinical trials has been suggested.15,20,21 This would afford investigators the opportunity to compare changes in dyspnoea across both study design and the pharmacologic agent used.
Therapeutic trials must consider the impact of AHFS characteristics on their inclusion and exclusion criteria and ability to recruit patients. Moreover, when studying a novel therapeutic agent targeting dyspnoea improvement, investigators should consider that patients in Eastern and Western Europe may differ from those in the USA with regard to the severity of their dyspnoea and the magnitude of its improvement in response to standard therapy in the first 6–24 h.10 Similar treatment and intervention strategies will likely result in different success rates between regions depending on the severity of underlying disease.
These findings have important implications for comparisons of observational studies as well. Observational trials should consider differences in background therapy (beta-blockers), co-morbidities and AHFS aetiology (coronary artery disease, acute coronary syndromes, arrhythmias), and severity of initial presentation (pulmonary oedema and ischaemic ECG changes) when evaluating novel diagnostic and prognostic tools and their association with outcomes. In combination with previously published findings, these differences in AHFS presentations suggest that results from international diagnostic, prognostic, and therapeutic studies may only be applicable to specific subsets of patients.1,11,12
Although heterogeneity in AHFS presentations and aetiologies were apparent in this study, there are also many striking similarities. First and foremost, congestion (on physical exam and CXR) is commonly present at initial evaluation. Second, while patient-perceived dyspnoea was greater upon presentation in Eastern and Western Europe, patients in all three regions had similar levels of dyspnoea within 6 h of initial treatment, highlighting the perceived importance and congruity of early, sustained dyspnoea relief to both patients and physicians. Third, targeting congestion relief with intravenous diuretics is a cornerstone of therapy in all regions. Those patients in Eastern and Western Europe also received intravenous vasodilators significantly more often, possibly contributing to the similar dyspnoea scores at the 6 h assessment. Finally, inotropes and vasopressors are used in a minority of patients. These subtleties are important, especially when evaluating dyspnoea improvement or resolution as an endpoint in a therapeutic trial.
Limitations
Our results suggest important differences in the presentation and management of patients with AHFS, but the results should be interpreted considering the limitations of our data collection. Patients were enrolled prospectively and the majority of data were collected prospectively, which avoids biases inherent in many registries and retrospective chart reviews. However, based on the availability of study staff, only a convenience sample of patients was enrolled, and only at participating EDs, which may not be representative of the entire health system within a geographic region. It is not possible to extrapolate beyond the sample to comment on population-level of health system differences, nor does the sampling method allow us to comment on the disease burden among the populations served by each ED. Nonetheless, crude comparisons of treatment rates and demographics between regions provide sufficient evidence to formulate hypotheses about likely differences between regions. Moreover, the comparisons demonstrate that treatment trials conducted in one geographic region are not necessarily generalizable to other regions since practice patterns, populations, and disease state may all vary between regions. Indeed, the sampling methodology, both in selecting participating EDs and in enrolling patients, may partly explain some of the differences observed between the findings in this study and those from registries such as ADHERE, OPTIMIZE, and EuroHeart Failure.3,5,22 Whether the advantages of prospective data collection in ED patients outweigh the disadvantages of retrospective data collection in only those patients admitted, is unclear.
If there was circadian variability in AHFS presentations at the participating centres, this may have introduced bias. Further, we enrolled patients who were able to give consent, perhaps eliminating the most acute presentations. However, the proportion of patients with pulmonary oedema on CXR, VAS scores, and number of ICU admissions suggest that the continuum of disease severity was well represented. There was an unclear or missing diagnosis at the 6 h mark in about 20% of our study population. This missing data may have impacted the overall results if these patients differed from the included patients in any way. However, given the clear trends in the data we consider this possibility unlikely, and similarities between included and excluded patients on baseline variables further suggest this is unlikely to have been a cause of significant bias; there were no clinically significant differences in age (64 vs. 69 years), male sex (55.5% vs. 56.2%), systolic blood pressure (135 vs. 144 mmHg), heart rate (92 vs. 90 beats per minute), or respiratory rate (23 vs. 22 breaths per minute).
Several comparisons are somewhat limited by the disproportionately small number of patients in one or more of the groups. Although comparisons such as use of inotropic agents, bumetanide, and sublingual ACE-I appear to be statistically different between the three regions, they were used in such a small proportion of patients that the clinical significance of this is unclear.
Treatment for AHFS was not protocolized in this study and was at the discretion of the treating physician. The use of intravenous vasodilators may suggest increased disease severity in Eastern and Western Europe, but this may also reflect different practice patterns, different types of hospitals (tertiary care vs. community), and different health-care resources available such as the ICU or telemetry beds and the means of non-invasive ventilation. Further, admission decisions may have been influenced by systematic differences in bed availability rather than geographic location. However, the type of hospital bed and bed availability were not documented in the current study. Further, disease severity may have been impacted if AHFS patients with chronic heart failure bypassed the ED and were admitted directly to the hospital. Direct admissions were not tracked in this study. Differences in physician diagnoses rather than actual patient characteristics may also account for some of the observed results. Because there was no central criterion standard for the diagnosis of AHFS, there may have been a systematic bias between sites and regions in how they defined AHFS, which has the potential to confound our results. Patients were diagnosed as AHFS by treating physicians at the various sites, and recent findings suggest that the acute care physician's accuracy is over 90% for predicting an AHFS diagnosis, using the cardiology chart review as the criterion standard.23 This would suggest any confounding by misclassification is likely to be small.
Conclusion
The presentation and early management of patients with AHFS differs between the USA, Western Europe, and Eastern Europe. These differences should be considered when designing and interpreting clinical investigations, since standard therapy, co-morbidities, risk profile, and disease severity may influence outcomes and the generalizability of results from one geographic region to another.
Funding
Supported in part by National Heart, Lung and Blood Institute grant K23HL085387 and PDL BioPharma.
Conflict of interest: S.P.C.: Prior and Current Grant/Research Support: Biosignetics, Inovise Medical Inc., Abbott Point-of-Care, NIH/NHLBI, Corthera, BRAHMS. Prior and current consulting: Abbot Point-of-Care, PDL BioPharma, Astellas, Otsuka Pharmaceuticals, Bayer. Prior and current scientific advisory board: Astellas, The Medicines Company, Corthera, Actelion, Speaker's Bureau: none. P.S.P.: Consultant: Astellas, Bayer, EKR Therapeutics, J&J, the Medicines Company, Otsuka, Palatin Technologies, PDL BioPharma, Pericor Therapeutics, and Solvay Pharmaceuticals. Honoraria: BiogenIdec, Corthera, Ikaria, Nile Therapeutics. Research support: Merck and PDL BioPharma. A.B.S.: Current Grant Support Corthera, PDL BioPharma, Abbott Diagnostics, Meso Scale Discovery, NIH / NHLBI (R01HL088459-02), Centers for Disease Control. Current Consultant: Abbott Diagnostics, Ortho-Clinical Diagnostics, Sanofi-Aventis. J.E.H.: Grant/Research Support: Biosite, Siemens, Nanosphere, Brahms. Salary/Consultant Fee: sanofi-Aventis, BMS, Molecular Insights. Board/Committee Membership: None. Stocks/Bonds: Mutual funds with various holdings. Honorarium/Expenses: sanofi-Aventis, BMS. C.D.M.: Research support: Johnson & Johnson/Scios, Inc., PDL, Biopharma, GlaxoSmithKline, Schering-plough, Biosite, Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership. Spoken at events underwritten by: Sanofi-Aventis, Schering-Plough. R.N.: Grant Support: Bmeye, Nanosphere, Brahms, Mitsubishi. F.P.: Advisory Board: Abbott, Beckman-coulter, Biosite, EKR, The Medicines Co. Research Grant: Abbott, Beckman-coulter, Biosite, BRAHMS, Heartscape, EKR, The Medicines Co. Speaker's Bureau: Abbott, Biosite. Ownership: Vital Sensors. M.T.: Research Support: PDL Biopharma. A.M.: consultant: Abbott, Orion Pharma, Bayer Pharma and The Medicine Company; lecture fees: Abbott, Guidant, Inverness and Edwards Life Sciences. M.G.: Consultant: Abbott Labs, Astellas, Astra Zeneca, Bayer Schering Pharma AG, CorThera, Inc., Cytokinetics, Inc., DebioPharm S.A., Errekappa Terapeutici (Milan, Italy), Glaxo Smith Kline, Ikaria, Johnson & Johnson, Medtronic, Merck, Novartis Pharma AG, Otsuka Pharmaceuticals, Palatin Technologies, Pericor Therapeutics, Protein Design Laboratories, Sanofi-Aventis, Sigma Tau, Solvay Pharmaceuticals, Trevena Therapeutics. Research Support: Medtronic, Novartis Pharma AG, Otsuka Pharmaceuticals, Sigma Tau, Solvay Pharmaceuticals, DebioPharm S.A., Pericor Therapeutics.
References
- 1.Blair JE, Zannad F, Konstam MA, Cook T, Traver B, Burnett JC, Jr, Grinfeld L, Krasa H, Maggioni AP, Orlandi C, Swedberg K, Udelson JE, Zimmer C, Gheorghiade M. Continental differences in clinical characteristics, management, and outcomes in patients hospitalized with worsening heart failure results from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan) program. J Am CollCardiol. 2008;52:1640–1648. doi: 10.1016/j.jacc.2008.07.056. doi:10.1016/j.jacc.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 2.Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola VP, Hochadel M, Komajda M, Lassus J, Lopez-Sendon JL, Ponikowski P, Tavazzi L. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006;27:2725–2736. doi: 10.1093/eurheartj/ehl193. doi:10.1093/eurheartj/ehl193. [DOI] [PubMed] [Google Scholar]
- 3.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. doi:10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 4.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, Dietz R, Gavazzi A, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, van Gilst WH, Widimsky J, Freemantle N, Eastaugh J, Mason J. The EuroHeart Failure survey programme—a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24:442–463. doi: 10.1016/s0195-668x(02)00823-0. doi:10.1016/S0195-668X(02)00823-0. [DOI] [PubMed] [Google Scholar]
- 6.McMurray JJ, Teerlink JR, Cotter G, Bourge RC, Cleland JG, Jondeau G, Krum H, Metra M, O'Connor CM, Parker JD, Torre-Amione G, van Veldhuisen DJ, Lewsey J, Frey A, Rainisio M, Kobrin I. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. J Am Med Assoc. 2007;298:2009–2019. doi: 10.1001/jama.298.17.2009. doi:10.1001/jama.298.17.2009. [DOI] [PubMed] [Google Scholar]
- 7.Gheorghiade M, Konstam MA, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. J Am Med Assoc. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332. doi:10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 8.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. J Am Med Assoc. 2002;287:1531–1540. doi: 10.1001/jama.287.12.1531. doi:10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 9.Pang PS, Tavares M, Collins SP, Cleland JG, Hollander J, Nieminen M, Miller C, Courtney DM, Kirk JD, Masip J, Parkhomenko A, Macarie C, Peacock WF, Spinar J, Nowak R, Raev D, Storrow AB, Spisak V, Hamdy A, Mebazaa A, Gheorghiade M. Design and rationale of the URGENT Dyspnea study: an international, multicenter, prospective study. Am J Ther. 2008;15:299–303. doi: 10.1097/MJT.0b013e31816b436e. doi:10.1097/MJT.0b013e31816b436e. [DOI] [PubMed] [Google Scholar]
- 10.Mebazaa A, Pang PS, Tavares M, Collins SP, Storrow AB, Laribi S, Andre S, Courtney DM, Hasa J, Spinar J, Masip J, Peacock WF, Sliwa K, Gayat E, Filippatos G, Cleland JG, Gheorghiade M. The impact of early standard therapy on dyspnoea in patients with acute heart failure: the URGENT-dyspnoea study. Eur Heart J. 2010;31:832–841. doi: 10.1093/eurheartj/ehp458. doi:10.1093/eurheartj/ehp458. [DOI] [PubMed] [Google Scholar]
- 11.Pitt B, Zannad F, Gheorghiade M, Martinez F, Love TE, Daniel C, Ahmed A. Transatlantic similarities and differences in major natural history endpoints of heart failure after acute myocardial infarction: a propensity-matched study of the EPHESUS trial. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.03.036. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gheorghiade M, Khan S, Blair JE, Harinstein ME, Krum H, Mukherjee R, Pitt B. The effects of eplerenone on length of stay and total days of heart failure hospitalization after myocardial infarction in patients with left ventricular systolic dysfunction. Am Heart J. 2009;158:437–443. doi: 10.1016/j.ahj.2009.07.003. doi:10.1016/j.ahj.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Fonarow GC, Heywood JT, Heidenreich PA, Lopatin M, Yancy CW. Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2007;153:1021–1028. doi: 10.1016/j.ahj.2007.03.012. doi:10.1016/j.ahj.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Nicol ED, Fittall B, Roughton M, Cleland JG, Dargie H, Cowie MR. NHS heart failure survey: a survey of acute heart failure admissions in England, Wales and Northern Ireland. Heart (British Cardiac Society) 2008;94:172–177. doi: 10.1136/hrt.2007.124107. [DOI] [PubMed] [Google Scholar]
- 15.Metra M, Cleland JG, Weatherley BD, Dittrich HC, Givertz MM, Massie BM, O'Connor CM, Ponikowski P, Teerlink JR, Voors AA, Cotter G. Dyspnoea in patients with acute heart failure: an analysis of its clinical course, determinants, and relationship to 60-day outcomes in the PROTECT pilot study. Eur J Heart Fail. 12:499–507. doi: 10.1093/eurjhf/hfq021. doi:10.1093/eurjhf/hfq021. [DOI] [PubMed] [Google Scholar]
- 16.Cotter G, Metra M, Milo-Cotter O, Dittrich HC, Gheorghiade M. Fluid overload in acute heart failure–re-distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail. 2008;10:165–169. doi: 10.1016/j.ejheart.2008.01.007. doi:10.1016/j.ejheart.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Cotter G, Metzkor E, Kaluski E, Faigenberg Z, Miller R, Simovitz A, Shaham O, Marghitay D, Koren M, Blatt A, Moshkovitz Y, Zaidenstein R, Golik A. Randomised trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary oedema. Lancet. 1998;351:389–393. doi: 10.1016/S0140-6736(97)08417-1. doi:10.1016/S0140-6736(97)08417-1. [DOI] [PubMed] [Google Scholar]
- 18.Cotter G, Felker GM, Adams KF, Milo-Cotter O, O'Connor CM. The pathophysiology of acute heart failure–is it all about fluid accumulation? Am Heart J. 2008;155:9–18. doi: 10.1016/j.ahj.2006.02.038. doi:10.1016/j.ahj.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 19.Levy P, Compton S, Welch R, Delgado G, Jennett A, Penugonda N, Dunne R, Zalenski R. Treatment of severe decompensated heart failure with high-dose intravenous nitroglycerin: a feasibility and outcome analysis. Ann Emerg Med. 2007;50:144–152. doi: 10.1016/j.annemergmed.2007.02.022. doi:10.1016/j.annemergmed.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 20.De Luca L, Mebazaa A, Filippatos G, Parissis JT, Bohm M, Voors AA, Nieminen M, Zannad F, Rhodes A, El-Banayosy A, Dickstein K, Gheorghiade M. Overview of emerging pharmacologic agents for acute heart failure syndromes. Eur J Heart Fail. 2008;10:201–213. doi: 10.1016/j.ejheart.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Pang PS, Cleland JG, Teerlink JR, Collins SP, Lindsell CJ, Sopko G, Peacock WF, Fonarow GC, Aldeen AZ, Kirk JD, Storrow AB, Tavares M, Mebazaa A, Roland E, Massie BM, Maisel AS, Komajda M, Filippatos G, Gheorghiade M. A proposal to standardize dyspnoea measurement in clinical trials of acute heart failure syndromes: the need for a uniform approach. Eur Heart J. 2008;29:816–824. doi: 10.1093/eurheartj/ehn048. doi:10.1093/eurheartj/ehn048. [DOI] [PubMed] [Google Scholar]
- 22.Fonarow GC, Heywood JT, Heidenreich PA, Lopatin M, Yancy CW, Fonarow GC, Heywood JT, Heidenreich PA, Lopatin M, Yancy CW Committee ASA Investigators. Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2007;153:1021–1028. doi: 10.1016/j.ahj.2007.03.012. doi:10.1016/j.ahj.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Collins SP, Peacock WF, Lindsell CJ, Clopton P, Diercks DB, Hiestand B, Hogan C, Kontos MC, Mueller C, Nowak R, Chen WJ, Huang CH, Abraham WT, Amsterdam E, Breidthardt T, Daniels L, Hasan A, Hudson M, McCord J, Naz T, Wagoner LE, Maisel A. S3 detection as a diagnostic and prognostic aid in emergency department patients with acute dyspnea. Ann Emerg Med. 2009;53:748–757. doi: 10.1016/j.annemergmed.2008.12.029. doi:10.1016/j.annemergmed.2008.12.029. [DOI] [PubMed] [Google Scholar]