Abstract
The L1 adhesion molecule functions in axon growth and guidance, but a role in synaptic development of cortical inhibitory interneurons is largely unexplored. L1 mediates adhesion by engaging the actin cytoskeleton through binding the actin/spectrin adapter protein ankyrin. Loss of L1-ankyrin interaction impaired process elaboration/branching by GABAergic interneurons, including basket cells, and reduced the number of perisomatic synapses in the cingulate cortex as shown in L1 mutant mice (L1YH) with a mutation in the ankyrin-binding site, either alone or intercrossed with GAD67-enhanced green fluorescence protein reporter mice. Electron microscopy revealed that perisomatic inhibitory synapses but not excitatory synapses in the neuropil were specifically affected. In wild-type cingulate cortex, L1 colocalized with perisomatic synaptic markers, whereas L1 phosphorylation on Tyr1229 decreased postnatally, correlating with increased ankyrin binding and synaptic development. These results suggest a novel role for L1 engagement with the actin cytoskeleton in development of inhibitory connectivity within the cingulate cortex.
Keywords: ankyrin, basket interneuron, frontal cortex, GABAergic inhibitory synapse, L1
Introduction
The L1 family of cell adhesion molecules (L1-CAMs) contains 4 structurally related transmembrane glycoproteins in vertebrates: L1 (NgCAM in chicken), Close Homolog of L1 (CHL1), NrCAM, and Neurofascin (reviewed in Maness and Schachner 2007). Each member of the family has 6 immunoglobulin (Ig)-like domains linked to 4–5 fibronectin III repeats (FNIII) in their divergent extracellular regions, a transmembrane domain, and a highly conserved cytoplasmic domain of ∼110 residues. The extracellular domains of L1-CAMs bind to neighboring cells or the extracellular matrix through multiple homophilic and heterophilic interactions, whereas the cytoplasmic domain engages the actin cytoskeleton. L1-CAMs are expressed in neuronal and glia cells, as well as in some nonneuronal cells, and they are known to mediate diverse cellular responses including adhesion, neurite outgrowth, axon fasciculation, and migration (Maness and Schachner 2007). Studies in mutant mice demonstrate that these functions are impaired by mutation or deletion of L1-CAM genes, and L1 human mutation are associated with a pleiotypic X-linked mental retardation disorder, termed the L1 syndrome (Kenwrick et al. 2000).
L1 reversibly binds to ankyrin, an actin/spectrin adapter protein (Bennett and Chen 2001), through a conserved motif (FIGQY) in the L1 cytoplasmic domain (Davis and Bennett 1994; Bennett and Baines 2001; Hortsch et al. 2009). Multiple ank repeats within the ankyrin molecule organize protein complexes within specialized membrane domains of the neurons, including the axon initial segment and node of Ranvier, by recruiting adhesion molecules, ion channels, and transporters (Bennett and Healy 2008). L1-ankyrin binding promotes stationary behavior of cells in culture (Gil et al. 2003) and neurite initiation (Nishimura et al. 2003) by inhibiting retrograde actin flow, but its function in vivo is poorly understood. Phosphorylation of L1 on Tyr1229, or the homologous tyrosine in other L1-CAMs, leads to disengagement of ankyrin and correlates with enhanced neurite outgrowth in vitro (Garver et al. 1997; Tuvia et al. 1997; Gil et al. 2003; Whittard et al. 2006). Mutation of tyrosine1229 to histidine in the FIGQY motif of L1, which is a human pathological mutation (Kenwrick et al. 2000), also causes ankyrin disengagement (Needham et al. 2001). L1-CAMs can be phosphorylated at this motif dependent on signaling of fibroblast growth factor (Chen et al. 2001), epidermal growth factor (Whittard et al. 2006), or ephrin B- (Zisch et al. 1997) receptor activation.
Although a role for L1 in neurite outgrowth is well established, a potentially new function for L1 and its interaction with ankyrin in synaptic development is largely unexplored. The L1 homolog in Drosophila, Neuroglian, has been shown to mediate the development of presynaptic terminals of a central synapse (Godenschwege et al. 2006), raising the possibility that L1 might have a related function in the mammalian neocortex. In addition, the interaction of neurofascin with ankyrin directs basket-cell axons to synaptic targets on the axon initial segment of cerebellar Purkinje cells (Ango et al. 2004). To investigate a potential function for L1-ankyrin interaction in synaptic development of mammalian cortical neurons in vivo, an L1 knock-in mouse, termed L1YH, was generated that expresses the pathological L1 point mutation (Tyr1229His), which specifically disrupts L1-ankyrin interaction (Buhusi et al. 2008). The mutant displays defective topographic mapping of retinal ganglion cell axons to targets in the superior colliculus (Buhusi et al. 2008), but it has not been analyzed for defects in synapse formation. Dysregulation of another Ig-class neural adhesion molecule, neural cell adhesion molecule (NCAM), has been shown to perturb synaptic development of GABAergic interneurons in the mouse prefrontal (cingulate) cortex (Brennaman and Maness 2008); thus, it was of interest to explore a related role for L1 in developing interneurons. GABAergic interneurons in the prefrontal cortex are important in furnishing spatially and temporally coordinated inhibitory input to regulate circuits involved in pyramidal cell synchrony and oscillations that underlie cognitive behaviors such as working memory (Klausberger and Somogyi 2008).
We report here that the disruption of L1-ankyrin binding alters the regulation of neuronal branching and leads to decrease in perisomatic synapses of GABAergic inhibitory interneurons in the developing mouse cingulate cortex. These results disclose an essential role of L1-ankyrin interaction in the development of inhibitory circuitry in the mammalian neocortex.
Materials and Methods
Mice
L1YH knock-in mice (Buhusi et al. 2008) and GAD67-enhanced green fluorescence protein (EGFP) bacterial artificial chromosome (BAC) transgenic mice (Chattopadhyaya et al. 2004) have been described previously. To obtain L1YH/GAD67-EGFP mice and wild-type littermate controls, L1YH mutant mice were intercrossed with GAD67-EGFP mice. Because the L1 gene is located on the X-chromosome, it was convenient to use male mice, which carry only one L1 allele, in all of the experiments. All animals were used according to University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee policies and in accordance with National Institutes of Health guidelines.
Antibodies
For immunohistochemistry, immunoprecipitation, and immunoblotting experiments, the following antibodies were used: Mouse anti-GAD65 mAb (GAD-6) (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), rabbit antisynaptophysin pAb and goat antigephryin pAb (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-NeuN mAb (A60) (Millipore, Billerica, MA), rabbit anti-GFP pAb and mouse anti-Ankyrin B mAb (clone 2.20) (Invitrogen, Carlsbad, CA), mouse anti-L1 mAb (clone 2C2) (Abcam, Cambridge, MA), mouse anti-L1 mAb (clone 5G3) (BD Bioscience, San Jose, CA), and mouse anti-β-tubulin mAb (clone TUJ1) (Covance, Emeryville, CA). Antibodies against phosphorylated mouse L1 (p-L1) were raised in rabbit against a phosphorylated peptide with the sequence NEDGSFIGQ(pY)SGKKE corresponding to the ankyrin-binding site of cytoplasmic domain of L1, essentially as described (Jenkins et al. 2001). The production and affinity purification of antibodies was done by Bethyl Laboratories (Montgomery, TX).
Western Blotting and Immunoprecipitation
Forebrains were collected from mice at designated ages and homogenized in RIPA buffer containing protease inhibitors and phosphatase inhibitors (0.15 M NaCl, 5 mM ethylenediaminetetraacetic acid, 1 mM ethyleneglycol-bis(2-aminoethylether)-N,N,N′,N′-tetra aceticacid, 1% NP-40, 1% sodium deoxcholate, 0.1% sodium dodecyl sulfate in 20 mM Tris–HCl, pH 7.0 containing 10 μg/mL leupeptin, 0.11 TIU/mL aprotinin, 0.2 mM sodium orthovanadate, and 10 mM sodium fluoride). Brain lysates were clarified by centrifugation at 14 000 rpm. Protein concentration was measured (Pierce Biotechnology, Rockford, IL). Brain lysates (75 μg) were subjected to 8.5% SDS-PAGE and transferred to nitrocellulose membranes (Schleicher and Schuell Bioscience Inc., Keene, NH). After blocking with 5% milk in tris buffered saline with Triton X-100, membranes were incubated at 4 °C with primary antibodies: mouse anti-L1 (1:1000), mouse anti-Ankyrin B (1:500), rabbit anti-pL1 (1:400), and mouse anti-β-tubulin (1:1000) followed by incubation of peroxidase-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA) and Western lightning plus-ECL (Perkin-Elmer, Waltham, MA) detection.
Immunoprecipitation of L1 was performed as described with minor modification (Buhusi et al. 2008). Brain lysates (1000 μg) from WT and L1YH mice were precleared with protein A/G-Sepharose (Pierce Biotechnology) for 30 min at 4 °C. Precleared lysates were incubated with a mixture of L1 monoclonal antibodies 2C2 (2.5 μg; Abcam) and 5G3 (1.25 μg; BD Bioscience) for 2 h at 4 °C followed by incubation of protein A/G-Sepharose for 30 min at 4 °C. Beads were washed with ice-cold RIPA buffer, and proteins were eluted by boiling in SDS-PAGE sample buffer and subjected to SDS-PAGE followed by western blotting analysis.
Immunohistochemistry and Confocal Microscopy
Mice were anesthetized and perfused transcardially with 4% paraformaldehyde in phosphate buffer, pH 7.4. The brains were postfixed in the same fixative for 2 days, and 60-μm-thick coronal sections were cut using a vibratome (Leica VT1200S; Leica, Nussloch, Germany). Brain sections at equivalent rostrocaudal levels were blocked in 10% normal goat serum (NGS) and 1% Triton X-100 followed by incubation of primary antibodies in 10% NGS and 0.1% Triton X-100: GAD65, 1:40; Synaptophysin, 1:150; NeuN, 1:400; GFP, 1:200, L1, 1:100; and Gephyrin, 1:50. Sections were then incubated with fluorescein isothiocyanate and/or Cy3-conjugated goat or donkey IgG (1: 100; Jackson Immuno-Research) and mounted in Vectashield (Vector Laboratories, Burlingame, CA). For some experiments, sections were imaged by confocal microscopy (Zeiss LSM510; Carl Zeiss, Tokyo, Japan) with a ×63 oil immersion objective at the University of North Carolina Microscopy Facility (Dr Robert Bagnell, Director, Department of Pathology, University of North Carolina School of Medicine). Scans from each channel were collected in the multiple-track mode and subsequently merged. Eight random images from layers II/III of the cingulate cortex region were taken and analyzed. For each image, the perisomatic signals (puncta rings) or neuropil area from at least 6 randomly selected pyramidal neurons (pyramidal cell somata were identified by NeuN immunofluorescence) were delineated, and then pixel density within the delineated area was calculated using NIH imageJ software and averaged as described previously (Huang et al. 1999; Brennaman and Maness 2008). Perisomatic boutons around a pyramidal cell soma were counted within a region 2 μm away from the soma membrane (Chattopadhyaya et al. 2004). For morphometric analysis of basket interneurons, EGFP-labeled basket interneurons in layers II/III of the cingulate cortex of L1YH/GAD67-EGFP and wild-type GAD67-EGFP littermate mice at P10, P21, and 2-month were imaged and reconstructed on Olympus FV500 confocal microscopy using ×20 objective and ×2 optical zoom. A z-stack across EGFP-labeled basket interneurons was projected using a 1.0-μm optical section interval and 30–60 sections per z-stack. For each mouse, 30–50 individual basket interneurons were fully imaged and reconstructed, and neurons were traced from confocal z-stack images using Neurolucida software (MBF Bioscience, Williston, VT), then analyzed using the NeuroExplorer module. Morphological features including soma area, number of processes, total number of ends, branching index, and Sholl analysis were measured as described previously (Brennaman and Maness 2008). Branching analysis did not extend to the most distal segments of processes, where the signal to noise ratio of EGFP is too low to reliably measure. At least 4 animals were used for each developmental age. Data were expressed as the mean ± standard error of the mean (SEM) and compared using Student's t-test for statistical significance at P < 0.05.
Colocalization analysis of L1 with pre and postsynaptic markers was done according to methods described previously with modification (Ango et al. 2008). Briefly, the 2 channels of L1 and GAD65/gephyrin double staining were transformed into 8-bit grayscale images and thresholded. The grayscale images of L1 and GAD65/gephyrin were then merged, and the total pixels of L1 (X), GAD65/gephyrin (Y), and merged (Z) images were measured using Image J software. The percentage of GAD65/gephyrin puncta that colocalized with L1 was obtained as (X + Y − Z)/Y × 100. For this analysis, 20 pyramidal cells were analyzed for each case. Values were expressed as mean ± SEM.
Electron Microscopy
Two-month-old L1YH mice and wild-type littermates were deeply anesthetized and were transcardially perfused with phosphate buffer (0.15 M sodium phosphate, pH 7.4) followed by 2% paraformaldehyde and 2.5% glutaraldehyde in phosphate buffer. The brains were postfixed in the same fixative for 2 days, and 100-μm-thick coronal vibratome sections were cut using Leica VT1200S vibratome. The vibratome sections were postfixed in 1% osmium tetroxide with 1.25% potassium ferrocyanide in phosphate buffer for 20 min, dehydrated in series of ethanol, and flat embedded in epoxy resin. Semithin sections (1 μm) were cut, stained with toluidine blue, and used for orientation purposes. Ultrathin (70 nm) sections of cingulate cortex were cut using a Leica Ultracut UCT microtome (Leica Microsystems, Inc., Bannockburn, IL) and mounted on 200-mesh copper grids (Electron Microscopy Sciences, Hatfield, PA). Ultrathin sections were contrasted with uranyl acetate and lead citrate and analyzed with a LEO EM 910 transmission electron microscope (Carl Zeiss SMT, Inc., Thornwood, NY) at the University of North Carolina Microscopy Facility (Dr Robert Bagnell, Director, Department of Pathology, University of North Carolina School of Medicine). Synapses were defined by the presence of a clear postsynaptic density facing a number of synaptic vesicles. Data were expressed as the mean ± SEM and compared using Student's t-test for statistical significance at P < 0.05.
Results
Synaptic Development Is Impaired in Prefrontal Cortex of L1YH Mutant Mice
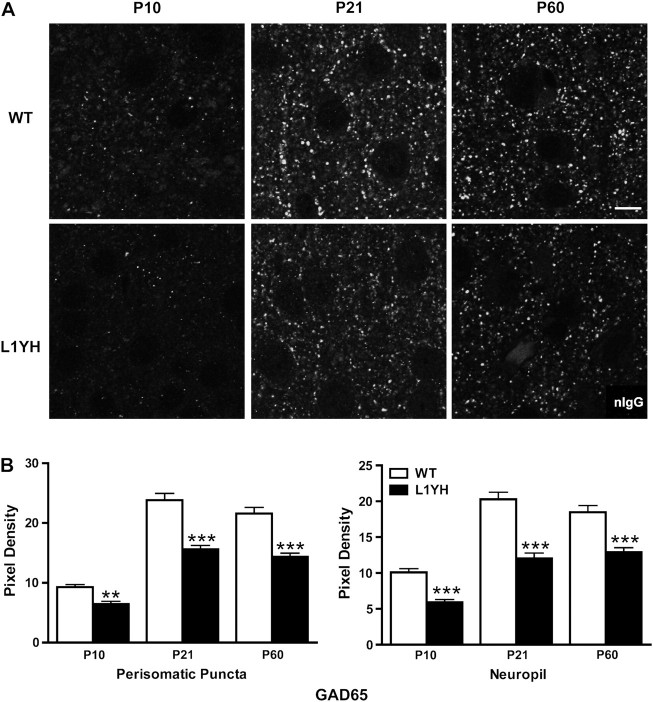
To investigate whether loss of L1-ankyrin interaction impaired synaptic development, the expression of synaptophysin, a presynaptic terminal marker, was analyzed in layer II/III of cingulate cortex of WT and L1YH mice at P10 (neonatal), P21 (adolescent), and P60 (adult) stages. As shown in Figure 1A, synaptophysin immunofluorescence in the cingulate cortex of WT mice was present as puncta rings around cell soma with additional punctate staining in the neuropil. In the cingulate cortex, synaptophysin immunofluorescence in perisomatic puncta as well as in neuropil was significantly decreased in L1YH mice compared with age-matched WT littermates at each of the stages examined in equivalent rostrocaudal sections (Fig. 1A and quantification at Fig. 1B). Because synaptophysin localizes in presynaptic terminals of both interneurons and pyramidal cells, it was of interest to investigate whether inhibitory and/or excitatory synapses were affected in L1YH mice. The 65-kDa isoform of glutamic acid decarboxylase (GAD65), which is enriched in presynaptic terminals of GABAergic inhibitory neurons (Feldblum et al. 1993; Esclapez et al. 1994), was compared for expression in L1YH mice and WT cingulate cortex. In layers II/III of the cingulate cortex of WT mice, prominent GAD65 immunofluorescence was evident in perisomatic puncta rings, which represented presynaptic boutons of GABAergic interneurons innervating soma of pyramidal and/or nonpyramidal neurons (Fig. 2A). Quantitative measurement of fluorescent pixel density of puncta rings surrounding the soma and neuropil showed that in WT cingulate cortex, the GAD65 immunofluorescence intensity increased about 2-fold from P10 to P21, then remained at elevated levels in adult, an accord with previous findings (Brennaman and Maness 2008). In L1YH mice, GAD65 immunofluorescence at perisomatic puncta rings and neuropil was significantly decreased compared with age-matched WT mice at all ages examined (Fig. 2A and quantification at Fig. 2B). These results suggested that loss of L1-ankyrin interaction decreased GAD65-positive inhibitory synapses surrounding cell soma and in neuropil of cingulated cortex.
Figure 1.
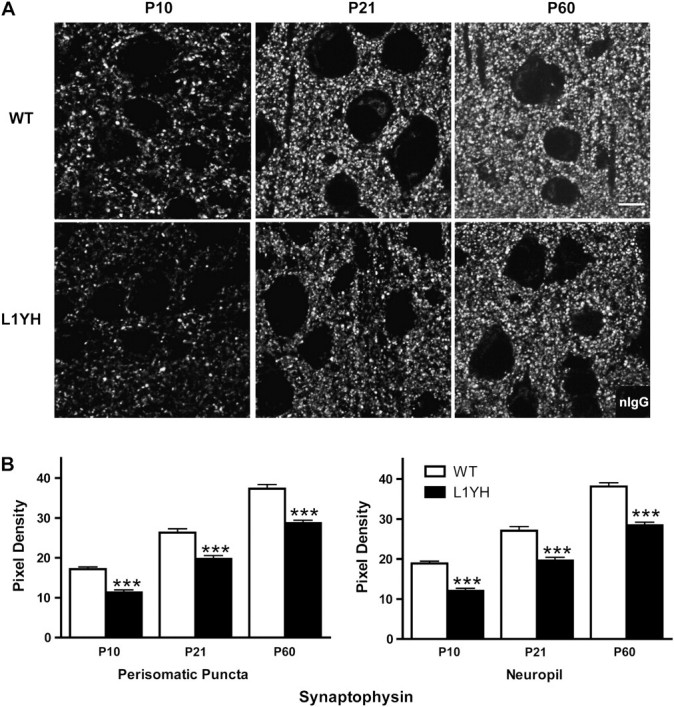
Synaptic puncta in the mouse prefrontal cortex increase postnatally during normal development but are reduced in L1YH mutant mice. (A) Representative confocal images of synaptophysin immunoreactivity from WT and L1YH cingulate cortex (layers II/III) at postnatal ages. Scale bar: 10 μm. (B) Pixel densities of synaptophysin immunoreactivity in perisomatic puncta and neuropil. In L1YH mice, synaptophysin immunofluorescence intensities in perisomatic puncta rings and neuropil were significantly lower than those of age-matched WT mice at all ages examined. Error bars represent SEM; ***P < 0.001 relative to WT mice. Four animals per genotype per stage were analyzed.
Figure 2.
Developmental increase in GAD65-postive perisomatic puncta and neuropil is decreased in L1YH mutant prefrontal cortex. (A) Representative confocal images of GAD65 immunoreactivity from WT and L1YH cingulate cortex (layers II/III) at postnatal ages. Scale bar: 10 μm. (B) Pixel densities of GAD65 immunoreactivity in perisomatic puncta and neuropil. In L1YH mice, GAD65 immunofluorescence intensities in perisomatic puncta rings and neuropil were significantly lower than those of age-matched WT mice at all ages examined. Error bars represent SEM; **P < 0.01 and ***P < 0.001 relative to WT mice. Four animals per genotype per stage were analyzed.
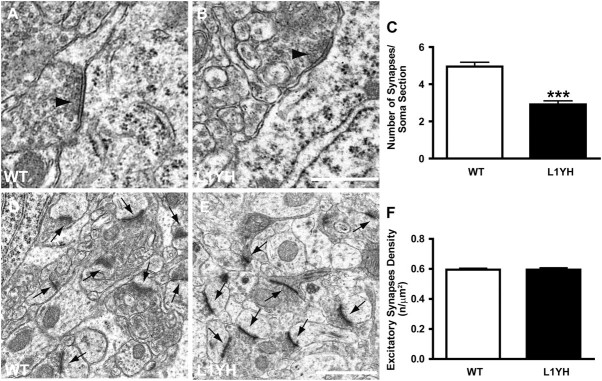
To confirm the loss of synapses by examining ultrastructure in the cingulate cortex of L1YH mice, electron microscopy was performed focusing on perisomatic synapses. The perisomatic region of pyramidal cells receives almost exclusively GABAergic synapses (Freund and Katona 2007). Innervation of pyramidal cell soma by interneurons was indicated by the presence of symmetric synapses along the cell membrane, which exhibited varicosities containing flattened vesicles (arrow heads in Fig. 3A and B). The density of perisomatic boutons with symmetric synapses in layers II/III of cingulate cortex was quantified from serial ultrathin sections of P60 WT and L1YH mutant mice. In L1YH mice, terminal boutons with symmetric synapses were morphologically normal but the density of symmetric synapses around cell soma was reduced by ∼40% compared with WT littermates (WT, 4.95 ± 0.23 synapses/soma section vs. L1YH, 2.92 ± 0.19 synapses/soma section, P < 0.001, Fig. 3C). These results suggested that in L1YH mice, the basket interneurons still contacted pyramidal cells and formed synapses but at significantly reduced efficiency and density. In contrast, the density of asymmetric synapses, which represent excitatory synapses in the neuropil of L1YH cingulate cortex (layers II/III), was the same as in WT (arrows in Fig. 3D,E and quantification at Fig. 3F). This result suggested that inhibitory synapses were preferentially affected in cingulate cortex of L1YH mutant mice.
Figure 3.
Reduced perisomatic inhibitory synapses but not excitatory synapses in L1YH mice revealed by electron microscopy analysis. Electron microscopy was performed in layers II/III cingulate cortex of L1YH mice and WT littermates at P60. At least 20 randomly selected pyramidal neurons and 6 neuropil areas per animal (n = 4 per genotype) were analyzed. (A,B) Ultrastructural analysis showed perisomatic inhibitory synapses (arrow heads in A, B) along pyramidal cells in cingulate cortex of L1YH mice (B), with largely normal morphology and organelle organization compared with WT (A). Scale bar: 0.5 μm. (C). Quantification of perisomatic inhibitory synapses along the somal perimeter of pyramidal cells in cingulate cortex of L1YH mice revealed an approximately 40% reduction compared with WT. Error bars represent SEM; ***P < 0.001. (D,E) Representative images of excitatory synapses (arrows in D, E) in neuropil areas in cingulate cortex of L1YH mice (E) and WT littermates (D). Scale bar: 1μm. (F) Quantification of excitatory synapses density in neuropil areas in cingulate cortex of L1YH mice and WT littermates. No difference was observed between WT and L1YH mice. n/μm2 = number of synapses per square micron of somal perimeter. Error bars represent SEM; ***P < 0.001.
Impaired Branching of Cortical Basket Interneurons in Developing L1YH Cortex
To explore the possible mechanism of reduced inhibitory innervation in L1YH mice, L1YH heterozygous females were crossed with GAD67-EGFP BAC transgenic males (Chattopadhyaya et al. 2004) to generate L1YH/GAD67-EGFP mice and WT/GAD67-EGFP littermates, which enabled visualization of GABAergic basket interneurons. In GAD67-EGFP mice, a subpopulation of parvalbumin-positive basket interneurons expresses EGFPdriven by the GAD67 promoter (Chattopadhyaya et al. 2004). These cells target the soma of pyramidal cells, as shown in the primary visual cortex (Chattopadhyaya et al. 2004). Immunofluorescence staining of L1 in layers II/III of the cingulate cortex of P10 WT/GAD67-EGFP mice showed that L1 colocalized in part with EGFP in processes and soma of basket interneurons but was also expressed throughout the EGFP-negative neuropil (Fig. 4A). Moreover, double immunofluorescence staining of L1 and the presynaptic inhibitory synapse marker GAD65 (Feldblum et al. 1993; Esclapez et al. 1994) in cingulate cortex of non-EGFP expressing WT mice at P10 showed that L1 colocalized in part with GAD65 (Fig. 4B). Quantitation of double staining using a thresholding method (Ango et al. 2008) indicated that the percent of GAD65 immunolabeling that overlapped with L1 immunolabeling at perisomatic sites was approximately 55.7 ± 2.4%. Double staining of L1 and the postsynaptic inhibitory synapse maker gephyrin (Sassoe-Pognetto and Fritschy 2000; Sassoe-Pognetto et al. 2000) showed much less colocalization (Fig. 4C). Quantitation of L1 and gephyrin double immunostaining showed that the percent of gephyrin immunolabeling that overlapped with L1 immunolabeling was approximately 9.5 ± 1.7%. These results suggested that L1 localized to basket interneurons in cingulate cortex and overlapped with presynaptic and to a lesser extent with postsynaptic inhibitory markers at perisomatic regions where basket-cell terminals are located.
Figure 4.
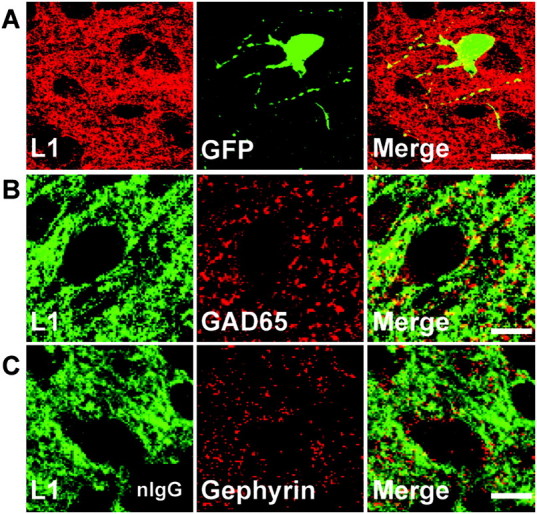
L1 localizes to GABAergic inhibitory synaptic puncta in postnatal mouse cingulate cortex. (A) Double immunofluorescence staining of L1 and GFP in cingulate cortex (layers II/III) of WT/GAD67-EGFP mice at P10 showed L1 localized along processes and somal membranes of EGFP-positive basket interneurons. Scale bar: 20 μm. (B) Double staining of L1 and GAD65, a presynaptic inhibitory synapse marker, in cingulate cortex (layers II/III) of WT mice at P10 revealed L1 colocalized in part with GAD65. Scale bar: 10 μm. (C) Double staining of L1 and gephyrin, a postsynaptic inhibitory synapse marker, in cingulate cortex (layers II/III) of WT mice at P10 showed less colocalization of L1 with gephyrin. Scale bar: 10 μm.
To investigate whether the loss of L1–ankyrin interaction affects process extension and arborization of basket interneurons, EGFP-positive interneurons of L1YH/GAD67-EGFP mice and WT/GAD67-EGFP littermates were analyzed by confocal microscopy during development at ages of P0, P10, P21, and P60, representing newborn, neonatal, adolescent, and adult stages, respectively. Images of EGFP-labeled basket interneurons in the cingulate cortex layers II/III were reconstructed in confocal z-stacks and analyzed using Neurolucida software. Both dendrites and axons of basket cells are densely arborized in the prefrontal cortex, and cannot be easily distinguished because their dendrites have few spines (Hartwich et al. 2009), thus both types of processes were analyzed as a group. EGFP-positive basket interneurons extended primary processes from the cell soma in both WT and L1YH mice at P10 with no obvious morphological differences. Basket interneurons in WT cingulate cortex developed extensively branched arbors from P21 to P60, whereas L1YH basket interneurons showed much less branching (Fig. 5). Quantification of basket interneuron arborization showed that in WT/GAD67-EGFP mice, the branching index (the total number of ends/total number of primary processes for each neuron in z-stack images) of basket interneurons increased from P10 to P21 and P60, whereas in L1YH/GAD67-EGFP mice, it did not increase with age and was significantly lower than that of WT at P21 and P60 (Fig. 6A). Similarly, Sholl analysis revealed that the number of crossings by L1YH basket interneuronal processes at a given distance from the soma was similar at P10 but significantly less than that of WT basket interneurons at P21 and P60 (Fig. 6B). The reduced branching index of L1YH basket interneurons was due neither to reduced numbers of primary processes emerging from the soma (Fig. 6C) nor to somal area (Fig. 6D). Moreover, basket-cell density in layers II/III of cingulate cortex was not altered in L1YH mice compared with age-matched WT littermates at any age (P10, P21, and P60) (Fig. 6E).
Figure 5.
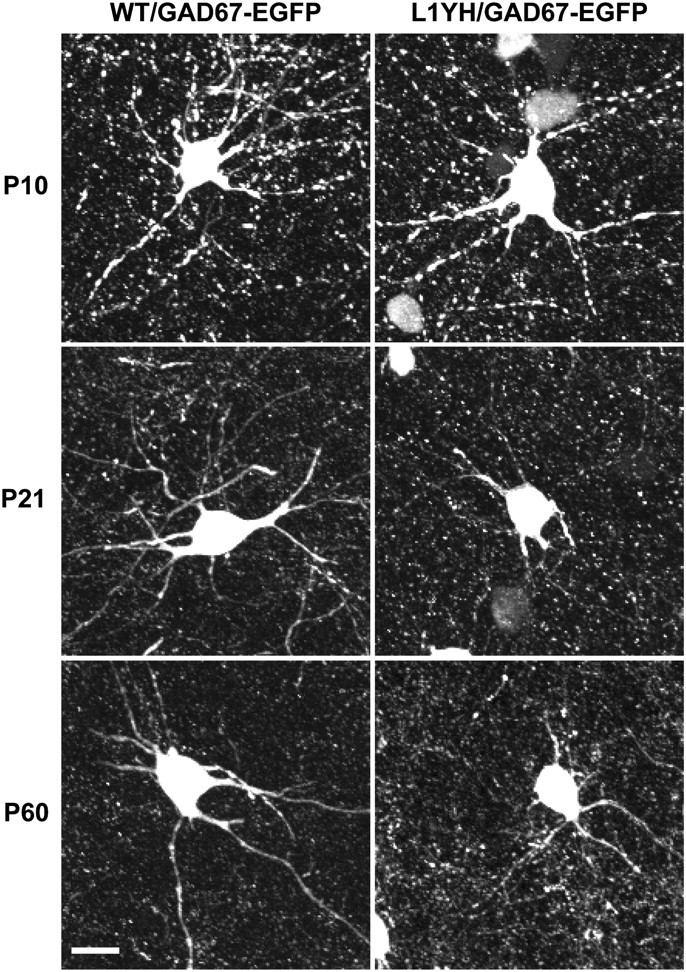
Reduced process elaboration by basket interneurons in cingulate cortex in L1YH/GAD67-EGFP mice. Individual EGFP-labeled basket interneurons from layers II/III of the cingulate cortex of L1YH/GAD67-EGFP and WT/GAD67-EGFP mice were imaged and reconstructed from confocal z-stacks. Representative images of basket interneurons at each stage are shown for each time point (P10, P21, and P60). EGFP-positive basket interneurons initiated primary processes (axons and dendrites exiting the soma) in both WT and L1YH mice at P10, and no morphological difference was observed between WT and L1YH mice (upper panel). WT basket interneurons developed extensively branched arbors from P21 to P60, whereas L1YH basket interneurons show much less branching (middle and lower panels). Scale bar: 20μm.
Figure 6.
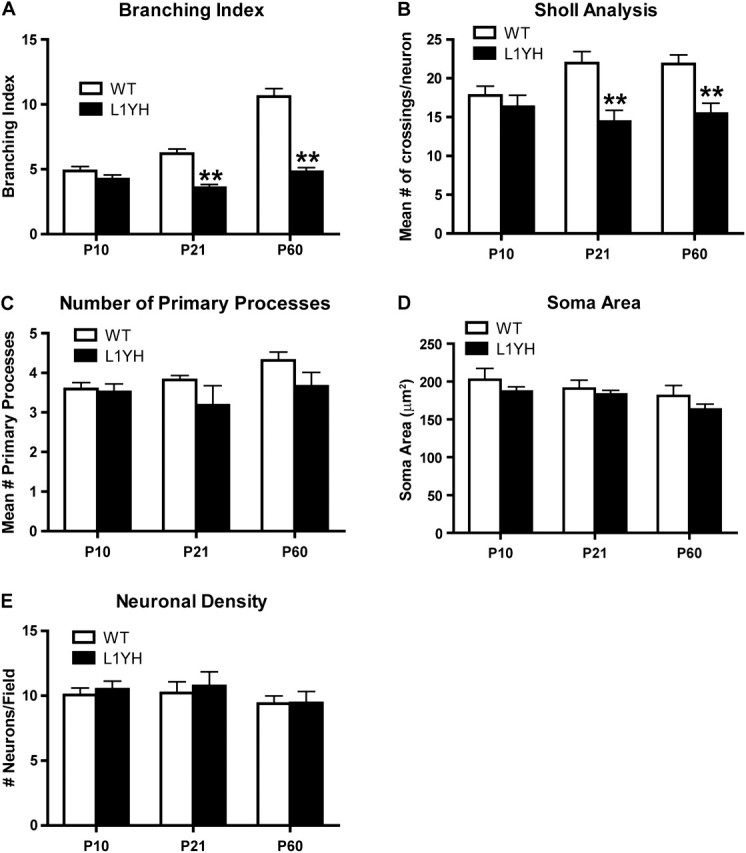
Quantification of basket interneuron arborization in the cingulate cortex of WT and L1YH/GAD67-EGFP mice. Individual EGFP-labeled basket interneurons from layers II/III of the cingulate cortex of L1YH/GAD67-EGFP and WT/GAD67-EGFP mice were imaged and reconstructed from confocal z-stacks, then analyzed using Neurolucida software. At least 60 EGFP-labeled basket interneurons from 6 random selected fields per animal were analyzed, using 4 mice per stage and genotype. (A) In L1YH/GAD67-EGFP mice, the branching index (the total number of ends/total number of primary processes for each neuron evident in the z-stack images) of basket interneurons did not increase with age and was significantly lower than that of WT at P21 and P60. (B) Sholl analysis revealed that in L1YH/GAD67-EGFP mice, the numbers of crossings by basket interneurons processes at a given distance from the soma were similar at P10 but significantly less than that of WT basket interneurons at P21 and P60 (mean number of crossings per neuron at Sholl distance of 30 μm was shown). (C–E) The mean numbers of primary processes per neuron (C), mean soma area (D), and neuronal density (E) were not affected in L1YH mice. Error bars represent SEM; **P < 0.01.
The Developmental Regulation of L1 Expression and Phosphorylation in Brain
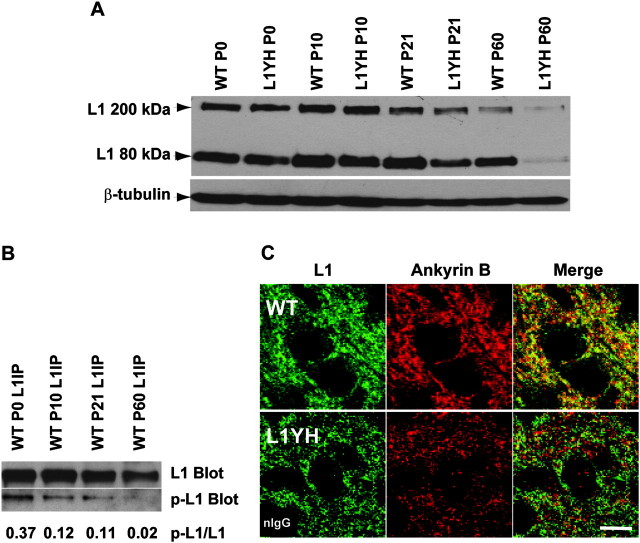
The reduced inhibitory synapse density and impaired basket interneuron branching in L1YH mice cingulate cortex suggested that L1–ankyrin interaction plays an important role in inhibitory synapse development. Double immunofluorescence staining of L1 and ankyrin B at P10 revealed a broad expression of L1 and ankyrin B in layers II/III of cingulate cortex, including colocalization at perisomatic sites in WT mice; however, there appeared to be less colocalization in L1YH cortex (Fig. 4B). Because the L1-ankyrin interaction is regulated by L1 phosphorylation (Garver et al. 1997; Tuvia et al. 1997; Gil et al. 2003; Whittard et al. 2006) in vivo, the developmental regulation of L1 expression and phosphorylation in the mouse brain was further examined (Fig. 7). Western blotting of brain lysates using an antibody against intracellular domain of L1 protein (2C2, Abcam) detected full-length L1 (FL-L1, 200–220 kDa) and an 80-kDa C-terminal plasmin-cleavage product (Burgoon et al. 1991; Nayeem et al. 1999) in WT and L1YH mutant brain from P0 to P60. The expression levels of L1 in WT and L1YH forebrain were similar from P0 to P21 relative to β-tubulin as a control; however, the L1 level in L1YH was lower than that in WT at P60 (adult stage) (Fig. 4C). The phosphorylation of L1 at Tyr1229 was examined by immunoprecipitation of L1 from brain lysates followed by immunoblotting with antibodies specific for L1 phosphorylated at Tyr1229 (p-L1) in WT brain at different ages. As shown in Figure 4D, the phosphorylation of L1 occurred at highest level at P0 (p-L1/L1 ratio = 0.37), decreasing at P10 and P21 (p-L1/L1 ratio = 0.11–0.12) and almost undetectable at P60 (p-L1/L1 ratio = 0.02). The striking decrease in L1 phosphorylation at Tyr1229 corresponded to stages of elevated inhibitory synaptogenesis/remodeling (P20) and synaptic maturation (P40) (Vincent et al. 1995; Flames et al. 2004). These results suggested that in WT mice, dephosphorylation of L1 at Tyr1229, resulting in increased L1 binding to the cytoskeleton through ankyrin, promotes inhibitory synaptogenesis and stabilization.
Figure 7.
Regulation of L1 expression and phosphorylation in postnatal brain development. (A) Developmental regulation of L1 expression in mouse forebrain. Lysates of mouse forebrain (75 μg) were analyzed at ages of P0, P10, P21, and P60 for L1 expression by SDS-PAGE and immunoblotting. The expression levels of L1 in WT and L1YH forebrain were similar from P0 to P21; however, the L1 level in L1YH brain was lower than WT at P60 (adult). The data shown were representative of three independent brain lysates. Blots were reprobed for β-tubulin as an internal control. (B) Developmental regulation of L1 phosphorylation on Tyr1229 in forebrain. L1 was immunoprecipitated from lysates of P0, P10, P21, and P60 WT forebrain and subjected to SDS-PAGE and immunoblotting with antibody specific for L1 phosphorylated at Tyr1229 (p-L1 Blot), then reprobed for total L1 with antibody specific for L1 protein (L1 Blot). Blots were densitometrically scanned, and band densities were analyzed using NIH Image J software. The ratios of phospho-L1 to L1 intensities are indicated below. (C) L1 colocalizes with ankyrin B in WT but not L1YH cingulate cortex. Double immunofluorescence staining of L1 and ankyrin B in layers II/III of cingulate cortex of P10 WT and L1YH mice shows colocalization in WT but not L1YH cortex in merged confocal images. Scale bar: 10 μm. Insert in lower left panel shows control staining of L1YH cortex with nonimmune IgG (nIgG).
Discussion
Our results provide evidence that a point mutation that disrupts ankyrin binding in the adhesion molecule L1 leads to impaired postnatal development of GABAergic perisomatic inhibitory neurons in the mouse cingulate cortex. Loss of L1–ankyrin interaction in L1YH mutant mice caused a striking decrease in arborization of neuronal processes and a reduction in the number of synapses in a subpopulation of basket cells whose axons target pyramidal cell soma. Electron microscopy revealed that perisomatic inhibitory synapses, but not excitatory synapses in the neuropil, were specifically affected. In the cingulate cortex of wild-type mice, L1 colocalized with presynaptic, and to a lesser extent postsynaptic, inhibitory markers at perisomatic sites. During the major postnatal period of basket-cell synapse formation in the cingulate cortex, L1 phosphorylation on Tyr1229 decreased, correlating with increasing ankyrin binding. Taken together, these results suggest a novel role for L1 engagement with the actin cytoskeleton in development of basket-cell connectivity within the mouse cingulate cortex.
The reduction in perisomatic synapses of basket interneurons in L1YH mutant mice might arise as a consequence of decreased branching of processes during postnatal development or of impairment in the formation and/or stabilization of synapses. Decreased numbers of axonal branches of the otherwise densely arborized basket cell would lead to correspondingly reduced numbers of perisomatic synaptic puncta, altering inhibitory/excitatory balance in the cortex. A possible involvement of L1–ankyrin binding in branch initiation of basket-cell axons in vivo is consistent with the demonstrated role of L1–ankyrin association in initiation of neurites in neuron cultures (Nishimura et al. 2003). L1 interaction with ankyrins might instead function during synaptogenesis either to promote initial adhesive interactions between pre and postsynaptic components or to stabilize synaptic contacts. Presynaptic ankyrin (Koch et al. 2008; Pielage et al. 2008), as well as spectrin (Pielage et al. 2005), is required for synaptic stability. A role for L1-ankyrin binding in synapse stabilization is consistent with the finding that the optic nerve of ankyrin B knockout mice grows normally but later degenerates as L1 is downregulated from the axons (Scotland et al. 1998). The decrease in perisomatic puncta of basket interneurons in the L1YH cingulate cortex is in accord with a reduction in the number of perisomatic inhibitory synapses in the hippocampus of L1 total knockout mice, which decreases inhibitory transmission (Saghatelyan et al. 2004). Although we have not directly measured neurotransmission in L1YH mice, functional evidence for L1 in development of cortical as well as hippocampal circuitry is suggested by the deficits in sensorimotor gating and spatial learning in L1 knockout mice (Fransen et al. 1998; Irintchev et al. 2004). It will be important to evaluate functional consequences of the L1YH mutation directly in future studies.
A role for related L1-CAMs in synaptic development of GABAergic neurons has also been demonstrated for CHL1, which is required for targeting of stellate GABAergic axons to Purkinje cell dendrites (Ango et al. 2008). Synapse formation between stellate cell axons and Purkinje cells cannot be maintained in older CHL1 null mutant mice and results in atrophy of axon terminals in accord with a role in synaptic stabilization (Ango et al. 2008). In addition, Neurofascin and its binding partner ankyrin G cooperate to direct axons of GABAergic basket interneurons in the cerebellum to the axon initial segment of Purkinje neurons (Ango et al. 2004). Although L1–CAMs are the only Ig family members that associate with the actin cytoskeleton through ankyrin, neural cell adhesion molecule NCAM couples to actin by binding to spectrin (Sytnyk et al. 2006) and promotes perisomatic innervation by basket interneurons in the cingulate cortex (Brennaman and Maness 2008). Involvement of ankyrin in neuronal branching and/or synaptic contacts is likely due to its ability to stabilize L1 in the plasma membrane and to inhibit retrograde actin flow (Gil et al. 2003; Nishimura et al. 2003; Whittard et al. 2006).
A mutant allele of the Drosophila L1 family ortholog, Neuroglian (Nrg849), which contains a Ser213Leu mutation in the extracellular Ig2 domain, disrupts the morphology of presynaptic terminals at a motor neuron synapse and impairs neurotransmission (Godenschwege et al. 2006). This mutation affects homophilic adhesion but also induces a 50% decrease in phosphorylation of tyrosine residue Y1234, which is homologous to L1Y1229. Some Nrg849 mutants exhibit pathfinding errors resulting in fewer synapses, similar to the L1YH phenotype. However, expression of nonphosphorylatable NrgY1234F mutant in Nrg849 mutant flies did not rescue the Nrg849 mutant phenotype, whereas expression in wild-type flies caused synaptic impairment (Godenschwege et al. 2006). Because NrgY1234F binds ankyrin at half of normal levels (Hortsch et al. 1998), unlike L1Y1229H, which does not detectably bind ankyrin (Buhusi et al. 2008), the reduced ankyrin binding of mutant NrgY1234F may not be sufficient to rescue the Nrg849 phenotype, whereas synapse formation in a wild-type background may be disrupted by competitive interactions of NrgY1234F with wild-type Nrg. In any case, it is difficult to directly contrast these studies due to differences in organism (mammalian vs. Drosophila), neuron type (GABAergic interneuron vs. motor neuron), and synapse (perisomatic synapses in mouse cingulate cortex vs. giant fiber synapses). Moreover, the functions of L1 and Nrg may not be identical because there are 4 members of the mammalian L1 family, which have diverse functions and Nrg is the single L1 ortholog in flies.
Basket interneurons control synchronous firing of pyramidal neuron groups (Freund and Katona 2007) and are important for regulating prefrontal cortical functions such as working memory (Spencer et al. 2003; Wang et al. 2004; Fuchs et al. 2007; Touzani et al. 2007). Dysfunction of GABAergic inhibitory interneurons is increasingly implicated in schizophrenia and bipolar disorder (Brambilla et al. 2003; Benes et al. 2008; Kim and Webster 2008; Lewis et al. 2008), diseases that have been linked to altered regulation of L1 (Kurumaji et al. 2001; Laifenfeld et al. 2005; Wakabayashi et al. 2008). The present findings show that L1, through ankyrin/cytoskeletal engagement, is required for appropriate regulation of neuronal branching leading to proper development of inhibitory GABAergic interneuron synapses in the mouse cingulate cortex, thus contributing to development of cortical inhibitory circuitry.
Funding
NIMH Silvio O. Conte Center for the Neuroscience of Mental Disorders (MH064065 P.F.M.).
Acknowledgments
The authors thank Dr L.H. Brennaman, Dr P.M.F. Siesser, and Dr G.P. Demyanenko for technical assistance during the study. We are grateful to Dr R. Bagnell and Ms V. J. Madden for help obtaining electron microscopic images. Conflict of Interest: None declared.
References
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–272. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Ango F, Wu C, Van der Want JJ, Wu P, Schachner M, Huang ZJ. Bergmann glia and the recognition molecule CHL1 organize GABAergic axons and direct innervation of Purkinje cell dendrites. PLoS Biol. 2008;6:e103. doi: 10.1371/journal.pbio.0060103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Subburaju S, Walsh JP. Circuitry-based gene expression profiles in GABA cells of the trisynaptic pathway in schizophrenics versus bipolars. Proc Natl Acad Sci U S A. 2008;105:20935–20940. doi: 10.1073/pnas.0810153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- Bennett V, Chen L. Ankyrins and cellular targeting of diverse membrane proteins to physiological sites. Curr Opin Cell Biol. 2001;13:61–67. doi: 10.1016/s0955-0674(00)00175-7. [DOI] [PubMed] [Google Scholar]
- Bennett V, Healy J. Organizing the fluid membrane bilayer: diseases linked to spectrin and ankyrin. Trends Mol Med. 2008;14:28–36. doi: 10.1016/j.molmed.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Boiko T, Vakulenko M, Ewers H, Yap CC, Norden C, Winckler B. Ankyrin-dependent and -independent mechanisms orchestrate axonal compartmentalization of L1 family members neurofascin and L1/neuron-glia cell adhesion molecule. J Neurosci. 2007;27:590–603. doi: 10.1523/JNEUROSCI.4302-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8:721–737. doi: 10.1038/sj.mp.4001362. 715. [DOI] [PubMed] [Google Scholar]
- Brennaman LH, Maness PF. Developmental regulation of GABAergic interneuron branching and synaptic development in the prefrontal cortex by soluble neural cell adhesion molecule. Mol Cell Neurosci. 2008;37:781–793. doi: 10.1016/j.mcn.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi M, Schlatter MC, Demyanenko GP, Thresher R, Maness PF. L1 interaction with ankyrin regulates mediolateral topography in the retinocollicular projection. J Neurosci. 2008;28:177–188. doi: 10.1523/JNEUROSCI.3573-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoon MP, Grumet M, Mauro V, Edelman GM, Cunningham BA. Structure of the chicken neuron-glia cell adhesion molecule, Ng-CAM: origin of the polypeptides and relation to the Ig superfamily. J Cell Biol. 1991;112:1017–1029. doi: 10.1083/jcb.112.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Ong B, Bennett V. LAD-1, the Caenorhabditis elegans L1CAM homologue, participates in embryonic and gonadal morphogenesis and is a substrate for fibroblast growth factor receptor pathway-dependent phosphotyrosine-based signaling. J Cell Biol. 2001;154:841–855. doi: 10.1083/jcb.200009004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JQ, Bennett V. Ankyrin binding activity shared by the neurofascin/L1/NrCAM family of cell adhesion molecules. J Biol Chem. 1994;269:27163–27166. [PubMed] [Google Scholar]
- Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR. Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci. 1994;14:1834–1855. doi: 10.1523/JNEUROSCI.14-03-01834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldblum S, Erlander MG, Tobin AJ. Different distributions of GAD65 and GAD67 mRNAs suggest that the two glutamate decarboxylases play distinctive functional roles. J Neurosci Res. 1993;34:689–706. doi: 10.1002/jnr.490340612. [DOI] [PubMed] [Google Scholar]
- Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, Lai C, Rubenstein JL, Marin O. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Fransen E, D'Hooge R, Van Camp G, Verhoye M, Sijbers J, Reyniers E, Soriano P, Kamiguchi H, Willemsen R, Koekkoek SK, et al. L1 knockout mice show dilated ventricles, vermis hypoplasia and impaired exploration patterns. Hum Mol Genet. 1998;7:999–1009. doi: 10.1093/hmg/7.6.999. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Garver TD, Ren Q, Tuvia S, Bennett V. Tyrosine phosphorylation at a site highly conserved in the L1 family of cell adhesion molecules abolishes ankyrin binding and increases lateral mobility of neurofascin. J Cell Biol. 1997;137:703–714. doi: 10.1083/jcb.137.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil OD, Sakurai T, Bradley AE, Fink MY, Cassella MR, Kuo JA, Felsenfeld DP. Ankyrin binding mediates L1CAM interactions with static components of the cytoskeleton and inhibits retrograde movement of L1CAM on the cell surface. J Cell Biol. 2003;162:719–730. doi: 10.1083/jcb.200211011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godenschwege TA, Kristiansen LV, Uthaman SB, Hortsch M, Murphey RK. A conserved role for Drosophila neuroglian and human L1-CAM in central-synapse formation. Curr Biol. 2006;16:12–23. doi: 10.1016/j.cub.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Hartwich K, Pollak T, Klausberger T. Distinct firing patterns of identified basket and dendrite-targeting interneurons in the prefrontal cortex during hippocampal theta and local spindle oscillations. J Neurosci. 2009;29:9563–9574. doi: 10.1523/JNEUROSCI.1397-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortsch M, Homer D, Malhotra JD, Chang S, Frankel J, Jefford G, Dubreuil RR. Structural requirements for outside-in and inside-out signaling by Drosophila neuroglian, a member of the L1 family of cell adhesion molecules. J Cell Biol. 1998;142:251–261. doi: 10.1083/jcb.142.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortsch M, Nagaraj K, Godenschwege FZ. The interaction between L1-type proteins and ankyrins-a master switch for L1-type CAM function. Cell Mol Biol Lett. 2009;14:57–69. doi: 10.2478/s11658-008-0035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Irintchev A, Koch M, Needham LK, Maness P, Schachner M. Impairment of sensorimotor gating in mice deficient in the cell adhesion molecule L1 or its close homologue, CHL1. Brain Res. 2004;1029:131–134. doi: 10.1016/j.brainres.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Jenkins SM, Kizhatil K, Kramarcy NR, Sen A, Sealock R, Bennett V. FIGQY phosphorylation defines discrete populations of L1 cell adhesion molecules at sites of cell-cell contact and in migrating neurons. J Cell Sci. 2001;114:3823–3835. doi: 10.1242/jcs.114.21.3823. [DOI] [PubMed] [Google Scholar]
- Kenwrick S, Watkins A, De Angelis E. Neural cell recognition molecule L1: relating biological complexity to human disease mutations. Hum Mol Genet. 2000;9:879–886. doi: 10.1093/hmg/9.6.879. [DOI] [PubMed] [Google Scholar]
- Kim S, Webster MJ. Correlation analysis between genome-wide expression profiles and cytoarchitectural abnormalities in the prefrontal cortex of psychiatric disorders. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.99. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch I, Schwarz H, Beuchle D, Goellner B, Langegger M, Aberle H. Drosophila ankyrin 2 is required for synaptic stability. Neuron. 2008;58:210–222. doi: 10.1016/j.neuron.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Laifenfeld D, Karry R, Klein E, Ben-Shachar D. Alterations in cell adhesion molecule L1 and functionally related genes in major depression: a postmortem study. Biol Psychiatry. 2005;57:716–725. doi: 10.1016/j.biopsych.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Morris HM. Cell and receptor type-specific alterations in markers of GABA neurotransmission in the prefrontal cortex of subjects with schizophrenia. Neurotox Res. 2008;14:237–248. doi: 10.1007/BF03033813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- Nayeem N, Silletti S, Yang X, Lemmon VP, Reisfeld RA, Stallcup WB, Montgomery AM. A potential role for the plasmin(ogen) system in the posttranslational cleavage of the neural cell adhesion molecule L1. J Cell Sci. 1999;112:4739–4749. doi: 10.1242/jcs.112.24.4739. [DOI] [PubMed] [Google Scholar]
- Needham LK, Thelen K, Maness PF. Cytoplasmic domain mutations of the L1 cell adhesion molecule reduce L1-ankyrin interactions. J Neurosci. 2001;21:1490–1500. doi: 10.1523/JNEUROSCI.21-05-01490.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Yoshihara F, Tojima T, Ooashi N, Yoon W, Mikoshiba K, Bennett V, Kamiguchi H. L1-dependent neuritogenesis involves ankyrinB that mediates L1-CAM coupling with retrograde actin flow. J Cell Biol. 2003;163:1077–1088. doi: 10.1083/jcb.200303060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielage J, Cheng L, Fetter RD, Carlton PM, Sedat JW, Davis GW. A presynaptic giant ankyrin stabilizes the NMJ through regulation of presynaptic microtubules and transsynaptic cell adhesion. Neuron. 2008;58:195–209. doi: 10.1016/j.neuron.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielage J, Fetter RD, Davis GW. Presynaptic spectrin is essential for synapse stabilization. Curr Biol. 2005;15:918–928. doi: 10.1016/j.cub.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Saghatelyan AK, Nikonenko AG, Sun M, Rolf B, Putthoff P, Kutsche M, Bartsch U, Dityatev A, Schachner M. Reduced GABAergic transmission and number of hippocampal perisomatic inhibitory synapses in juvenile mice deficient in the neural cell adhesion molecule L1. Mol Cell Neurosci. 2004;26:191–203. doi: 10.1016/j.mcn.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Fritschy JM. Mini-review: gephyrin, a major postsynaptic protein of GABAergic synapses. Eur J Neurosci. 2000;12:2205–2210. doi: 10.1046/j.1460-9568.2000.00106.x. [DOI] [PubMed] [Google Scholar]
- Sassoe-Pognetto M, Panzanelli P, Sieghart W, Fritschy JM. Colocalization of multiple GABA(A) receptor subtypes with gephyrin at postsynaptic sites. J Comp Neurol. 2000;420:481–498. doi: 10.1002/(sici)1096-9861(20000515)420:4<481::aid-cne6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Scotland P, Zhou D, Benveniste H, Bennett V. Nervous system defects of AnkyrinB (−/−) mice suggest functional overlap between the cell adhesion molecule L1 and 440-kD AnkyrinB in premyelinated axons. J Cell Biol. 1998;143:1305–1315. doi: 10.1083/jcb.143.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytnyk V, Leshchyns'ka I, Nikonenko AG, Schachner M. NCAM promotes assembly and activity-dependent remodeling of the postsynaptic signaling complex. J Cell Biol. 2006;174:1071–1085. doi: 10.1083/jcb.200604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzani K, Puthanveettil SV, Kandel ER. Consolidation of learning strategies during spatial working memory task requires protein synthesis in the prefrontal cortex. Proc Natl Acad Sci U S A. 2007;104:5632–5637. doi: 10.1073/pnas.0611554104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuvia S, Garver TD, Bennett V. The phosphorylation state of the FIGQY tyrosine of neurofascin determines ankyrin-binding activity and patterns of cell segregation. Proc Natl Acad Sci U S A. 1997;94:12957–12962. doi: 10.1073/pnas.94.24.12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SL, Pabreza L, Benes FM. Postnatal maturation of GABA-immunoreactive neurons of rat medial prefrontal cortex. J Comp Neurol. 1995;355:81–92. doi: 10.1002/cne.903550110. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Uchida S, Funato H, Matsubara T, Watanuki T, Otsuki K, Fujimoto M, Nishida A, Watanabe Y. State-dependent changes in the expression levels of NCAM-140 and L1 in the peripheral blood cells of bipolar disorders, but not in the major depressive disorders. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32:1199–1205. doi: 10.1016/j.pnpbp.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Tegner J, Constantinidis C, Goldman-Rakic PS. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci U S A. 2004;101:1368–1373. doi: 10.1073/pnas.0305337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittard JD, Sakurai T, Cassella MR, Gazdoiu M, Felsenfeld DP. MAP kinase pathway-dependent phosphorylation of the L1-CAM ankyrin binding site regulates neuronal growth. Mol Biol Cell. 2006;17:2696–2706. doi: 10.1091/mbc.E06-01-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisch AH, Stallcup WB, Chong LD, Dahlin-Huppe K, Voshol J, Schachner M, Pasquale EB. Tyrosine phosphorylation of L1 family adhesion molecules: implication of the Eph kinase Cek5. J Neurosci Res. 1997;47:655–665. doi: 10.1002/(sici)1097-4547(19970315)47:6<655::aid-jnr12>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]