Abstract
Background
Birt–Hogg–Dubé syndrome (BHDS) is an inherited autosomal genodermatosis characterised by fibrofolliculomas of the skin, renal tumours and multiple lung cysts. Genetic studies have disclosed that the clinical picture as well as responsible germline FLCN mutations are diverse.
Objectives
BHDS may be caused by a germline deletion which cannot be detected by a conventional genetic approach. Real-time quantitative polymerase chain reaction (qPCR) may be able to identify such a mutation and thus provide us with a more accurate clinical picture of BHDS.
Methods
This study analysed 36 patients with multiple lung cysts of undetermined causes. Denaturing high performance liquid chromatography (DHPLC) was applied for mutation screening. If no abnormality was detected by DHPLC, the amount of each FLCN exon in genome was quantified by qPCR.
Results
An FLCN germline mutation was found in 23 (63.9%) of the 36 patients by DHPLC and direct sequencing (13 unique small nucleotide alterations which included 11 novel mutations). A large genomic deletion was identified in two of the remaining 13 patients by qPCR (one patient with exon 14 deletion and one patient with a deletion encompassing exons 9 to 14). Mutations including genomic deletions were most frequently identified in the 3′-end of the FLCN gene including exons 12 and 13 (13/25=52.0%). The BHDS patients whose multiple cysts prompted the diagnosis in this study showed a very low incidence of skin and renal involvement.
Conclusions
BHDS is due to large deletions as well as small nucleotide alterations. Racial differences may occur between Japanese and patients of European decent in terms of FLCN mutations and clinical manifestations.
Keywords: Tumour suppressor gene syndrome, folliculin, genodermatosis, familial pneumothorax, real-time quantitative PCR, diagnostics tests, clinical genetics, genetic screening/counselling, respiratory medicine, diffuse parenchymal lung disease
Introduction
Birt–Hogg–Dubé syndrome (BHDS) is a rare, autosomal dominant inheritance genodermatosis characterised by multiple, asymptomatic, dome shaped skin papules involving the head, neck, chest, back, and arms.1 These skin manifestations are pathologically characterised as fibrofolliculomas, or hamartomas of the hair follicles.1 2 BHDS is associated with hereditary renal tumours, especially hybrid oncocytic renal tumours and chromophobe renal cell carcinoma, as well as with pneumothoraces and/or multiple lung cysts.3–5 A risk assessment study of BHD affected patients concluded that a diagnosis of BHDS conferred a 6.9-fold increased risk of developing renal neoplasia and a 50.3-fold increased risk of spontaneous pneumothorax.6 The folliculin gene (FLCN), also known as BHD, that lies within the chromosomal band 17p11.2 is mutated in patients with BHDS.7 The FLCN gene consists of 14 exons7 and is considered to be a tumour suppressor gene.8
Genetic testing, however, has disclosed that patients with BHDS show a clinical heterogeneity and do not always have the three characteristic phenotypes (involvement of skin, kidney, and lung). An isolated familial spontaneous pneumothorax without skin and renal involvements is caused by FLCN germline mutations.9–11 Among the three manifestations, lung involvement may be the earliest since spontaneous pneumothorax was reported to occur at 7 years of age in a patient with BHDS.12
This study extended our previous study10 and analysed the FLCN gene in patients with multiple cysts and/or spontaneous pneumothorax due to undetermined causes. A real-time quantitative polymerase chain reaction (qPCR) system was established for genetic testing of the FLCN gene since some patients with BHDS may have a large genomic deletion like other hereditary genetic disorders.13–16 The results demonstrated, for the first time, that not only a small nucleotide alteration, but also a genome deletion, causes BHDS and its clinical phenotype can be solely or mainly expressed in the lungs.
Subjects and methods
Study population
This study was approved by the ethics committees of Juntendo University. All patients who participated in this study provided their written informed consent. As previously reported, this was an extended study10 to analyse the FLCN gene in patients who had multiple lung cysts of undetermined causes with or without pneumothorax episodes. This study assessed 36 patients including 14 males and 22 females (the age at the first pneumothorax and the enrolment, 35.1±15.3 and 48.1±15.0 years old, respectively, mean±SD). Nine of 36 patients were smokers. They were enrolled for this study since multiple lung cysts were demonstrated by chest computed tomography (CT) when they had a pneumothorax or were noted to have an abnormality on their chest x-rays during a regular health check-up (for details see supplementary file).
Mutation analysis of the FLCN gene
Mutations of the FLCN gene were first screened by denaturing high performance liquid chromatography (DHPLC) followed by the direct sequencing of the FLCN exon which was suspected to have a mutation.10 If sequence alterations were located in the region of the splice signal sequence and supposed to affect mRNA splicing, reverse transcription of FLCN mRNA followed by PCR (RT-PCR) was performed using ThermoScript RT-PCR system (Invitrogen, Carsbad, California, USA).10 The primers used for RT-PCR are listed in supplementary table 1.
If no abnormality was detected by DHPLC, then the copy number of each exon of the FLCN gene in the genome was quantified using real-time qPCR to detect a large genomic deletion in the FLCN gene. The DNA copy number of 14 exons of the FLCN gene (exons 1 to 14) was determined with an iCycler real-time PCR detection system (Applied Biosystems, Foster City, California, USA) using the DNA binding dye SYBR Green I. If qPCR indicated a germline deletion in the FLCN gene, Southern blotting was performed to confirm the qPCR result (see supplementary file and supplementary table 1 listing the primers for qPCR).
Nucleotide alterations were described relative to a reference sequence of coding DNA according to the nomenclature recommended by den Dunnen and Antonarakis17: the nucleotide +1 in the coding DNA sequence of FLCN is the A of the ATG translation initiation codon.
Results
Germline mutation of the FLCN gene
An FLCN germline mutation was found in 23 (63.9%) of the 36 patients by DHPLC. The remaining 13 patients were examined by qPCR, and a genomic deletion was found in two patients (15.4%). Accordingly, an FLCN germline mutation was found in 25 (69.4%) of the 36 patients with multiple lung cysts (table 1).
Table 1.
The results of mutation analysis of the FLCN gene
| Patient | Location | Mutation | Predicted results |
| JBR4 | Exon 10 | c.1063-10_1065delTCTTGTTTAGGTC | Exon 10 skip (38 amino acid in-frame deletion) |
| JBR7 | Exon 10 | c.1063-10_1065delTCTTGTTTAGGTC | Exon 10 skip (38 amino acid in-frame deletion) |
| JBR8 | Exon 13 | c.1533G→A (W511X) | Protein truncation |
| JBR9 | Exon 12 | c.1347_1353dupCCACCCT | Frame shift, protein truncation |
| JBR10 | Exon 7 | c.769_771delTCC | In-frame deletion |
| JBR11 | Exon 9 | c.887C→A (S296X) | Protein truncation |
| JBR12 | Exon 13 | c.1533_1536delGATG | Frame shift, protein truncation |
| JBR13 | Exon 13 | c.1433-1G→T | Exon 13 skip (frame shift, protein truncation) |
| JBR14 | Exon 9 ∼ 14 | c.872-?_c.1740+?del | Protein truncation |
| JBR15 | Exon 12 | c.1347_1353dupCCACCCT | Frame shift, protein truncation |
| JBR16 | Exon 12 | c.1347_1353dupCCACCCT | Frame shift, protein truncation |
| JBR17 | Exon 10 | c.1063-2A→G | Exon 10 skip (38 amino acid in-frame deletion) |
| JBR18 | Exon 14 ∼ | c.1539-?_c.1740+?del | Protein truncation |
| JBR19 | Exon 6 | c.[564_565dupCC;566_577delTGCTGGGGAAGG] | Frame shift, protein truncation |
| JBR20 | Exon 5 | c.328C→T (Q110X) | Protein truncation |
| JBR21 | Exon 12 | c.1347_1353dupCCACCCT | Frame shift, protein truncation |
| JBR22 | Exon 12 | c.1347_1353dupCCACCCT | Frame shift, protein truncation |
| JBR23 | Exon 6 | c.397-7_399delCCTCCAGGTC | Exon 6 skip (74 amino acid in-frame deletion) |
| JBR24 | Exon 13 | c.1533_1536delGATG | Frame shift, protein truncation |
| JBR25 | Exon 12 | c.1347_1353dupCCACCCT | Frame shift, protein truncation |
| JBR26 | Exon 12 | c.1347_1353dupCCACCCT | Frame shift, protein truncation |
| JBR27 | Exon 12 | c.1347_1353dupCCACCCT | Frame shift, protein truncation |
| JBR28 | Exon 9 | c.997_998dupTC | Frame shift, protein truncation |
| JBR29 | Exon 11 | c.1177-5_1177-3delCTC | Exon 11 skip (frame shift, protein truncation) |
| JBR30 | Exon 7 | c.769-771delTCC | In-frame deletion |
DHPLC screening resulted in the identification of 13 unique mutations including three nonsense mutations, four deletions, two insertions, one composite mutation consisting of insertion and deletion, and three splice acceptor site mutations. No missense mutation was identified in the study population. Eleven of them were novel (supplementary figures 1 and 2). Five mutations including two splice acceptor site mutations were expected to cause abnormalities in FLCN mRNA splicing. The effect of a mutation on FLCN mRNA transcripts was examined using Epstein–Barr virus lymphoblastoid B cell line (EBV-LCL) established from the patient using RT-PCR,10 which confirmed that all five mutations generated the exon skipped transcript together with normally spliced transcript (supplementary figure 2). Most of the mutations identified in this study were expected to cause a frameshift leading to the premature protein truncation, but c.769–771delTCC and three of five mutations within the region of the splice signal sequence (supplementary figures 2A–C) were expected to cause in-frame amino acid deletions (table 1).
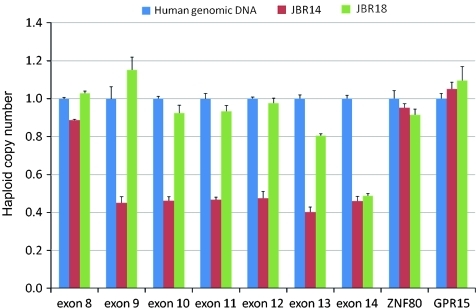
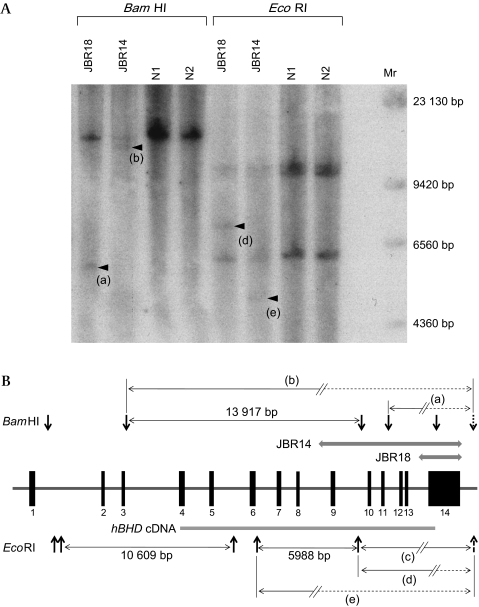
A large genomic deletion in the FLCN gene was identified in two patients, c.872-?_c.1740+?del and c.1539-?_c.1740+?del, using qPCR (figure 1). Patient JBR14 showed a decreased haploid copy number close to 0.5 in exons 9 to 14, suggesting a large genomic deletion spanning from exons 9 to 14. On the other hand, JBR18 showed a decreased haploid copy number close to 0.5 only in exon 14, suggesting a genomic deletion including exon 14 and 3′-untranslated region. These findings were confirmed with a conventional Southern blotting analysis (figure 2). BamHI digestion of normal controls generated a single positive fragment (figure 2A; 13 917 bp in size as expected in figure 2B) since other fragments are expected not to show up due to their small sizes. In patients JBR18 and JBR14, a smaller fragment, (a) and (b) in figure 2A, respectively, were demonstrated together with the fragment found in normal controls. EcoRI digestion yielded three fragments that were recognised in normal controls (figure 2A), two large fragments (10 609 bp and (c) as expected in figure 2B) and a smaller fragment (5988 bp as expected in figure 2B). In contrast, an aberrant fragment was identified (d) in patient JBR18 and (e) in JBR14, together with three fragments found in normal controls (figure 2A). The identical pattern of DNA fragments was confirmed in 10 normal controls when digested with BamHI and EcoRI.
Figure 1.
Analysis of the FLCN haploid copy number. FLCN copy number status (mean±SD) was determined by quantitative polymerase chain reaction (qPCR). The representative data from exon 8 to exon 14 of the FLCN gene, and the data of two reference genes, ZNF80 and GPR15, in two patients (JBR14 and JBR18) and normal genomic DNA (calibrator) are presented. JBR14 showed the gene dosage value close to 0.5 in 6 exons (exons 9 to 14), suggesting a large genomic deletion encompassing from exon 9 to exon 14. On the other hand, JBR18 showed the gene dosage value close to 0.5 only in exon 14, suggesting a genomic deletion of exon 14 and 3′-untranslated region. The gene dosage values of the remaining exons (exons 1 to 7) for JBR14 and JBR18 were close to 1.0 (data not shown).
Figure 2.
Southern blot analysis of the FLCN gene. Genomic DNA from two patients (JBR14 and JBR18) and two normal controls (N1 and N2) was digested with either BamHI or EcoRI and Southern blotting was performed using 32P-labelled hBHD cDNA probe25 (panel A). Size marker (Mr) was shown on the right. Distribution of 14 exons of the FLCN gene, restriction sites of BamHI and EcoRI, and expected length of fragment are schematically presented (panel B). The location of an undetermined restriction site which is involved in the generation of an aberrant band is indicated by a dotted arrow. Aberrant bands (a, b, c, d, and e) expected to be generated with a large genomic deletion (a thick shaded line) in JBR14 and JBR18 are depicted with the addition of a dotted line.
Clinical features of the BHDS patients in whom pneumothorax and/or multiple lung cysts are the presenting feature
The clinical features of 25 patients who were shown to carry an FLCN germline mutation in this study are summarised in table 2. In addition, to delineate the clinical features of patients with BHDS more precisely, five previously reported patients were included.10 Accordingly, 30 patients with BHDS included six males and 24 females. FLCN mutations were more frequently identified in females although BHDS is believed to have no gender predilection. This seems to be a selection bias since our hospital is the main institution in Japan where patients with suspected or diagnosed lymphangioleiomyomatosis are most often referred to. The age at enrolment to the study was 45.7±15.4 years old (mean±SD). All but one patient (29/30, 96.7%) had pneumothorax. Patient JBR19 had no information on the side that experienced pneumothorax and then 20 of 28 (71.4%) experienced pneumothorax in both lungs. The majority of patients (25 of 29; 86.2%) had pneumothorax repeatedly by the time of their enrolment. Only one patient had no episode of pneumothorax, but had multiple lung cysts. Only four patients experienced one pneumothorax. The total median number of pneumothorax episodes was three (range 0–8). No tendency for the side of pneumothorax episodes was recognised. The most frequent combination of phenotypes in the current group was lung cysts without skin lesions and renal disease, and this was demonstrated in 21 patients (70.0%). The median age of the 21 patients whose manifestation was only multiple lung cysts was 38 years old (range 23–85 years). All patients were screened for skin lesion by dermatologists. Skin lesions were detected in seven patients and only two patients had a histological confirmation: JBR16 (fibrofolliculomas) and JBR30 (seborrhoeic keratosis). All patients were screened for renal tumour by utilising either ultrasonography or CT. Renal tumours were very rare in this group and were found in only two patients, JBR15 with a single angiomyolipoma in the right kidney and JBR19 with a renal cancer (histopathological information was unavailable). Three patients had carcinoma in a different organ, including the breast, thyroid, and colon in their medical history.
Table 2.
Clinical data of the 30 subjects who carry pathogenic FLCN mutation
| Patient* | Sex | Age† | No. and location of PTX episodes‡ | Skin lesion¶ | Renal disease | Family history of PTX | Family history of skin lesion | Family history of renal disease | Medical history** | Smoking history |
| JBR1(B1) | F | 23(30) | L(1) R(1) | None | None | Yes | No | No | Endometriosis, PCOS, ovarian cyst, lipoma | Never |
| JBR2(B2) | F | 16(38) | L(3) R(3) | None | None | Yes | No | No | Myoma of the uterus, | Ex-smoker 2.2 pack-years |
| JBR3(B3) | F | 25(40) | L(3)R(2) | None | None | Yes | No | Yes | None | Never |
| JBR4 | F | 49(80) | R1 | None | None | No | No | No | None | Never |
| JBR5(B6) | F | 35(37) | L(1)R(1) | None | None | Yes | No | No | Myoma of the uterus | Never |
| JBR6(B7) | F | 28(38) | L(2)R(1) | None | None | Yes | No | Yes | Vocal cord nodules, myoma of the uterus, carcinoma of the thyroid gland | Ex-smoker 0.6 pack-years |
| JBR7 | F | 52(53) | L(3) | None | None | Yes | No | No | Breast carcinoma | Never |
| JBR8 | M | 33(33) | R(1) | None | None | No | No | No | Non-functioning adenoma of adrenal gland | Never |
| JBR9 | F | (31) | Never (lung cyst) | None | None | No | No | No | None | Never |
| JBR10 | F | 78(85) | L(1)R(1) | None | None | No | No | No | Colon cancer | Never |
| JBR11 | M | 35(38) | R(2) | Nose | None | Yes | No | No | None | Never |
| JBR12 | F | 57(68) | L(1)R(2) | Face, neck | Hydronephrosis (L) | No | No | No | Endometriosis | Never |
| JBR13 | F | 27(32) | L(2)R(2) | None | None | Yes | Yes | No | None | Never |
| JBR14 | F | 27(31) | L(1)R(6) | None | None | No | No | No | None | Never |
| JBR15 | F | 38(43) | L(1)R(2) | None | AML(R) | No | No | No | None | Ex-smoker 2.7 pack-years |
| JBR16 | F | 26(54) | L(4) | FFs | None | Yes | Yes | No | None | Never |
| JBR17 | M | 34(34) | L(5)R(2) | None | None | No | No | No | None | Ex-smoker 18.8 pack-years |
| JBR18 | F | 16(46) | L(4)R(4) | None | None | Yes | No | No | None | Never |
| JBR19 | F | unknown(53) | no details (2) | None | Cancer | Yes | No | No | None | Never |
| JBR20 | F | 26(38) | L(2)R(2) | None | None | No | No | No | None | Ex-smoker 7.5 pack-years |
| JBR21 | F | 20(34) | L(1)R(3) | Chest | None | Yes | No | No | Endometriosis | Never |
| JBR22 | F | 41(59) | R(1) | Face, neck | None | Yes | No | No | None | Never |
| JBR23§ | M | 25(40) | L(1)R(2) | None | None | Yes | No | No | None | Never |
| JBR24 | F | 52(52) | L(1) | None | None | No | No | No | None | Never |
| JBR25 | M | 29(32) | L(1)R(3) | None | None | Yes | No | No | None | Never |
| JBR26 | F | 21(23) | L(2)R(2) | None | None | No | No | No | None | Never |
| JBR27 | F | 65(66) | R(2) | None | None | Yes | No | No | None | Never |
| JBR28 | F | 24(44) | L(2)R(1) | None | None | Yes | No | No | None | Never |
| JBR29 | F | 51(53) | L(1)R(1) | Neck | None | Yes | Yes | No | None | Ex-smoker 4.7 pack-years |
| JBR30 | M | 29(66) | L(3)R(3) | Seborrhoeic keratosis | None | Yes | No | No | Gallbladder polyp | Never |
JBR16 had FFs on the nose and JBR30 had seborrhoeic keratosis on the face and the neck.
Family history was obtained by an interview with the patient. FLCN mutation status of the family member was undetermined since they did not wish the genetic testing.
The description in parentheses indicates the correspondence of the patient whose clinical data were already reported in the previous study.10
Age (years) at the first PTX is shown. Age at the enrolment in this study is indicated in parenthesis.
The number of pneumothorax episode in parentheses.
The detailed clinical pictures of this case are described elsewhere.18
The locations of skin papules observed are indicated if the patient has no skin biopsy.
Only medical histories considered to be relevant for BHDS are listed.
FFs, fibrofolliculomas; L, left sided; PCOS, polycystic ovarian syndrome; PTX, pneumothorax; R, right sided.
Spectrum of germline FLCN mutations
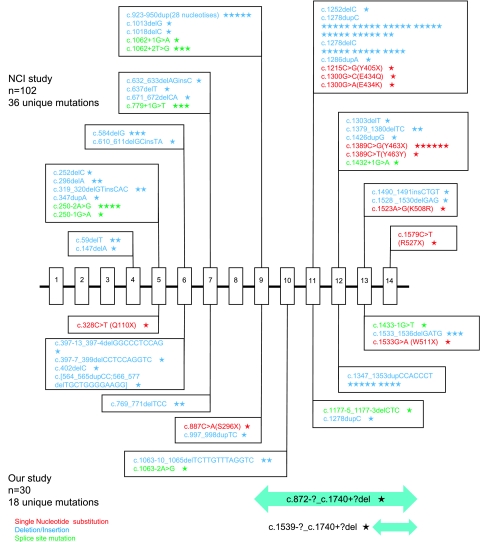
FLCN mutations identified in 30 Japanese patients whose presenting feature is pneumothorax and/or multiple lung cysts are summarised and the features were compared with those reported by the National Cancer Institute (NCI) group (figure 3).19 20 There were 18 unique mutations including two genomic deletions in the current study population, while the NCI group found 36 unique mutations. Deletion and insertion were the mutation type that was most frequently detected in both groups. The missense mutation, c.1523A>G (K508R), was detected in the NCI group, but not in the current study population. The mutations were distributed over the entire region in both groups. However, the mutation seems to be found more frequently in the 3′ region of the FLCN gene, especially after exon 9, in Japanese patients with BHDS (23/30, 76.7%). Both exon 12 and 13 seem to be a hot spot in Japanese BHD patients since c.1347_1353dupCCACCCT in exon 12 (9/30 = 30.0%) and c.1533_1536delGATG in exon13 (3/30 = 10.0%) are the mutations most frequently detected. On the other hand, exon 11 is a hot spot and represents 50.0% (51/102) of the BHD families in the NCI group. The majority of mutations in exon 11 occurred within the mononucleotide tract of eight cytosine resides, 1278dupC or 1278delC, in the NCI group. However, only two patients (2/30, 6.7%) in the current group had mutations in exon 11, while only one patient with 1278dupC was identified.
Figure 3.
Comparison of FLCN germline mutation spectrum reported by the National Cancer Institute (NCI) group and the current study group. The upper half shows the result of NCI group19 20 while the lower half is the result of the current study (combined with the previously reported result10 and the present study). Symbols: red, single nucleotide substitution; blue, deletion and/or insertion; green, splice site mutation; the bars on the bottom, genomic deletion; a star, one patient.
Discussion
This study demonstrated, for the first time, that not only a small nucleotide alteration but also a large genomic deletion in the FLCN gene causes BHDS. All of the previously reported FLCN mutations have been identified with direct sequencing of each exon of the FLCN gene. Recently, an FLCN mutation database has been introduced: http://shingenedatabase.com/.21 However, no large genomic deletion in the FLCN gene has yet been found in this online database. However, the current study has clearly shown that genetic testing for BHDS should include methods to detect a germline deletion in the FLCN gene.
qPCR has recently evolved as a valuable alternative to Southern blotting to demonstrate a genomic deletion and has successfully been applied for a genetic testing of Von-Hippel-Lindau disease,13 22 familial hypercholesterolaemia,14 and long QT syndrome.16 qPCR analysis of the FLCN gene will hereafter become one of the methods to be incorporated into genetic testing for BHDS as well. Other techniques for the detection of copy number changes or large deletions include the multiplex ligation dependent probe amplification (MLPA) method consisting of ligation of oligonucleotide probes having identical end sequences and subsequent multiplex PCR amplification using only one primer pair.23 MLPA has been successfully applied for the detection of large genomic deletion in LDL receptor gene,14 the PROSI gene in protein S deficiency,15 and the KCNQ1 and KCNH2 genes in long QT syndrome.16 Since the MLPA kit for the FLCN gene has recently become commercially available, it may therefore be an alternative for qPCR especially if a large number of samples are required to be screened.14
The Japanese patients in the present hospital based study appear to have a distinct spectrum of FLCN mutations in comparison to that of Caucasian patients with BHDS. FLCN mutations seem to frequently occur in the 3′-end of the FLCN gene in the Japanese. The 3′-end of the FLCN gene seems to have a significant relevance with FLCN function. Baba et al identified the FLCN interacting protein, FNIP1, which binds to the C-terminal region of FLCN.24 Takagi et al25 and Hasumi et al26 identified FNIP-L/FNIP2, another FLCN interacting protein with homology to FNIP1, and reported that the FNIP-L/FNIP2-binding domain is also present in the C-terminal region of FLCN. Germline FLCN mutations in the Japanese may directly impair the interaction between FLCN and its binding proteins and result in their disease phenotype.
The common mutation between the NCI group and the current study is only the mutation of a cytosine insertion in the C8 tract of exon 11. Accordingly, not only the distribution of mutations but also the nucleotide alterations are different between Caucasians and Japanese. One possible explanation for the apparent difference in the FLCN mutations may be due to the difference in an enrolment of the patients between the two groups. NCI group enrolled primarily patients who had some skin lesions or some renal tumours while the current study enrolled patients with multiple lung cysts with or without pneumothorax. In this context, the mutations detected in the present study may be predisposed to the lung phenotype of BHDS, although no phenotype–genotype correlation has been found so far.19 20 Another possibility may be the racial difference between Caucasians and Japanese since some of the inherited tumour suppressor gene syndromes are reported to show racial differences in mutations or clinical manifestations—for example, APC gene mutations causing familial adenomatous polyposis27 28 and BRCA1/BRCA2 gene mutations seen in African Americans and Caucasians.29 30
The most frequent combination of phenotypes in the present study group was lung cysts without skin lesions and renal diseases, and was demonstrated in 21 patients (70.0%). All of the patients had multiple lung cysts, and all but one patient had a history of pneumothorax. Although various nucleotide alterations were found in the FLCN gene including a germline deletion, no apparent correlation between the BHD phenotype, even in the lung phenotype, and genotype was found. Interestingly, two patients with a germline deletion had pneumothorax repeatedly and showed the highest number of occurrences of pneumothorax in the study population; JBR14 had seven pneumothorax episodes and JBR18 had eight. Further accumulation of patients with a germline deletion is required to determine if it confers the severe lung phenotype.
Toro et al20 and Schmidt et al19 in NCI group reported that they found 33 patients (32.4%) with three manifestations (skin lesion, renal tumour, lung cysts/pneumothorax), but there were no patients with three lesions in the present population. In their study population, lung cysts were found on chest CT imaging in 80.4% (82/102) of families and 66.0% (144/218) of individuals with BHDS, respectively. A history of spontaneous pneumothorax was demonstrated in 49.0% (50/102) of families and 34.1% (98/287) of individuals with BHDS, respectively. Although lung cysts/pneumothorax is frequently demonstrated in the NCI group, they had no patient with BHDS whose phenotype is limited to the lung. As in the findings of the spectrum of germline FLCN mutations, the differences in phenotype may also result from the method of enrolment of subjects into the study. Alternatively, racial difference or age at the time of enrolment into the study may be involved. Age related development of phenotype was reported in BHDS. Skin papules are reported to develop after the age of 30 years,1 while patients with renal tumours increase after the age of 40, although pneumothorax frequently occurs in patients younger than 40 years.6 More than half of the patients (16/30; 53.3%) in the current population were more than 40 years old and the majority (29/30; 96.7%) were more than 30 years old, respectively, at the time of enrolment into this study. More patients with the skin and renal phenotype should have been included, but the current population must be carefully followed to determine if they develop any skin and renal disease in the future.
The radiological features of pulmonary cysts that can help to differentiate BHDS from other cystic lung diseases have been identified on chest CT images.31 Accordingly, pulmonologists should be aware of BHDS, especially if patients have a family history of pneumothorax and characteristic cysts on chest CT images irrespective of the skin and renal diseases. Genetic testing, including qPCR of the FLCN gene, should therefore be performed in order to make an earlier diagnosis of BHDS while, in addition, subsequent care for the future development of renal tumours may also be warranted.
Acknowledgments
We would like to thank the physicians listed in the supplementary file for referring patients to us.
Footnotes
Funding: Grant-in-Aid for Scientific Research No. 18659242 (Seyama K) and in part by the High Technology Research Center Grant from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Competing interests: None.
Ethics approval: This study was conducted with the approval of the Juntendo University.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Birt AR, Hogg GR, Dube WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol 1977;113:1674–7 [PubMed] [Google Scholar]
- 2.Vincent A, Farley M, Chan E, et al. Birt-Hogg-Dube syndrome: a review of the literature and the differential diagnosis of firm facial papules. J Am Acad Dermatol 2003;49:698–705 [DOI] [PubMed] [Google Scholar]
- 3.Roth JS, Rabinowitz AD, Benson M, et al. Bilateral renal cell carcinoma in the Birt-Hogg-Dube syndrome. J Am Acad Dermatol 1993;29:1055–6 [DOI] [PubMed] [Google Scholar]
- 4.Toro JR, Glenn G, Duray P, et al. Birt-Hogg-Dube syndrome: a novel marker of kidney neoplasia. Arch Dermatol 1999;135:1195–202 [DOI] [PubMed] [Google Scholar]
- 5.Binet O, Audefray D, Beltzer-Garelly E, et al. [Haber's syndrome. First French family (2 cases)]. Ann Dermatol Venereol 1986;113:43–50 [PubMed] [Google Scholar]
- 6.Zbar B, Alvord WG, Glenn G, et al. Risk of renal and colonic neoplasms and spontaneous pneumothorax in the Birt-Hogg-Dube syndrome. Cancer Epidemiol Biomarkers Prev 2002;11:393–400 [PubMed] [Google Scholar]
- 7.Nickerson ML, Warren MB, Toro JR, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dube syndrome. Cancer Cell 2002;2:157–64 [DOI] [PubMed] [Google Scholar]
- 8.Vocke CD, Yang Y, Pavlovich CP, et al. High frequency of somatic frameshift BHD gene mutations in Birt-Hogg-Dube-associated renal tumors. J Natl Cancer Inst 2005;97:931–5 [DOI] [PubMed] [Google Scholar]
- 9.Graham RB, Nolasco M, Peterlin B, et al. Nonsense mutations in folliculin presenting as isolated familial spontaneous pneumothorax in adults. Am J Respir Crit Care Med 2005;172:39–44 [DOI] [PubMed] [Google Scholar]
- 10.Gunji Y, Akiyoshi T, Sato T, et al. Mutations of the Birt Hogg Dube gene in patients with multiple lung cysts and recurrent pneumothorax. J Med Genet 2007;44:588–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Painter JN, Tapanainen H, Somer M, et al. A 4-bp deletion in the Birt-Hogg-Dube gene (FLCN) causes dominantly inherited spontaneous pneumothorax. Am J Hum Genet 2005;76:522–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bessis D, Giraud S, Richard S. A novel familial germline mutation in the initiator codon of the BHD gene in a patient with Birt-Hogg-Dube syndrome. Br J Dermatol 2006;155:1067–9 [DOI] [PubMed] [Google Scholar]
- 13.Hoebeeck J, van der Luijt R, Poppe B, et al. Rapid detection of VHL exon deletions using real-time quantitative PCR. Lab Invest 2005;85:24–33 [DOI] [PubMed] [Google Scholar]
- 14.Damgaard D, Nissen PH, Jensen LG, et al. Detection of large deletions in the LDL receptor gene with quantitative PCR methods. BMC Med Genet 2005;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson AM, Hillarp A, Sall T, et al. Large deletions of the PROS1 gene in a large fraction of mutation-negative patients with protein S deficiency. Thromb Haemost 2005;94:951–7 [DOI] [PubMed] [Google Scholar]
- 16.Eddy CA, MacCormick JM, Chung SK, et al. Identification of large gene deletions and duplications in KCNQ1 and KCNH2 in patients with long QT syndrome. Heart Rhythm 2008;5:1275–81 [DOI] [PubMed] [Google Scholar]
- 17.den Dunnen JT, Antonarakis SE. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat 2000;15:7–12 [DOI] [PubMed] [Google Scholar]
- 18.Ishii H, Oka H, Amemiya Y, et al. A Japanese family with multiple lung cysts and recurrent pneumothorax: a possibility of Birt-Hogg-Dubé syndrome. Int Med 2009;48:1413–7 [DOI] [PubMed] [Google Scholar]
- 19.Schmidt LS, Nickerson ML, Warren MB, et al. Germline BHD-mutation spectrum and phenotype analysis of a large cohort of families with Birt-Hogg-Dube syndrome. Am J Hum Genet 2005;76:1023–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toro JR, Wei MH, Glenn GM, et al. BHD mutations, clinical and molecular genetic investigations of Birt-Hogg-Dube syndrome: a new series of 50 families and a review of published reports. J Med Genet 2008;45:321–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei MH, Blake PW, Shevchenko J, et al. The folliculin mutation database: an online database of mutations associated with Birt-Hogg-Dube syndrome. Hum Mutat 2009;30:E880–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hattori K, Teranishi J, Stolle C, et al. Detection of germline deletions using real-time quantitative polymerase chain reaction in Japanese patients with von Hippel-Lindau disease. Cancer Sci 2006;97:400–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schouten JP, McElgunn CJ, Waaijer R, et al. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res 2002;30:e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baba M, Hong SB, Sharma N, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci U S A 2006;103:15552–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takagi Y, Kobayashi T, Shiono M, et al. Interaction of folliculin (Birt-Hogg-Dube gene product) with a novel Fnip1-like (FnipL/Fnip2) protein. Oncogene 2008;27:5339–47 [DOI] [PubMed] [Google Scholar]
- 26.Hasumi H, Baba M, Hong SB, et al. Identification and characterization of a novel folliculin-interacting protein FNIP2. Gene 2008;415:60–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plawski A, Slomski R. APC gene mutations causing familial adenomatous polyposis in Polish patients. J Appl Genet 2008;49:407–14 [DOI] [PubMed] [Google Scholar]
- 28.Gavert N, Yaron Y, Naiman T, et al. Molecular analysis of the APC gene in 71 Israeli families: 17 novel mutations. Hum Mutat 2002;19:664. [DOI] [PubMed] [Google Scholar]
- 29.Pal T, Permuth-Wey J, Holtje T, et al. BRCA1 and BRCA2 mutations in a study of African American breast cancer patients. Cancer Epidemiol Biomarkers Prev 2004;13(11 Pt 1):1794–9 [PubMed] [Google Scholar]
- 30.Shen D, Wu Y, Subbarao M, et al. Mutation analysis of BRCA1 gene in African-American patients with breast cancer. J Natl Med Assoc 2000;92:29–35 [PMC free article] [PubMed] [Google Scholar]
- 31.Tobino K, Gunji Y, Kurihara M, et al. Characteristics of pulmonary cysts in Birt-Hogg-Dubé syndrome: Thin-section CT findings of the chest in 12 patients. Eur J Radiol 2009. Sep 24. [Epub ahead of print]. [DOI] [PubMed]