Abstract
Background
Recent guidelines on iron deficiency anaemia (IDA) have confirmed the aetiological role of Helicobacter pylori (H pylori), but the relationship still remains controversial.
Methods
Starting in May 2009, searches of the following databases were undertaken: Medline (1966 to April 2009), Embase (1980 to April 2009), the Cochrane library (1800 to June 2008), Cochrane Central Register of Controlled Trials, Premedline, Healthstar, CBMdisc and the Chinese National Knowledge Infrastructure Database (January 1970 to April 2009). Changes in haemoglobin (Hb) concentrations and serum ferritin (SF) concentrations were recorded for intervention and control groups. The meta-analysis used random effect models and subgroup analyses were performed to explain heterogeneity.
Results
Eight studies met the inclusion criteria. All studies were performed in Asia, an area with a high incidence of IDA and H pylori. The pooled analysis of eight studies showed that H pylori eradication therapy can improve IDA, since changes in Hb and SF concentrations in the intervention groups were higher than in controls. The weighted mean difference (WMD) of Hb was 12.88 g/l (95% CI 6.03 to 19.74 g/l, p<0.00001); the WMD of SF was 10.05 μg/l (95% CI 5.48 to 14.63 μg/l, p<0.00001).
Conclusions
H pylori eradication therapy combined with iron administration is more effective than iron administration alone for the treatment of IDA. Eradication therapy has different effects on adults and children. Bismuth based triple therapy has a better response in terms of increased Hb and SF concentrations than proton pump inhibitor (PPI) based triple therapy.
Keywords: Iron deficiency; Helicobacter pylori; hemoglobin; serum ferritin; meta-analysis; bacteriology, gastroenterology; anaemia; nutritional support
Iron deficiency anaemia (IDA) is a global public health problem affecting both developing and developed countries, with major consequences for human health as well as social and economic development, especially in Asia.1–3 The World Health Organization estimates the number of people with IDA worldwide to be a staggering one billion.4Many risk factors for IDA have been identified, most of which are related to dietary habits.2 4In the past decades, the association between Helicobacter pylori (H pylori) infection and IDA has been controversial.5–7 However, eradication of H pylori has not been clearly demonstrated to improve IDA. The American College of Gastroenterology8 suggests adequately powered research studies are needed to assess whether H pylori eradication offers benefit to patients with IDA, especially unexplained IDA.
We performed a meta-analysis of randomised controlled trials (RCTs) to determine whether H pylori eradication treatment is an effective therapeutic strategy to improve IDA, especially serum haemoglobin (Hb) concentrations and serum ferritin (SF) concentrations. (Meta-analysis is the process of combining several study results that can be used to draw conclusions about therapeutic effectiveness or to plan new studies which also avoids the possibility of prejudice caused by a single study.9)
Methods
Search strategy and identification of studies
We performed the meta-analysis in accordance with the standard protocol recommended by the Quality of Reporting of Meta-analyses Group (QUOROM).10 The following databases were included in the search: Medline (1966 to April 2009), Embase (1980 to April 2009), the Cochrane Central (1800 to June 2008), the Cochrane Library (1800 to June 2008), Cochrane Central Register of Controlled Trials, Premedline, Healthstar, CBMdisc and the Chinese National Knowledge Infrastructure Database (January 1970 to April 2009). The search was limited to RCTs, using the following index terms: ‘H pylori’, ‘iron deficiency anemia’, ‘sideropenic refractory anemia’, ‘ferritin’, ‘iron’ and ‘hemoglobin’. Articles in all languages were included in the search, and translated as necessary. We sought information about unpublished and ongoing RCTs from authors of the included RCTs, and experts in the field. The Chinese National Knowledge Infrastructure Database was searched in Chinese. The CBMdisc was searched in Chinese and English. Only reference lists of selected publications were also individually searched for any other relevant articles.
Inclusion criteria
Inclusion criteria were established before identifying the articles to avoid any selection bias. RCTs on the relationship between H pylori infection and IDA, including the data describing the changes of the blood parameters (Hb and SF concentrations) between baseline and after treatments, were considered eligible for meta-analysis. Results of the studies included in this analysis were described in terms of baseline and end point blood parameters of both intervention and control groups, with mean variables stated as mean±SD. The participants in the RCTs must have had both H pylori infection and IDA, without other types of manifest causes of IDA. The Jadad11 score was utilised to screen out RCTs of low quality (Jadad score <2).
Quality assessment
The quality of the studies was assessed using the scoring system proposed by Jadad,11 including: selection bias12 (randomisation procedure and allocation concealment); attrition bias13 (withdrawals, dropouts); and detection bias14 15 (placebo utilisation). For those studies that did not describe randomisation methods, we contacted the authors to solicit this information and then reassessed the scores.
Data extraction
Two investigators (Huang XL and Qu XH) independently extracted data by using a structured form. Discrepancies were resolved by discussion and consultation with two other reviewers (Yan WL and Huang YL). The following information was sought from each report: year of publication, sample size, demographic information including ethnicity, age, and sex, country and region, method of ascertainment of H pylori, eradication therapy for H pylori, and changes in mean (SD) concentrations of Hb and SF in both the intervention and control groups.
Data analysis and statistical methods
We collected the baseline blood parameter values of both the intervention and control groups, and the reassessment data after H pylori eradication therapy. Mean changes in blood parameters between baseline and end point were calculated. The SD of Hb and SF changes were back-calculated when not provided. We calculated SD using the formula provided by Follmann et al.16 The correlation coefficient was calculated as 0.13.
With the intention of comparing mean changes in Hb and SF between intervention and control groups, we used weighted mean difference (WMD) and 95% CIs17 to evaluate the differences between each set of values. Pooled estimates of WMD of changes in Hb and SF concentrations between the control and intervention groups were calculated with Review Manager Version 5,18 and STATA vision 1019 was used to calculate Egger's test in publication bias.
Heterogeneity assessing
Meta-analysis should only be considered when a group of trials is sufficiently homogeneous in terms of participants, interventions and outcomes to provide a meaningful summary.20 We examined heterogeneity between trials with a standard Q test statistic (testing the hypothesis of homogeneity) and present the I2 value,21 which can be interpreted as the percentage of total variation across several studies due to heterogeneity.
For results showing significant heterogeneity (I2 >50%), a random effects meta-analysis was also performed using the method of DerSimonian and Laird.22 The underlying assumption of the model is that the studies are from a hypothetical population and that inter-study variance can be represented by a single estimate. This estimate allows differential weighting of studies based on precision. Compared with the fixed effects model, the random effects model gives greater weight to less precise studies that may have undesired effects on the summary estimates.23
Subgroup analysis
We utilised subgroup analyses to explore the factors that could impact the pooled estimates and cause heterogeneity. The difference of subgroups was tested with t tests. We separated the studies into groups we assumed to be methodologically or biologically heterogeneous. Subgroups included: sample size, age, country and region, degree of IDA, method of ascertainment of H pylori, eradication therapy for H pylori, and end point of blood reinspection. According to the classification standard by WHO, we defined people <18 years old as the children group and people >18 years old as the adult group. According to the diagnosis standard for IDA constituted by WHO,33 we defined those with Hb value of 80–120 g/l as the mild to moderate anaemia group, and those with a Hb value 60–80 g/l as the marked (pronounced) anaemia group.
Sensitivity analysis and publication bias
In sensitivity analysis, we calculated the main Cochrane Q* contributor. We then excluded this main contributor and reassessed the result to check the stability of the meta-analysis. Publication bias was assessed by the Egger's tests.24
Results
Search results
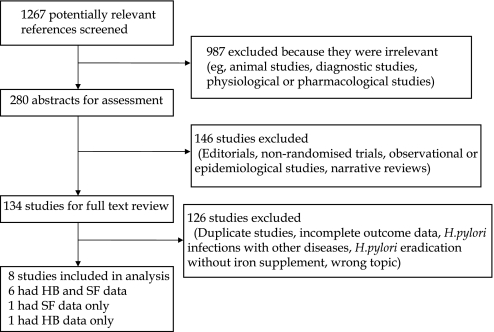
We identified 1267 potentially eligible articles. After excluding articles whose titles identified them as irrelevant, we used the abstracts of the remaining articles to further exclude studies that were not RCTs (editorials, non-randomised trials, observational or epidemiological studies, and narrative reviews). A final total of eight RCTs were identified as eligible for inclusion in the meta-analysis.25–27 29 31 32 Six RCTs25–32 provided Hb and SF data, one RCT28 provided only Hb changes, and one RCT30 provided only SF changes; figure 1 provides a description of these studies.
Figure 1.
Flow chart depicting the trial flow for selection of randomised controlled trials to be included. HB, haemoglobin; SF, serum ferritin.
Study characteristics
Our meta-analysis involved 450 participants, ranging in age from 2–76 years old. Most patients were children from Asia. Three RCTs26 27 32 involved patients who had mild to moderate anaemia, and another five RCTs25 28–31 involved patients who had marked anaemia. Five RCTs26 27 29 31 32 utilised proton pump inhibitor (PPI) based triple therapy to eradicate H pylori, and three RCTs25 28 30 used bismuth based triple therapy as their eradication regimen. The end point for blood parameter reinspection varied among the studies. Three RCTs26 27 29 selected 8 weeks as the time for reinspection. Three RCTs25 28 30 followed up blood parameters on sequential time nodes. Sarker et al32 selected 90 days as an end point in his study, and Vijayan et al31 selected 30 days (1 month) as an end point. Table 1 lists the characteristics of the eight reviewed studies.
Table 1.
Summary characteristics of studies and participants
| Reference | Year of publication | Age group | Country | Participants | Treatment therapies | H pylori test methods | Degree of anaemia | Time node for examination |
| Choe YH25 | 1999 | 10–17 | South Korea | 13 | B+A+M | Histology | Marked anaemia | Sequential |
| *Chen LH-128 | 2005 | 17–72 | China | 62 | B+A+M | UBT | Marked anaemia | Sequential |
| Lin Y27 | 2005 | 6–12 | China | 68 | O+A+C | UBT/histology | Moderate anaemia | 8 week |
| Huang LP26 | 2005 | 2–7 | China | 58 | O+A+M | Serum IgG test | Moderate anaemia | 8 week |
| Ren B29 | 2006 | 16–65 | China | 42 | O+A+M | Histology/rapid urease test | Marked anaemia | 8 week |
| †Chen LH-230 | 2007 | 18–76 | China | 86 | B+A+M | UBT | Marked anaemia | Sequential |
| Vijayan G31 | 2007 | >13 | India | 22 | L+T+C | Rapid urease test/histology | Marked anaemia | 30 days |
| Sarker SA32 | 2008 | 2–5 | Bangladesh | 99 | O+A+C | UBT | Moderate anaemia | 90 days |
Has only haemoglobin (Hb) data.
Has only serum ferritin (SF) data.
A, amoxicillin; B, bismuth; L, lansoprazole; M, metronidazole; O, omeprazole; T, tinidazole; UBT, urea breath test.
Summary estimates
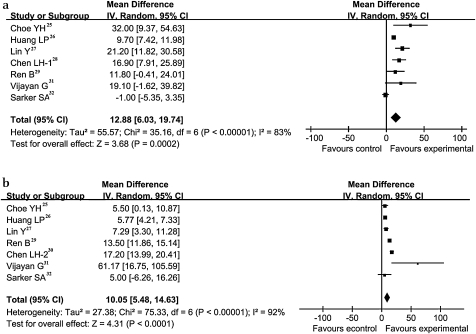
Hb and SF concentrations were selected to evaluate the therapeutic efficacy of H pylori as effect sizes. Among several biochemical tests that can reflect the metabolism of iron in the body, assessment of SF concentrations is the most specific in correlating with relative total body iron stores, particularly in the presence of chronic infection. We analysed these two continuous variables and performed a subgroup analysis for each. Seven of the eight RCTs provided data on changes in Hb, showing these changes to be higher in the intervention groups than in the control groups. The WMD of Hb concentrations from these seven studies was 12.88 g/l (95% CI 6.03 to 19.74 g/l, p<0.00001). Figure 2(a) shows the forest plots for the summary estimates of Hb changes between the intervention and control groups. The changes of SF in the intervention groups are higher than in control groups (figure 2(b)). The WMD of SF was calculated to be 10.05 μg/l (95% CI 5.48 to 14.63 μg/l, p<0.00001). Both summary estimates show significant heterogeneity in RCTs.
Figure 2.
Treatment effect and underlying connection between H pylori and IDA. (a) Weighted mean difference (WMD) forest plots of changes in hemoglobin (g/l) concentrations; (b) WMD forest plots of studies estimating changes in serum ferritin (μg/l) concentrations. Horizontal lines=95% CI. The size of the data marker corresponds to the weight of that study. The diamond and vertical broken line represent the summary estimate. The result favours experimental groups.
Subgroup analyses
Subgroup analyses to explore the heterogeneity sources among RCTs identified several methodological and biological sources of heterogeneity, which also allowed us to identify factors that could affect the pooled estimates. Table 2 shows the subgroups of Hb and SF studies and heterogeneity assessed by p value.
Table 2.
Summary estimates of effect of H pylori eradication on iron deficiency anaemia in subgroup analyses
| Summary estimate (WMD) | 95% CI | Heterogeneity (х2) | p Value* | |
| Haemoglobin (g/l) | ||||
| Age | ||||
| Children | 11.77 | 2.40 to 21.15 | 30.48 | <0.0001 |
| Adult | 15.11 | 7.87 to 22.35 | 0.43 | 0.51 |
| Treatment | ||||
| Bismuth | 20.73 | 7.85 to 33.61 | 1.48 | 0.02 |
| PPI | 10.42 | 2.75 to 18.08 | 27.63 | <0.0001 |
| Degree of anaemia | ||||
| Moderate anaemia | 9.13 | −0.40 to 18.66 | 26.20 | <0.0001 |
| Marked anaemia | 16.92 | 10.37 to 23.46 | 2.42 | 0.49 |
| Time period | ||||
| 4 weeks | 13.75 | 8.84 to 18.65 | 1.11 | 0.57 |
| 8 weeks | 15.47 | 8.98 to 21.97 | 10.76 | 0.03 |
| Serum ferritin (μg/l) | ||||
| Age | ||||
| Children | 5.93 | 4.53 to 7.32 | 0.54 | 0.91 |
| Adult | 15.08 | 11.49 to 18.67 | 4.05 | 0.04 |
| Therapy | ||||
| Bismuth | 11.55 | 0.09 to 23.01 | 13.61 | 0.0002 |
| PPI | 9.14 | 3.71 to 14.58 | 51.64 | <0.0001 |
| Degree of anaemia | ||||
| Moderate anaemia | 5.96 | 4.51 to 7.40 | 0.51 | 0.77 |
| Marked anaemia | 13.19 | 7.92 to 18.46 | 17.98 | 0.0004 |
| Time period | ||||
| 4 weeks | 2.24 | −2.37 to 6.85 | 7.05 | 0.03 |
| 8 weeks | 9.99 | 5.25 to 14.73 | 69.48 | <0.0001 |
The p value is test for heterogeneity of subgroup.
PPI, proton pump inhibitor; WMD, weighted mean difference.
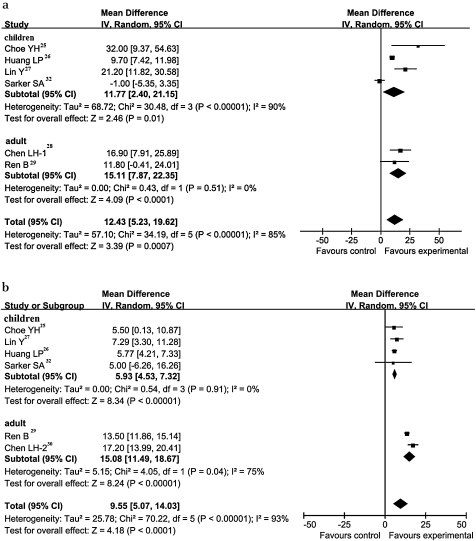
The WMD of Hb for children was 11.77 g/l (95% CI 2.40 to 21.15 g/l, p<0.00001), which was lower than the pooled estimates for adult individuals of 15.11 g/l (95% CI 7.87 to 22.35 g/l, p=0.51). The same situation occurred in the pooled analysis of SF. The WMD of SF for children was 5.93 μg/l (95% CI 4.53 to 7.32 μg/l, p=0.91), which was lower than the result for adults of 15.08 μg/l (95% CI 11.49 to 18.67 μg/l, p=0.04) (figure 3).
Figure 3.
Forest plot of age subgroup. (a) Weighted mean difference (WMD) forest plots of changes in haemoglobin (g/l) concentrations. (b) WMD forest plots of studies estimating changes in serum ferritin (μg/l) concentrations. Horizontal lines=95% CI. The size of the data marker corresponds to the weight of that study. The diamond and vertical broken line represent the summary estimate. The result favours experimental groups.
The baseline of Hb concentrations was divided into the mild to moderate anaemia group and the marked anaemia group by the boundary of 80 g/l. The WMD of the mild to moderate anaemia group was 9.13 g/l (95% CI −0.40 to 18.66 g/l, p<0.0001) and the WMD of the marked anaemia group was 16.92 g/l (95% CI 10.37 to 23.46 g/l, p=0.49). The marked anaemia group achieved a wider rising range than the mild to moderate anaemia group.
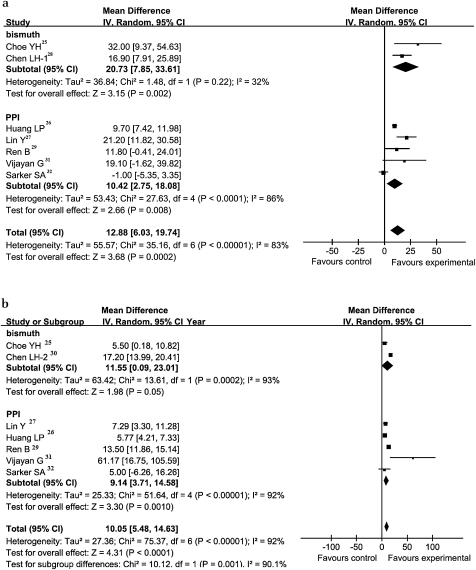
To examine the therapeutic regimen, we separated the studies into bismuth and PPI triple therapy. Bismuth triple therapy showed an obvious advantage over PPI triple therapy, particularly when used together with oral ferrous sulfate for H pylori patients with IDA. The WMD of Hb in the bismuth triple therapy group was 20.73 g/l (95% CI 7.85 to 33.61 g/l, p=0.02) versus 10.42 g/l (95% CI 2.75 to 18.08 g/l, p<0.0001) in the PPI triple therapy group. As for WMD of SF, bismuth triple therapy also showed obvious advantages over PPI triple therapy: 11.55 μg/l (95% CI 0.09 to 23.01 μg/l, p=0.0002) versus 9.14 μg/l (95% CI 3.71 to 14.58 μg/l, p<0.0001) (figure 4).
Figure 4.
Forest plot of therapy regimen subgroup. (a) Weighted mean difference (WMD) forest plots of changes in haemoglobin (g/l) concentrations. (b) WMD forest plots of studies estimating changes in serum ferritin (μg/l) concentrations. Horizontal lines=95% CI. The size of the data marker corresponds to the weight of that study. The diamond and vertical broken line represent the summary estimate. The result favours experimental groups.
The time period for blood parameter reinspection was also identified as having methodological heterogeneity and we performed a subanalysis. The WMD of Hb of the 4 week group was 13.75 g/l (95% CI 8.84 to 18.65 g/l, p=0.57) compared with the WMD of the 8 week group of 15.47 g/dl (95% CI 8.98 to 21.97 g/dl, p=0.03). The WMD of SF of the 4 week group was 2.24 μg/l (95% CI −2.37 to 6.85 μg/l, p=0.03), compared with 9.99 μg/l (95% CI 5.25 to 14.73 μg/l, p<0.0001) for the 8 week group. It seemed that the contrast between the intervention group and the control group increased with time.
Sensitivity analysis
Sensitivity analysis was performed for both Hb and SF data. Diversiform means was utilised to test the steadiness of the results we reached. The results show that most of the heterogeneity was caused by the study of Sarker et al32 (Q=23.8) in Hb changes, and Huang et al26 (Q=55.32) in SF changes. Hence, we conducted a sensitivity analysis by excluding these studies and reassessing the results. The sensitivity analyses showed that the results we reached were in general robust to the exclusion of the heterogeneity contributors from the meta-analysis. The WMD of Hb was 15.58 g/l (95% CI 9.57 to 21.60 g/l, p=0.04); the WMD of SF was 11.11 μg/l (95% CI 6.63 to 15.60 μg/l, p<0.00001).
Publication bias
The bias coefficient of the Hb studies, as calculated with Egger's24 test by STATA 10, was 1.44 (p=0.350). The bias coefficient of the SF studies was 1.32 (p=0.586), which showed a lack of publication bias.
Discussion
Our meta-analysis, evaluating a total of eight RCTs, shows that H pylori eradication treatment is an effective therapeutic strategy to improve IDA, especially Hb and SF concentrations. The WMD of Hb was 12.88 g/l (95% CI 6.03 to 19.74 g/l, p<0.00001); the WMD of SF was 10.05 μg/l (95% CI 5.48 to 14.63 μg/l, p<0.00001).
The association between IDA and H pylori infection and whether eradication of H pylori could treat IDA has been widely debated. An earlier review by Muhsen and Cohen34 included observational studies only. It suggests an association between H pylori and iron deficiency (pooled OR 1.38, 95% CI 1.16 to −1.65) or IDA (pooled OR 2.8, 95% CI 1.9 to 4.2). However, whether eradication of H pylori prevents or treats IDA has not been determined. In this meta-analysis, we try to answer this clinical research question.
In our study, internal validity and external validity were considered during our study procedure. To avoid selection bias, we searched not only the Medline database but also Embase, the Cochrane Library, Cochrane Central Register of Controlled Trials, Premedline, Healthstar, and CBMdisc for relevant articles. To minimise bias in the selection of studies and in data extraction, reviewers who were blinded to the journal, author, institution and date of publication, independently selected articles on the basis of the inclusion criteria. To ensure all the selected articles were high quality, the studies was assessed using the scoring system proposed by Jadad et al.11 All of the studies included had a Jadad score >2.
Eight RCTs, including high quality Chinese trials, satisfied the inclusion criteria. Almost all RCTs were performed in areas with a high incidence of H pylori and IDA (China, South Korea, India and Bangladesh), but all studies were performed in Asia. Thus, the external validity of our findings may be limited. Also, all the RCTs only compared the increase in Hb and SF concentrations achieved with H pylori eradication (plus iron) treatment and with iron administration alone in patients with IDA.
Our test for heterogeneity was significant, and hence we utilised a random effects model that accounts for inter-study variation. Compared with the fixed effects model, the random effects model evenly distributes weight among studies, minimising the impact of heterogeneity.23 So we utilised subgroup analyses to explore the factors that could affect the pooled estimates and cause heterogeneity.
Age was an important determinant of heterogeneity. Because the prevalence of both H pylori infection and IDA is significantly age dependent, assessment of the effect of H pylori infection on the IDA age matched group is meaningful.4 36–38 Iron requirements on a body weight basis are proportional to growth velocity. Accordingly, iron requirements of children are larger than adults and iron deficiency is most common in the preschool years and during puberty.4 During the preschool years and puberty, because of the large demand and poor dietary pattern, a given diet may be low in iron or may contain adequate amounts of iron which are of low bioavailability. Other nutrients necessary for haematopoiesis may also be deficient.39 In addition, vulnerability to iron deficiency varies greatly with each stage of life. In general, children aged 6 months to 5 years are the most vulnerable.40 To test this hypothesis, we separated individuals into an adult group and a children group. The result indicated that the adult group achieved a better response from the eradication of H pylori than the children group.
Heterogeneity can be also caused by a variety of eradication methods. The way in which the RCTs chose to eradicate H pylori can make a difference in pooled analysis. Bismuth based triple therapy achieved a much better response in regard to iron intake than PPI based triple therapy, probably by inhibiting intragastric proteolysis.35
All the RCTs took 2 weeks to eradicate H pylori, but the time for iron supplementation varied from study to study. Four weeks and 8 weeks were considered as time points to test the response to the intervention, so we divided the studies into 4 weeks and 8 weeks and undertook a subanalysis. It turned out that the 8 week iron supplement group had a much better response than the 4 week group. We suggest that further studies should be undertaken to evaluate the long term effect of H pylori eradication on correcting IDA.
Other patient characteristics (extent of H pylori infection and sex distribution) are also important for diagnostic accuracy, but variation in data presentation and incomplete reporting of the data made it impossible to study the effect of these variables. In this meta-analysis, we also attempted to reveal the relationship between the country of origin and the diagnostic accuracy, the result showing no significant statistical difference.
Our meta-analysis has several limitations. Only eight RCTs were available, and all the studies were performed in Asia. Eradication treatment without ferrous sulfate can illustrate the character H pylori plays in the IDA in a better way, but there is only few studies relevant, so we excluded these studies. This concept should be discussed in the future. We expect further investigations will take study design into consideration.
In conclusion, on the basis of the best available evidence, our meta-analysis suggests that H pylori eradication can improve IDA. These findings support the guidelines of the American College of Gastroenterology.8 Refractory IDA patients should be considered for assessment and treatment of H pylori. A strategy of population based screening and treatment for H pylori infection to prevent IDA is not currently recommended, but this concept should be discussed in the future. Eradication therapy has different effect on adults and adolescents. Bismuth based triple therapy has a better response in terms of increased Hb and SF concentrations than proton pump inhibitor (PPI) based triple therapy.
Main messages.
H pylori eradication (plus iron administration) therapy is more effective than iron administration alone for the treatment of IDA.
Refractory IDA patients should be considered for assessment and treatment of H pylori.
A strategy of population based screening and treatment for H pylori infection to prevent IDA is not currently recommended.
Eradication therapy has different effect on adults and children
Bismuth based triple therapy has a better response in terms of increased Hb and SF concentrations than proton pump inhibitor based triple therapy
Current research questions.
To explore the actual relationship between IDA and H pylori.
To investigate whether incidence of IDA will decline after H pylori eradication.
To investigate whether H pylori eradication without iron will improve IDA.
To explain the mechanism of the role of H pylori in IDA.
Acknowledgments
Our thanks to Professor Qiu YL(Department of Public health, Shanghai Jiaotong University School of Medicine, Shanghai, China) for the generosity in providing us with the information and knowledge, and to Stephen Walter for his helpful advice on statistical analysis.
Footnotes
Funding: The article included in this analysis were sponsored by several foundations, National Natural Science Foundation of China, Grant No. 30770599; Foundation of China Postdoctoral Science Foundation, Grant No.2005038143; Foundation of Shanghai Municipal Education Commission, Grant No.09YZ82; Foundation of Shanghai Leading Academic Discipline Project, Grant No. S30203; Natural Science Foundation of Shanghai Jiaotong University School of Medicine, Grant No. 2008XJ024.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.World Health Organization World prevalence of anaemia 1993-2005. WHO Global Database on Anaemia. World Health Organization; http://whqlibdoc.who.int/publications/2008/9789241596657_eng.pdf, (accessed 2008). [Google Scholar]
- 2.Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet 2007;370:511–20(PMID: 17693180), doi: 10.1016/S0140-6736(07)61235-5 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Control of iron deficiency anaemia in South-East Asia. Report of an intercountry workshop. http://whqlibdoc.who.int/searo/1994-99/SEA_NUT_134.pdf (accessed 1995).
- 4.World Health Organization, United Nations University, The United Nations Children's Fund Iron deficiency anaemia. Assessment, prevention and control: A guide for programme managers. http://whqlibdoc.who.int/hq/2001/WHO_NHD_01.3.pdf (accessed 2001). [Google Scholar]
- 5.Malfertheiner P, Megraud F, O'Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007;56:772–81 (PMID: 17170018), doi: 10.1136/gut.2006.101634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DuBois S, Kearney DJ. Iron-deficiency anemia and Helicobacter pylori infection: a review of the evidence. Am J Gastroenterol 2005;100:453–9 (PMID: 15667507), doi: 10.1111/j.1572-0241.2005.30252.x [DOI] [PubMed] [Google Scholar]
- 7.Annibale B, Marignani M, Monarca B, et al. Reversal of iron deficiency anemia after Helicobacter pylori eradication in patients with asymptomatic gastritis. Ann Intern Med 1999;131:668–72 (PMID:10577329). [DOI] [PubMed] [Google Scholar]
- 8.Chey WD, Wong BC. Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology Guideline on the Management of Helicobacter pylori Infection. Am J Gastroenterol 2007;102:1808–25 (PMID:17608775), doi: 10.1111/j.1572-0241.2007.01393.x [DOI] [PubMed] [Google Scholar]
- 9.L'Abbé KA, Detsky AS, O'Rourke K. Meta-analysis in clinical research. Ann Intern Med. 1987;107:224–33 (PMID: 3300460). [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality reporting of Meta-Analyses. Lancet 1999;354:1896–900 (PMID: 10584742), doi: 10.1016/S0140-6736(99)04149-5 [DOI] [PubMed] [Google Scholar]
- 11.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12 (PMID: 8721797), doi: 10.1016/0197-2456(95)00134-4 [DOI] [PubMed] [Google Scholar]
- 12.Kunz AR, Oxman AR. Empirical evidence of selection bias in studies of the effects of health care: a systematic review. Presented at the Cochrane Colloquium, Oslo, 1995 [Google Scholar]
- 13.Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias. JAMA 1995;273: 408–12 (PMid:7823387), doi: 10.1001/jama.273.5.408 [DOI] [PubMed] [Google Scholar]
- 14.Karlowski TR, Chalmers TC, Frenkel LD, et al. Ascorbic Acid for common cold: a prophylactic and therapeutic trail. JAMA 1975;231:1038–42 (PMid:163386), doi: 10.1001/jama.231.10.1038 [PubMed] [Google Scholar]
- 15.Colditz GA, Miller JN, Mosteller F. How study design affects outcomes in camparisons of therapy. I: medical. Star Med 1989;8:441–54 (PMid:2727468), doi: 10.1002/sim.4780080408 [DOI] [PubMed] [Google Scholar]
- 16.Follmann D, Elliott P, Suh I, et al. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 1992;45:769–73 (PMID: 1619456), doi: 10.1016/0895-4356(92)90054-Q [DOI] [PubMed] [Google Scholar]
- 17.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326:219 (PMid:12543843), doi: 10.1136/bmj.326.7382.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JPT,Green S: Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration 2008http://www.cochrane.org/resources/handbook [Google Scholar]
- 19.Sterne JAC, Bradburn MJ, Egger M. Meta-analysis in STATATM. In: Egger M, Smith GD, Altman DG, eds. Systematic Reviews in Health Care: Meta-analysis in Context. London: BMJ Publishing, 2001;347–69doi: 10.1002/9780470693926.ch18 [Google Scholar]
- 20.Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101–29 doi: 10.2307/3001666 [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60 (PMid:12958120), doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta analysis in clinical trials. Controlled Clin Trials 1986;7:177–88 (PMid:3802833), doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 23.Poole C, Greenland S. Random-effects meta-analyses are not always conservative. Am J Epidemiol 1999;150: 469–75 (PMID: 1672966). [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–34 (PMid:9310563). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choe YH, Kim SK, Son BK, et al. Randomized placebo-controlled trial of Helicobacter pylori eradication for iron-deficiency anemia in preadolescent children and adolescents. Helicobacter 1999;4:135–9 (PMid:10382128), doi: 10.1046/j.1523-5378.1999.98066.x [DOI] [PubMed] [Google Scholar]
- 26.Huang LP, Zhuang ML, Bei GP. Clinical treatment study of iron deficiency anemia associated Helicobacter pylori infection. J appal clin ediatr. 2005;20:30–1 (in chinese). [Google Scholar]
- 27.Lin Y, Wang WG, Wang SZ. Treatment of iron-deficiency anemia in patients with concomitant Helicobacter pylori infection:experience of 68 cases. Chin J Contemp Pediatr. 2005;7:429–31 (in chinese). [Google Scholar]
- 28.Chen LH, Luo HS. Clinical study on Helicobacter pylori influence therapeutics of Iron deliciency anemia in adults. Chin J Mod Med.2005;15:2969–71 (in chinese). [Google Scholar]
- 29.Ren B. Study on relationship between iron deficiency anemia and Helicobacter pylori infection. J Clin Re 2006;11:1805.– (in chinese). [Google Scholar]
- 30.Chen LH, Luo HS. Effects of H pylori therapy on erythrocytic and iron parameters in iron deficiency anemia patients with H pylori -positive chronic gastristis. World J Gastroenterol 2007;13:5380–3 (PMid:17879411). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vijayan G, Sundaram RC, Bobby Z, et al. Increased plasma malondialdehyde and fructosamine in anemic H pylori infected patients: effect of treatment. World J Gastroenterol 2007;13:796–800 (PMid:17278206). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarker SA, Mahmud H, Davidsson L, et al. Causal relationship of Helicobacter pylori with iron-deficiency anemia or failure of iron supplementation in children. Gastroenterology 2008;135:1534–42 (PMid:18775429), doi: 10.1053/j.gastro.2008.07.030 [DOI] [PubMed] [Google Scholar]
- 33.Michael Dobson. World health organization hemoglobin color scale, a practical answer to a vital need. Update in Anaesthesia 2002;6/1–6/2 [Google Scholar]
- 34.Muhsen K, Cohen D. Helicobacter pylori infection and iron stores: a systematic review and meta-analysis. Helicobacter 2008;13:323–40 (PMid:19250507), doi: 10.1111/j.1523-5378.2008.00617.x [DOI] [PubMed] [Google Scholar]
- 35.Hutchinson C, Geissler CA, Powell JJ, et al. Proton pump inhibitors suppress absorption of dietary non-haem iron in hereditary haemochromatosis. Gut 2007;56:1291–5 (PMid:17344278), doi: 10.1136/gut.2006.108613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol 2009;24:1587–600 (PMid:19788600), doi: 10.1111/j.1440-1746.2009.05982.x [DOI] [PubMed] [Google Scholar]
- 37.Huang JQ, Sridhar S. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet 2002;359:14–22 (PMID: 11809181), doi: 10.1016/S0140-6736(02)07273-2 [DOI] [PubMed] [Google Scholar]
- 38.Jolobe O. Guidelines for the management of iron deficiency anaemia. Gut 2001;48:283–4 (PMID: 11211859), doi: 10.1136/gut.48.2.283b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization, Food and Agricultural Organization of the United Nations Vitamin and mineral requirements in human nutrition. http://whqlibdoc.who.int/publications/2004/9241546123.pdf (accessed 2004).
- 40.Dallman PR, Siimes MA, Stekel A. Iron deficiency in infancy and childhood. Am J Clin Nutr 1980;33:86–118 (PMID 6986756). [DOI] [PubMed] [Google Scholar]