Abstract
Repetitive transcranial magnetic stimulation (rTMS) was applied to test the role of selected cortical regions in remediating sleep-deprivation–induced deficits in visual working memory (WM) performance. Three rTMS targets were chosen using a functional magnetic resonance imaging (fMRI)–identified network associated with sleep-deprivation–induced WM performance impairment: 2 regions from the network (upper left middle occipital gyrus and midline parietal cortex) and 1 nonnetwork region (lower left middle occipital gyrus). Fifteen participants underwent total sleep deprivation for 48 h. rTMS was applied at 5 Hz during a WM task in a within-subject sham-controlled design. The rTMS to the upper-middle occipital site resulted in a reduction of the sleep-induced reaction time deficit without a corresponding decrease in accuracy, whereas stimulation at the other sites did not. Each subject had undergone fMRI scanning while performing the task both pre- and postsleep deprivation, and the degree to which each individual activated the fMRI network was measured. The degree of performance enhancement with upper-middle occipital rTMS correlated with the degree to which each individual failed to sustain network activation. No effects were found in a subset of participants who performed the same rTMS procedure after recovering from sleep deprivation, suggesting that the performance enhancements seen following sleep deprivation were state dependent.
Keywords: facilitation, fMRI, sleep deprivation, TMS, working memory
Introduction
It has long been established that cognitive performance drops with sleep deprivation (for a review, see Dinges and Kribbs 1991). Performance generally suffers via decreases in attention, attributed to lapses into short-duration stage I sleep as well as general cognitive slowing. Such effects have been observed using the delayed match-to-sample (DMS) task, a variant of the working memory (WM) task of Sternberg (1969; Rypma et al. 2002). In this task, subjects respond as quickly as possible as to whether a test letter was included in a set of letters they had just seen. Following 48 h of total sleep deprivation in a group of young adults, reaction time (RT) in this task increased 25% from baseline levels, with a 61% increase in RT variability, a drop in accuracy of 14%, and percentage of nonresponses going from essentially none to 30% (Habeck et al. 2004). The subjects in the Habeck et al. study performed the DMS task while undergoing functional magnetic resonance imaging (fMRI) immediately before and at the end of sleep deprivation. Ordinal Trend Canonical Variates Analysis (Habeck et al. 2005; Moeller and Habeck 2006) was applied to the pre–post imaging data to identify an activation pattern whose expression decreased with sleep deprivation in as many subjects as possible (in this case, in 17 of 18 participants). Individual decreases in the activation of the pattern were correlated with individual levels of sleep-deprivation–induced performance deterioration, including reductions in accuracy, increases in RT variability, and increased lapsing. Thus, a brain network was identified using fMRI whose activation was related to resilience to sleep-deprivation–induced decrements in WM. However, this brain–behavior relationship was observed using a brain imaging technique and can only be correlational. One way to test causality in this relationship between neural reserve network expression and sustained performance is through the use of transcranial magnetic stimulation (TMS).
Prior work has demonstrated the utility of TMS as a noninvasive tool in testing the functional role of cortical regions associated with the performance of selected cognitive tasks (e.g., Walsh et al. 1998; Matthews et al. 2001; Luber, Peterchev, et al. 2007). In its initial applications, TMS was used to “disrupt” cognitive, motor, and perceptual functioning in cerebral cortex (e.g., Amassian et al. 1989; Pascual-Leone et al. 1991; Grafman et al. 1994). Disruptive action of TMS is clearly useful for establishing brain–behavior relationships and mapping function in health and disease (e.g., Luber, Peterchev, et al. 2007), but it is less clear how such action could be therapeutically applicable. However, TMS has also been reported to enhance performance in a number of tasks, including choice RT (Evers et al. 2001), picture naming (Topper et al. 1998), mental rotation of 3-dimensional objects (Klimesch et al. 2003), backward masking (Grosbras and Paus 2003), Stroop (Hayward et al. 2004), recognition memory (Kohler et al. 2004), and analogical reasoning (Boroojerdi et al. 2001). We recently reported that TMS could enhance performance on the DMS WM task (Luber, Kinnunen, et al. 2007). It is hypothesized that TMS-induced enhancements in these studies reflect facilitation of neural processing in localized cortical regions rather than disruption. Such facilitation could be useful in establishing brain–behavior relationships but might also have therapeutic value as illustrated by the reported antidepressant effects of TMS (e.g., Pascual-Leone et al. 1996; George et al. 1997; Avery et al. 2006).
A facilitatory effect of TMS could be especially useful in testing hypotheses regarding whether enhanced activity of a target region, or distributed network, could improve performance. Rather than relying on interindividual correlations between task performance and network expression, TMS-facilitation of activity in an imaging-identified network could be used to test whether increased activity of the network can actually improve performance within an individual. Such information would further validate the functional significance of the network and could also represent a potential avenue for therapeutic application.
We applied this approach to the set of cortical regions Habeck et al. (2004) identified whose decrease in activity with sleep deprivation correlated with worsened DMS task performance. Participants performed the DMS task during fMRI sessions prior to and at the end of a 2-day sleep deprivation period. Immediately following sleep deprivation, subjects underwent a TMS session in which 5-Hz repetitive transcranial magnetic stimulation (rTMS) was applied during the retention interval of the DMS task. This frequency and phase of application were selected based on our successful improvement of DMS performance at baseline with 5-Hz rTMS given during the retention phase (Luber, Kinnunen, et al. 2007). Stimulation sites were chosen from sites in the functional network identified in the Habeck et al. (2004) sleep deprivation study. Performance during active and sham rTMS stimulation was compared. We also computed the level of activation of the functional network related to DMS performance for each subject. These activation levels were then examined for correlations with performance during rTMS sessions, to test for a relationship between the fMRI change with sleep deprivation and rTMS effects. After recovery from sleep deprivation, the subjects returned for an identical rTMS session to determine whether any of the observed effects of rTMS were state dependent. We hypothesized that TMS administered to these sites in sleep-deprived individuals might reverse or remediate deterioration in task performance.
Materials and Methods
Subjects
Fifteen healthy male and female volunteers (5 female) with a mean age of 24.5 ± 2.7 years were recruited and signed written consent for participation in this study. Participants had an average of 16.2 ± 1.6 years of education. The group IQ was estimated by National Adult Reading Test and had a mean of 121.1 ± 4.2. The study was approved by the Columbia University and New York State Psychiatric Institute Investigational Review Board and was performed under an Food and Drug Administration-approved Investigational Device Exemption. Subjects were right handed (as determined by the modified Edinburgh Handedness Questionnaire), had normal or corrected-to-normal vision, and were native English speakers. Potential subjects were excluded if they had a history of current or past Axis I psychiatric disorder including substance abuse/dependence as determined by the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 4th Edition Axis I disorders (SCID-I/NP; First et al. 1998) or a history of neurological disease. All subjects were screened with physical and neurological examinations, blood and urine testing, urine drug screens, and pregnancy tests for women of childbearing capacity.
DMS Task
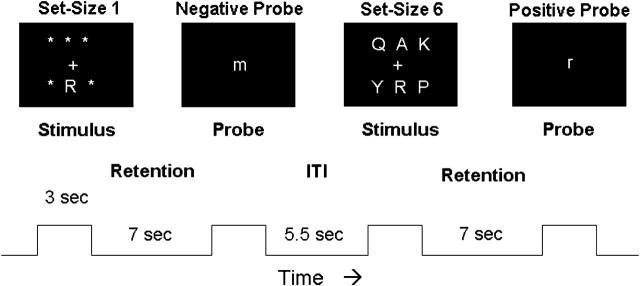
Participants were trained on the DMS task. Each trial was 20 s long according to the following sequence. First, an array of 1 or 6 uppercase letters was presented on a computer screen for 3 s (stimulus phase; see Fig. 1). Each letter subtended 1.1 degrees of visual angle. Next, the screen was blank for 7 s (retention phase), during which time the subjects were asked to fixate on the center of the screen and keep the stimulus items in mind. Finally, a test stimulus, a single lowercase letter, appeared for 3 s at the center of the screen (probe period). At this time, the subject was to indicate by a button press whether or not the probe letter matched a character in the stimulus array, using the right hand for matching probes and the left for nonmatches. Subjects were instructed to respond as quickly and as accurately as possible. Following the probe phase was a 5.5-s intertrial interval, during which the computer screen was again blank. Choice of set size (1 or 6) and positive or negative probe for an individual trial was pseudorandomized, with the restriction that there be 16 true positive and 16 true negative probes for each of the 2 set sizes over a block of 64 trials. The rTMS was applied for every other trial in a block. This interleaving of trials yielded an interval of 33 s between TMS trains, consistent with safety guidelines (Wassermann 1998).
Figure 1.
Schematic diagram of the DMS paradigm. Two trials are shown, the 1st with a set size of 1 and requiring a “no” response and the 2nd with a set size of 6 and requiring a “yes” response. The trial phases and their durations are listed beneath (ITI = intertrial interval).
Sleep Deprivation and MRI Procedures
Sleep deprivation was carried out over the course of 57 h, beginning at 7 AM on Day 1 and terminating by 3 PM on Day 3. MRI sessions occurred within 3 h of study start and within 4 h of leaving. MRI procedures are described in detail elsewhere (Habeck et al. 2004). Briefly, functional images were acquired using a 1.5-T magnetic resonance scanner (Philips Medical Systems, Eindhoven, The Netherlands). DMS task administration and data collection were controlled by computer using Psyscope software and synchronized with the MR scanner. Task stimuli were back projected onto a screen located at the foot of the MRI bed, with subjects viewing the screen via a mirror system located in the head coil. After the initial MRI session, participants were brought to a dedicated experimental testing room where they began a sequence of periodically administered cognitive tasks. Participants were admitted to the hospital at 6 PM on Day 1 where they entered the sleep disorders center. At that point, portable polysomnographic (PSG) monitoring and recording equipment, including electroencephalography (EEG) and electromyography (EMG) for sleep staging, was applied to the participants. Experimental personnel accompanied and monitored the participants continuously during day and evening hours. At night, participants were monitored by video camera and PSG when not in the direct presence of the technologists; otherwise, they remained with the technologists, who were trained to not allow sleep to occur in the participant. Participants were not allowed to consume caffeine or other stimulants. Nighttime cognitive testing was also monitored by the sleep technologist. While not participating in experimental protocols, participants had access to the Internet, music, and a TV with broadcast programing, movies, and video games. After the final MRI session on Day 3, subjects began a TMS session, which concluded the sleep deprivation period.
TMS Application
Subjects received both active and sham stimulation. The rTMS was applied using a vacuum-cooled figure-of-eight coil (5 cm diameter) powered by a Magstim Super-Rapid stimulator (Magstim Co., Whitland, South West Wales, UK). For sham rTMS, the coil was placed perpendicular to the subject's head (1 wing, 90° sham manipulation). When asked at the end of the session to make their best guesses as to which conditions were real TMS and which were sham, subjects were unable to identify sham blocks above chance level (54% correct, chi-square = 0.18, P < 0.67). Of note, all stimulation sites were at posterior locations, so subjects could not see any differences in coil positioning. In addition, subjects were told that the coil would be placed at different sites and that even very small differences in its exact location could result in very different sensations, depending on whether it was directly over a nerve or a muscle. The rTMS delivery was well tolerated. Side effects attributable to rTMS by the subjects were mostly absent: Over all sessions, only a single subject reported a slight headache. In addition, subject ratings of mood using a 5-item (happy, angry, nervous, sad, and tired) visual analog rating scale were not significantly different between the beginning and end of rTMS sessions.
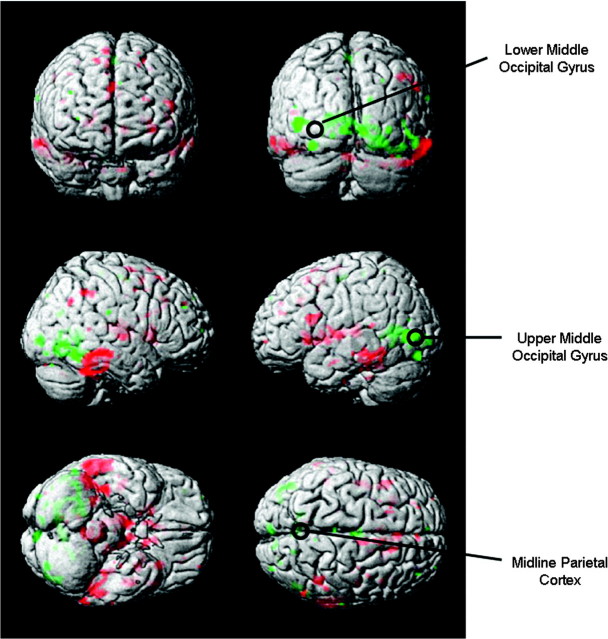
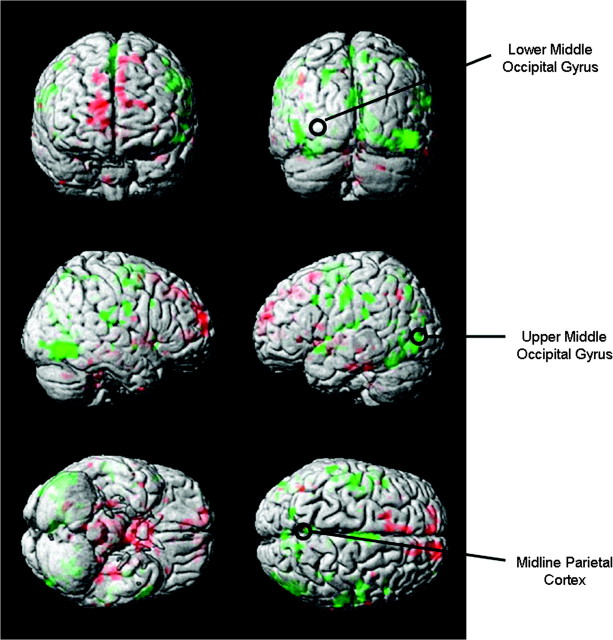
The rTMS stimulus intensity was set at 100% of motor threshold (MT) of the left hemisphere, which was defined as the lowest intensity needed to evoke motor potentials of at least 50 mV recorded from the 1st dorsal interosseous muscle in at least 5/10 stimulations. Three cortical sites were chosen for rTMS based on a functional cortical network of fMRI activation found in a previous study of sleep-deprived subjects performing the same DMS task (Habeck et al. 2004). Two of the target sites in this study, the upper part of the left middle occipital gyrus (UMO) in Brodmann Area 19 and midline inferior parietal cortex (PC), were located within this network, whereas a 3rd site not in the network, the lower part of the left middle occipital gyrus (LMO: about 3 cm posterior and below the other occipital site in Brodmann Area 18) was used as a control (see Fig. 2). The LMO site was used previously as a control site in Luber, Kinnunen, et al. (2007). The LMO site served as a control both by being outside the network and by being close enough to UMO so as to produce a similar scalp sensation, which was determined by subjective reports and ratings. During the TMS session, we asked subjects to describe the scalp sensation they felt due to the rTMS for each block. We began having them rate scalp sensation on a scale from 1 (no sensation) to 10 (quite painful) about halfway into the study. Overall, there was no agreement among subjects concerning which site produced the greatest sensation: 6 thought that LMO was greater, 4 that UMO was, and 5 that there was no difference between the 2. Of 8 subjects who gave numerical ratings, there were no significant differences in the ratings for LMO and UMO (t = 0.41, 7 degrees of freedom [df], P = 0.69). The sites were identified using high-resolution structural MRI scans obtained for each subject. Coil placement was guided by Brainsight, a computerized frameless stereotaxy system (Rogue Research, Montreal, Canada). This system uses an infrared camera to monitor the positions of reflective markers attached to the participant's head. Head locations are correlated in real time with the participant's MRI data after the data are coregistered to a set of anatomical locations. Reflective markers are attached to the coil and the subject, so that relative positions of the coil to the head (and the MRI) can be tracked, allowing precise positioning of the coil with respect to annotated MRI locations.
Figure 2.
Surface rendered projections of brain regions whose associated activation changed in most subjects from pre- to postsleep deprivation in a previous study (Habeck et al. 2004). Green indicates areas going down in activation with sleep deprivation. Red indicates areas going up in activation with sleep deprivation. The circles mark the 3 rTMS targets.
Eight blocks of 64 trials of the DMS task were run in each session. Two consecutive blocks were run at each of the 3 sites with active rTMS, and single blocks of sham rTMS were run at the beginning and end of each session. The order of sites was counterbalanced, and the sites of sham stimulation coincided with the 1st and last active sites used in a given session. Five-hertz active or sham rTMS was applied during the 7-s retention interval (35 pulses) of every other trial. Subjects were allowed breaks between each block, and their wakefulness was continuously monitored during task performance.
Nonsleep-Deprived Control Experiment
Eight of the 15 subjects (3 female) returned for a 2nd rTMS session after at least 2 weeks had gone by, allowing them time to recover and return to their normal sleep cycles. Upon their return, subjects were asked if they felt recovered from the sleep deprivation and if they had returned to their normal sleeping patterns. All answered that they had. They were then asked if they had a normal night's sleep the previous night, and all said they had. MT was reassessed when subjects returned for the nonsleep-deprived sessions, and when there was a difference, the new MT was used. There was no group difference between sleep-deprived and nonsleep-deprived MT (t = 0.86, 7 df, P = 0.42). The other rTMS treatment parameters were the same as in the sleep-deprived session: 5-Hz rTMS during the retention period of the DMS task, with trains applied every other trial. Sham rTMS was applied at the beginning and end of the session, just as it had been done during the sleep deprivation phase of the study. This design allowed us to compare sleep-deprived and nonsleep-deprived results within individual. The Brainsight stereotaxy system allowed precise repositioning of the TMS coil over the 3 sites used in the sleep deprivation session. The site order was changed for each subject from what it had been in the sleep-deprived session and was counterbalanced across the nonsleep-deprived subjects.
Analysis
Repeated-measures analyses of variance (ANOVAs) with factors of site (PC, LMO, and UMO), set size (1 and 6), and TMS (Active and Sham) were performed on the median RT and accuracy data. In initial analyses, no significant main effects or interactions due to gender were found. In addition, given our previous published results (Luber, Kinnunen, et al. 2007), we performed post hoc paired t-tests between active and sham at set size 6. This was done for the 3 sites, resulting in 3 tests. The significant alpha level, adjusted from 0.05, was 0.017.
The event-related fMRI data were analyzed using the ordinal trends covariance model (OrT) in order to extract a monotonic pattern of activity occurring pre- and postsleep deprivation, as was done in Habeck et al. (2004). OrT (Habeck et al., 2005; Moeller and Haybeck 2006) is predicated on event-related experimental designs in which incremental changes in task parameters are expected to produce monotonic trends in the activity of individually targeted signals. OrT performs an analysis with the aim of identifying topographic patterns that express ordinal trends on a subject-by-subject basis. OrT is a guided principal components analysis: a specially designed linear transformation is applied to the neuroimaging data with the effect that maximal salience is assigned to topographic patterns whose expressions are monotonic across a specified series of experimental conditions, corresponding to the positive incremental changes expected in the level of the targeted neural signal. The specific details of the analysis can be found in Habeck et al. (2004). In addition, the topographic network related to decreased performance in the DMS task during sleep deprivation found in a previous study (Habeck et al., 2004) was prospectively applied to the fMRI data of the subjects of the present study. This was obtained by performing the inner product of each subject-task scan with the Habeck et al. pattern in order to quantify pattern expression for individual subjects in the present group. The expression of the pattern for each subject was correlated with the difference between active and sham rTMS at each site, to examine the relationship between performance-related fMRI changes with sleep deprivation and rTMS-induced performance effects.
Results
Performance in the rTMS Session at the End of the Sleep Deprivation Period
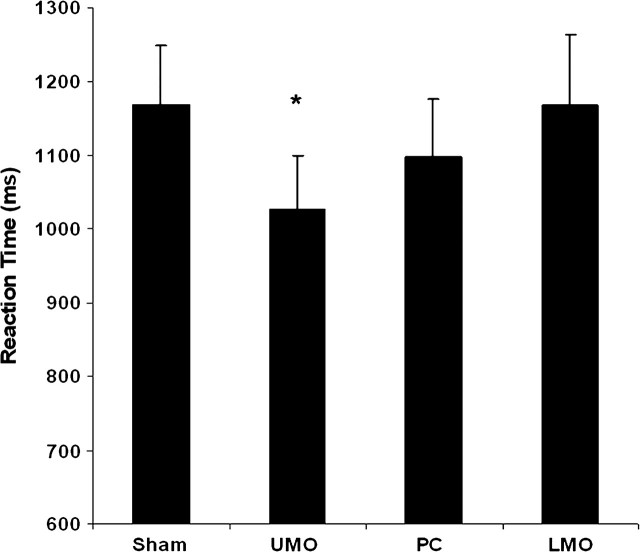
The average number of time-outs (i.e., trials in which participants made no response) in the sleep-deprived participants was 4.1 or 6.5% of trials, with no significant difference across rTMS target sites or between active and sham (Table 1). Participants maintained high levels of accuracy, averaging close to 90% correct for both set sizes (Table 2). There was no significant difference in accuracy across sites or between active and sham and no main effect of set size. For RT, the expected main effect of set size was found (F = 57.3, 1,14 df, P < 0.0001) as well as a site × rTMS interaction (F = 4.8, 3,12 df, P < 0.02). Post hoc testing revealed that rTMS given at UMO decreased RT for set size 6 by a mean difference of 143 ms (t14 = 2.98, P < 0.005; see Fig. 3). Thirteen of 15 sleep-deprived subjects were faster with rTMS stimulation to UMO. Stimulation at PC showed a slight trend for improvement (t14 = 1.29, P < 0.1), but the degree of improvement (72 ms faster) was half of that seen with stimulation at UMO. Stimulation at the active control site (LMO) produced no effect on RT, and there was no difference in RT between conditions at set size 1 at any site.
Table 1.
Mean number of time-outs (and standard error) for sleep-deprived participants
| Set size | Sham | LMO | PC | UMO |
| 1 | 3.7 (1.2) | 3.8 (0.9) | 5.1 (1.2) | 3.5 (0.8) |
| 6 | 4.4 (0.9) | 3.7 (0.8) | 5.1 (1.2) | 3.9 (0.8) |
Note: LMO, active TMS at the lower occipital site; PC, midline parietal site; and UMO, upper occipital site. Set size of 1 = 1 letter in the stimulus set to be remembered; set size of 6 = 6 letters. There were no significant differences in time-outs across site and set size.
Table 2.
Mean accuracy and RT (and standard error) for sleep-deprived participants
| Set size | Sham | LMO | PC | UMO |
| Accuracy | ||||
| 1 | 90.9 (2.6) | 86.8 (3.8) | 90.4 (2.2) | 90.1 (2.0) |
| 6 | 91.2 (2.1) | 88.1 (2.8) | 85.4 (3.7) | 88.9 (2.4) |
| RT | ||||
| 1 | 828 (55) | 861 (70) | 856 (66) | 833 (71) |
| 6 | 1169 (80) | 1169 (95) | 1097 (79) | 1026 (73)* |
Note: LMO, active TMS at the lower occipital site; PC, midline parietal site; and UMO, upper occipital site. Set size of 1 = 1 letter in the stimulus set to be remembered; set size of 6 = 6 letters. *UMO RT < sham RT, P < 0.05.
Figure 3.
Mean RT in the sleep-deprived group at set size 6 with active TMS at 3 scalp locations and for sham TMS. *P < 0.005.
Correlation of RT Performance with Network Expression
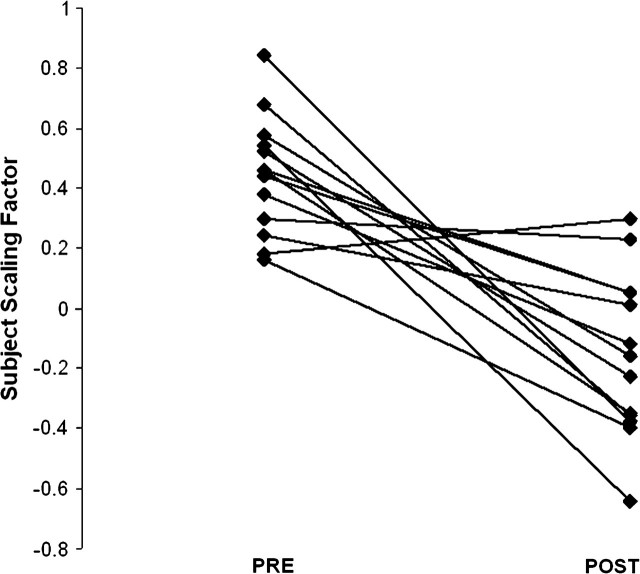
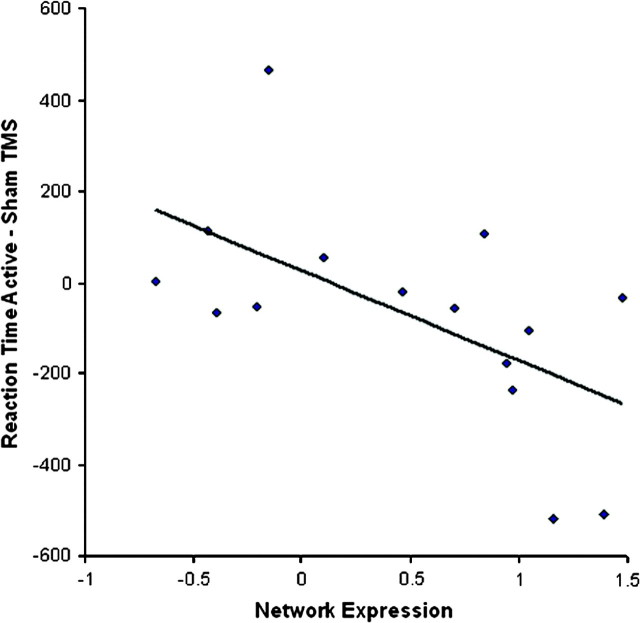
Using OrT, a covariance pattern was extracted from the event-related fMRI data measured during the probe phase of set size 6 whose expression decreased for 12 of the 13 subjects in the course of sleep deprivation (Fig. 4). The network is shown in Figure 5. The UMO and PC sites exhibited a decrease in activation, as they did in the original network (Fig. 2), and the LMO site once again did not demonstrate activation. However, the original network from Habeck et al. (2004) was based on the pre/postsleep deprivation fMRI in 18 subjects, was derived from the 1st principal component out of the analysis, and found that the individual expression of the network correlate with DMS performance measures. The fMRI network derived from the present group was based on 13 subjects, was based on a combination of the first 4 principal components, and did not predict performance measures. This network, while similar to the original, was therefore considered a less reliable estimate of the functional network underlying DMS performance during sleep deprivation. Instead, the original network was prospectively applied to the pre- and postsleep deprivation fMRI data of the group of subjects in the present study. As shown in Figure 6, the difference in individual expression of that network pre- and postsleep deprivation was correlated with the difference in median RT during active and sham postsleep deprivation rTMS at the superior occipital site (r = −0.58, P < 0.025). The negative correlation indicates that the greater the decrease in network activation, the more facilitated the RT relative to sham, that is, the more sensitive the neural network to sleep deprivation, the more it responded to the rTMS intervention. As expected, the correlations for LMO (r = −0.26, P < 0.34) and PC (r = −0.37, P < 0.17) were not significant. It should be pointed out that in the correlations between network expression and TMS effect in an entirely new data set, the probability for an erroneous chance association should be very close to the P level obtained from parametric distribution theory.
Figure 4.
Subject expression of the pattern resulting from the OrT analysis: 12/13 subjects decreased their expression of the activation pattern.
Figure 5.
Surface rendered projections of brain regions whose associated activation changed in most subjects of the present study from pre- to postsleep deprivation. Green indicates areas going down in activation with sleep deprivation. Red indicates areas going up in activation with sleep deprivation. The circles mark the 3 rTMS targets.
Figure 6.
The improvement of RT between active TMS to UMO and sham TMS correlates (r = −0.58, P < 0.025) with the degree to which fMRI network expression is depressed following sleep deprivation.
Control Experiment for State Dependency of Effects
Eight of the subjects who had previously taken part in a sleep-deprived rTMS session returned after 2 weeks for a nonsleep-deprived control session. There was no difference in MT found in the sleep-deprived and nonsleep-deprived states (t = 0.73, 7 df, P < 0.49), replicating the same finding in an earlier sleep deprivation study (Scalise et al. 2006). The group performance data are shown in Table 3. A repeated-measures ANOVA on accuracy yielded only the expected main effect of sleep state (F = 6.1, 1,7 df, P < 0.05). A repeated-measures ANOVA on RT yielded main effects of set size (F = 114.5, 1,7 df, P < 0.0001) and state (sleep deprived vs. sleep replete) (F = 10.8, 1,7 df, P < 0.015) and a site × rTMS × state interaction (F = 15.2, 1,7 df, P < 0.01). Post hoc testing found a significant effect between sham rTMS and active rTMS at site UMO for a set size of 6 in the sleep-deprived state, where RT was speeded by an average of 150 ms (t7 = 2.3, P < 0.03). This represents approximately the same degree of facilitation of RT as reported above for the entire sleep-deprived group. There was no significant effect between sham or active rTMS in the nonsleep-deprived state.
Table 3.
Mean RT (and standard error) for the 8 nonsleep-deprived participants compared with their sleep-deprived results
| Sham | LMO | UMO | PC | |
| Set size = 1 | ||||
| Sleep deprived | 801 ± 51 | 825 ± 62 | 800 ± 63 | 827 ± 63 |
| Not sleep deprived | 609 ± 44 | 644 ± 58 | 628 ± 57 | 604 ± 50 |
| Set size = 6 | ||||
| Sleep deprived | 1152 ± 67 | 1121 ± 84 | 1002 ± 74** | 1079 ± 81 |
| Not sleep deprived | 824 ± 76 | 887 ± 93 | 816 ± 62 | 809 ± 77 |
Note: LMO, active TMS at the lower occipital site; PC, midline parietal site; and UMO, superior occipital site. Set size of 1 = 1 letter in the stimulus set to be remembered; set size of 6 = 6 letters. ** P < 0.01.
Discussion
The rTMS delivered to UMO, but not to the active control region or the sham condition, reduced RT on a DMS WM task in sleep-deprived individuals. The degree of performance enhancement at UMO, but not at the other 2 sites, correlated with the degree to which the individual failed to sustain activation of the fMRI network associated with neural reserve to sleep-deprivation–induced impairments in the task. The facilitation only occurred in sleep-deprived subjects: No facilitation was seen when the same participants were not sleep deprived. Taken together, these results are consistent with the hypothesis that activity in a UMO-containing network plays a functional role in resilience to the effects of sleep deprivation.
Previous studies of the neural mechanisms of WM in imaging and in TMS have primarily emphasized the prefrontal cortex, especially dorsolateral prefrontal cortex, and to some extent inferior PC (e.g., Mottaghy et al. 2002; Herwig et al. 2003). WM has been conceived of as a faculty that holds and manipulates information representations (Baddeley 1986). As the present study suggests, it is worthwhile to observe that the “place” where this manipulation of representations occurs may be widely distributed across the brain, beyond PFC and PC, and include regions such as extrastriate occipital cortex, where such representations are known to be perceptually processed.
In 2 studies measuring the effects of rTMS on DMS task performance (the present study and Luber, Kinnunen, et al. 2007), the production of a facilitatory effect appears to be quite sensitive to the specific circumstances of the task and the cortex stimulated. Both the UMO and PC sites are elements of the network related to the cognitive effects of sleep deprivation (Habeck et al. 2004). However, the stimulation at UMO appears to facilitate recovery from the effects of sleep deprivation but does not enhance performance in nonsleep-deprived states, whereas the opposite was seen to occur with stimulation at PC. We previously reported 5-Hz rTMS to PC-enhanced WM performance in healthy subjects who had not been sleep deprived, where 5-Hz rTMS to the midline parietal site improved RT by an average of 88 ms, an 11% decrease in RT relative to sham rTMS (Luber, Kinnunen, et al. 2007). Here, we report that rTMS applied to PC in sleep-deprived individuals resulted in a group mean decrease of 72 ms in the sleep-deprived subjects (Table 2). However, this was only a 6% improvement relative to sham and did not significantly improve WM (though there was a minimal statistical trend [P < 0.1] toward improvement). Decreases in left occipital activity with sleep deprivation have been seen in a number of imaging studies (Drummond et al. 1999; Drummond and Brown 2001; Bell-McGinty et al. 2004; Chee and Choo 2004; Habeck et al. 2004; Choo et al. 2005). rTMS to this region may have enhanced visual processing involved in comparing representations of the memory items with the probe. This possibility is supported by the fact that performance facilitation was set size specific (i.e., RT was decreased with multiple-item rather than with single-item memory sets). On the other hand, although there was no observed facilitation at PC in the sleep-deprived state, the Luber, Kinnunen, et al. (2007) study found a facilitation of RT in nonsleep-deprived subjects with 5-Hz rTMS during the retention period of the DMS task at the same midline parietal site used in the present study. There it was suggested that performance improvements may have been related to increased efficiency of verbal strategic processes involved in the task. These site-specific differences in response to rTMS suggest that the cortical regions of the WM circuit are differentially sensitive to sleep deprivation and that this sensitivity is at least partially reversible.
It should be noted that the PC effect found in Luber, Kinnunen, et al. (2007) was not replicated in the nonsleep-deprived group in the present study, although they did show facilitation at UMO in a sleep-deprived state. Possible explanations for this rely on physiological and psychological factors. On the one hand, the region deactivated in sleep deprivation in the Habeck et al. network was relatively deep and possibly beyond the effective range of the figure-of-eight TMS coil used, whereas more superficial PC may have been involved in the effects observed in Luber et al. A future study using a more penetrating coil shape (e.g., a double-cone coil) might provide evidence for this possibility. On the other hand, the midline parietal site may have been sensitive to individual differences in DMS task strategy. Previous TMS studies of WM have stimulated more lateral parietal regions. The rTMS to right PC but not left has disrupted WM performance (Kessels et al. 2000; Herwig et al. 2003), whereas single-pulse TMS to right and left PC has disrupted both accuracy (Mottaghy et al. 2003) and RT (Oliveri et al. 2001) measures of WM. The lateral parietal targets were based on imaging studies in healthy young subjects. The parietal site stimulated here was targeted according to a network highlighted by sleep deprivation and may have had an adjunct function in the task, perhaps, as noted in the Luber, Kinnunen, et al. (2007) study, involving strategic processing. As such, it may have been more sensitive to individual differences, which could in turn affect its relative utilization among subjects. For example, in the DMS task, some subjects reported using a semantic mnemonic strategy, whereas others used acoustic rehearsal. There is evidence that parietal areas might contribute more to the former strategy and more temporal areas to the latter (Catani et al. 2005). Given such variability in strategic processing, a greater number of subjects than 8 might be required to consistently see an effect at the site. Evidence of a trend toward facilitation in the present nonsleep-deprived group can be seen in Table 4, comparing the number of subjects who showed some facilitation (active-sham RT <−50 ms, the approximate standard error in the RT measure) and those who did not, for each scalp site. Although signs of facilitation were uniformly distributed at UMO, a majority showed possible facilitation at PC. None did so at LMO.
Table 4.
Number of subjects who showed some facilitation (active-sham RT <−30 ms) versus those who did not, for each scalp site
| Active-sham RT | LMO | PC | UMO |
| ≤30 ms | 0 | 5 | 2 |
| −30 to 30 ms | 3 | 0 | 2 |
| >30 ms | 5 | 3 | 4 |
Note: LMO, active TMS at the lower occipital site; PC, midline parietal site; and UMO, upper occipital site.
Our demonstration that the expression of the DMS task–related network was correlated with rTMS-induced facilitation of WM is especially notable given that the network was originally identified in an independent group of sleep-deprived individuals. Forward application of experimentally derived covariance patterns from 1 group to another has proven useful in exploring potential functional topographies. For example, an alpha EEG topography related to performance of an episodic memory task in a group of young adults was found to also be present in a group of elderly adults (Luber et al. 2004). In the present case, Habeck et al. (2004) found an fMRI covariance network whose expression decreased over the course of sleep deprivation. The degree of decrease in the expression of this network was correlated with individual decreases in performance in the DMS task. When that network was forward applied to the fMRI data of the sleep-deprived individuals of the present study, we found that the greater the decrease in its expression, the larger the RT decrease caused by rTMS. The relationship of the covariance pattern with an independent performance measure in an entirely new group of subjects further validates the pattern as a functional topography involved in WM processes. It also suggests that coupling covariance analysis of imaging data with TMS intervention studies probing the identified networks represents a useful technique in examining functional networks related to cognition.
The relationship of individual differences in imaging activations with WM performance and with rTMS effects suggests both that the present sleep deprivation paradigm in young adults may be a useful model for understanding the neural bases of cognitive decline in the elderly and that rTMS may be potentially useful in exploring and treating cognitive deficits due to aging and neuropathology in general. Elderly persons show a broad range of individual differences in the degree to which they experience cognitive deficits. One hypothesis that has been formulated to explain this interindividual variability is cognitive reserve (Stern 2002). Age-related deficits in cognition are assumed to occur due to age-related neuropathology. The theory of cognitive reserve suggests that individuals with higher intelligence or education are better able to cope with age-related neuropathology than those with lower intelligence or education because they can call upon more neural resources. The neural mechanisms underlying cognitive reserve are thought to involve both the capacity and efficiency of long established neural networks (neural reserve) as well as the development of compensatory networks. In the present study, sleep deprivation produced a state in which some individuals suffered large deficits in WM performance, whereas others were much less affected. This was not a general effect such as arousal because the performance deficit was tied to the deterioration in activation of a specific cortical network activated by the WM task. This suggests that the activity of the network exhibited properties of neural reserve, where a greater capacity or efficiency in the network allowed some individuals to maintain performance in the face of a reversible “pathology.” Moreover, our results also suggest that rTMS was able to somehow enhance the network activity in those who were not able to maintain performance, artificially facilitating neural reserve.
There are a number of possible mechanisms that might be responsible for the performance-enhancing effects observed here. One facilitatory mechanism that would not reflect neural reserve has been observed in RT studies: intersensory facilitation (IF), a well-studied effect in which simple RT can be shortened if the cue signal is accompanied by a 2nd stimulation, such as the auditory click of a rTMS coil or the physical sensation rTMS causes in skin and superficial muscle. IF has been proposed to be the likely cause of facilitatory effects in studies using a simple RT task and single-pulse TMS (Terao et al. 1997) and a more complex choice RT task using rTMS during the retention phase of a WM task structured in a similar way to that used here (Nixon et al. 2004). However, IF is unlikely to be the cause of the facilitation found in the present study both because facilitation was not demonstrated in the sham rTMS condition (which had an identical auditory click as the active TMS) and it was not seen with active rTMS to other scalp locations. The LMO location produced a similar scalp sensation as the UMO location, thereby providing a control for the physical sensation of rTMS. Had the RT reductions with rTMS to the UMO been solely attributable to IF, they should have likewise been seen with LMO stimulation.
One possible mechanism that may reflect manipulation of neural reserve is the 1 in which TMS delivered to a cortical region that is necessary for task performance prior to its engagement in the task increases neural excitability in a way that can prime the involvement of that region and thereby enhance performance under some conditions. Trains of rTMS have been shown to produce lasting effects on cortical excitability as measured by electrophysiological response (Barardelli et al. 1998; Peinemann et al. 2000) and with positron-emission tomography imaging (Siebner et al. 2000). In the present study, the application of rTMS during the 7-s period immediately preceding probe presentation likewise may have increased the excitability of occipital neurons in a way that facilitated the subsequent memory search. The mechanism behind such facilitation may be a local increase in excitability, perhaps produced by a temporary increase in the amplitude of excitatory postsynaptic potentials (e.g., Iriki et al. 1989), which may lead to a larger neural response. A related possibility is that TMS may affect the oscillatory dynamics of brain networks, perhaps by generating a resonance with local activity in the alpha (Klimesch et al. 2003) or theta (Lakatos et al. 2005) EEG ranges. Of note, a number of reports have shown that a 5-Hz TMS frequency, which may affect theta activity as well as alpha activity (as a subharmonic in the alpha range), has resulted in facilitation of cognitive performance (Barardelli et al. 1998; Ragert et al. 2003; Luber, Kinnunen, et al. 2007). While intriguing, all of these possible mechanisms await substantiation.
This study also adds to the literature on the safety of large numbers of TMS pulses given to healthy subjects and to the safety of rTMS in the sleep-deprived state (Anderson et al. 2006). In the present study, we applied 6,750 magnetic pulses in a 2.5-h session, a total similar to a session in Anderson et al. Here, rTMS was applied after 54–57 h (2 nights) of sleep deprivation and the sessions were well tolerated, with no significant side effects.
Conclusion
This study has provided evidence for remediation of the effects of sleep deprivation on a WM task, using TMS targeted to a cortical location that was part of a functional network identified through fMRI. However, there are some limitations to this study. The sample size was relatively small, especially in the nonsleep-deprived control experiment. In terms of the design, the within-subject crossover design caused a risk of carryover TMS effects in the later conditions and within-subject active/sham conditions lead to a chance of unblinding subjects. Moreover, the DMS task represents only 1 aspect of WM function and other WM tasks need to be examined to establish the generality or specificity of the network explored here, as do TMS parameters such as stimulation frequency. Nonetheless, while the neural mechanisms behind the rTMS-aided facilitation remain speculative, its therapeutic potential carries important clinical and theoretical implications that merit further study. In particular, the results here demonstrate how rTMS can be applied to test the functional significance of fMRI-identified networks associated with resilience to cognitive decline produced experimentally via sleep deprivation. Future studies might productively apply this paradigm to test the functional role of such networks in clinical populations. If neural reserve-associated networks are found to play a functional role in clinical disorders, then they represent therapeutic targets for intervention development with focal brain stimulation.
Funding
Defense Advanced Research Projects Agency Award (DAAD19-02-0047).
Acknowledgments
Dr Lisanby has received research support from Magstim Company, Neuronetics, and Cyberonics. Conflict of Interest: None declared.
References
- Amassian VE, Cracco RQ, Maccabee PJ, Cracco JB, Rudell A, Eberle L. Suppression of visual perception by magnetic coil stimulation of human occipital cortex. Electroencephalogr Clin Neurophysiol. 1989;74:458–462. doi: 10.1016/0168-5597(89)90036-1. [DOI] [PubMed] [Google Scholar]
- Anderson B, Mishory A, Nahas Z, Borckardt JJ, Yamanaka K, Rastogi K, George MS. Tolerability and safety of high daily doses of repetitive transcranial magnetic stimulation in healthy young men. J ECT. 2006;22:49–53. doi: 10.1097/00124509-200603000-00011. [DOI] [PubMed] [Google Scholar]
- Avery DH, Holtzheimer PE, Fawaz W, Russo J, Neumaier J, Dunner DL, Haynor DR, Claypoole KH, Wajdik C, Roy-Burne P. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol Psychiatry. 2006;59:187–194. doi: 10.1016/j.biopsych.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory. Oxford (UK): Oxford University Press; 1986. [Google Scholar]
- Barardelli A, Inghilleri M, Rothwell JC, Romeo S, Curra A, Gilio F, Modugno N, Manfredi M. Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp Brain Res. 1998;122:79–84. doi: 10.1007/s002210050493. [DOI] [PubMed] [Google Scholar]
- Bell-McGinty S, Habeck C, Hilton HJ, Rakitin B, Scarmeas N, Zarahn E, Flynn J, DeLaPaz R, Basner R, Stern Y. Identification and differential vulnerability of a neural network in sleep deprivation. Cereb Cortex. 2004;14:496–502. doi: 10.1093/cercor/bhh011. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Phipps M, Kopylev L, Wharton CM, Cohen LG, Grafman J. Enhancing analogic reasoning with rTMS over the left prefrontal cortex. Neurology. 2001;56:526–528. doi: 10.1212/wnl.56.4.526. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Annals Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Choo W-C. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24:4560–4567. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo W-C, Lee W-W, Venkatraman V, Sheu F-S, Chee MWL. Dissociation of cortical regions modulated by both working memory load and sleep deprivation and by sleep deprivation alone. Neuroimage. 2005;25:579–587. doi: 10.1016/j.neuroimage.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Kribbs NB. Performing while sleepy: effects of experimentally induced sleepiness. In: Monk TH, editor. Sleep, sleepiness and performance. New York (NY): John Wiley and Sons; 1991. [Google Scholar]
- Drummond SP, Brown GG, Stricker JL, Buxton RB, Wong EC, Gillin JC. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. Neuroreport. 1999;10:3745–3748. doi: 10.1097/00001756-199912160-00004. [DOI] [PubMed] [Google Scholar]
- Drummond SPA, Brown GG. The effects of total sleep deprivation on cerebral responses to cognitive performance. Neuropsychopharmacol. 2001;25:S68–S73. doi: 10.1016/S0893-133X(01)00325-6. [DOI] [PubMed] [Google Scholar]
- Evers S, Bockermann I, Nyhuis PW. The impact of transcranial magnetic stimulation on cognitive processing: an event-related potential study. Neuroreport. 2001;12:2915–2918. doi: 10.1097/00001756-200109170-00032. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured clinical interview for DSM-IV axis I disorders- non-patient edition (SCID-I/NP, Version 2.0-8/98 revision) New York: Biometrics Research Department, NY State Psychiatric Institute; 1998. [Google Scholar]
- George MS, Wassermann EM, Kimbrell TA, Little JT, Williams WE, Danielson AL, Greenberg BD, Hallett M, Post RM. Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: A placebo-controlled crossover trial. Am J Psychiatry. 1997;154:1752–1756. doi: 10.1176/ajp.154.12.1752. [DOI] [PubMed] [Google Scholar]
- Grafman J, Pascual-Leone A, Alway D, Nichelli P, Gomez-Tortosa E, Hallett M. Induction of a recall deficit by rapid-rate transcranial magnetic stimulation. Neuroreport. 1994;5:1157–1160. doi: 10.1097/00001756-199405000-00034. [DOI] [PubMed] [Google Scholar]
- Grosbras M-H, Paus T. Transcranial magnetic stimulation of the human frontal eye field facilitates visual awareness. Eur J Neurosci. 2003;18:3121–3126. doi: 10.1111/j.1460-9568.2003.03055.x. [DOI] [PubMed] [Google Scholar]
- Habeck C, Krakauer JW, Ghez C, Sackeim HA, Eidelberg D, Stern Y, Moeller JR. A new approach to spatial covariance modeling of functional brain imaging data: ordinal trends analysis. Neural Computation. 2005;17:1602–1645. doi: 10.1162/0899766053723023. [DOI] [PubMed] [Google Scholar]
- Habeck C, Rakitin BC, Moeller J, Scarmeas N, Zarahn E, Brown T, Stern Y. An event-related fMRI study of the neurobehavioral impact of sleep deprivation on performance of a delayed-match-to-sample task. Cogn Brain Res. 2004;18:306–321. doi: 10.1016/j.cogbrainres.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Hayward G, Goodwin GM, Harmer CJ. The role of the anterior cingulate cortex in the counting Stroop task. Exp Brain Res. 2004;154:355–358. doi: 10.1007/s00221-003-1665-4. [DOI] [PubMed] [Google Scholar]
- Herwig U, Abler B, Schonfeldt-Lecuona C, Wunderlich A, Grothe J, Spitzer M, Walter H. Verbal storage in a premotor-parietal network: evidence from fMRI-guided magnetic stimulation. Neuroimage. 2003;20:1032–1041. doi: 10.1016/S1053-8119(03)00368-9. [DOI] [PubMed] [Google Scholar]
- Iriki A, Pavlides C, Keller A, Asanuma H. Long-term potentiation in the motor cortex. Science. 1989;245:1385–1387. doi: 10.1126/science.2551038. [DOI] [PubMed] [Google Scholar]
- Kessels RPC, d'Alfonso AAL, Postma A, de Haan EHF. Spatial working memory performance after high-frequency repetitive transcranial magnetic stimulation of the left and right posterior parietal cortex in humans. Neurosci Lett. 2000;287:68–70. doi: 10.1016/s0304-3940(00)01146-0. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Gerloff C. Enhancing cognitive performance with repetitive transcranial magnetic stimulation at human individual alpha frequency. Eur J Neurosci. 2003;17:1129–1133. doi: 10.1046/j.1460-9568.2003.02517.x. [DOI] [PubMed] [Google Scholar]
- Kohler S, Paus T, Buckner RL, Milner B. Effects of left inferior prefrontal stimulation on episodic memory formation: A two-stage fMRI-rTMS study. J Cogn Neurosci. 2004;16:178–188. doi: 10.1162/089892904322984490. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Shroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol. 2005;94:1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- Luber B, Kinnunen LH, Rakitin BC, Ellsasser R, Stern Y, Lisanby SH. Facilitation of performance in a working memory task with rTMS stimulation of the precuneus: frequency and time-dependent effects. Brain Res. 2007;1128:120–129. doi: 10.1016/j.brainres.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Luber B, Peterchev A, Nguyen T, Sporn A, Lisanby SH. Application of TMS in psychophysiological studies. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 3rd ed. New York: Cambridge University Press; 2007. [Google Scholar]
- Luber B, Trott CT, Friedman D, Moeller JR. A ghost of retrieval past: A functional network of alpha EEG related to source memory in elderly humans. Cog Brain Res. 2004;20:144–155. doi: 10.1016/j.cogbrainres.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Matthews N, Luber B, Qian N, Lisanby S. Transcranial magnetic stimulation differentially affects speed and direction judgments. Exp Brain Res. 2001;140:397–406. doi: 10.1007/s002210100837. [DOI] [PubMed] [Google Scholar]
- Moeller JR, Habeck CG. Reciprocal benefits of mass-univariate and multivariate modeling in brain mapping: applications to event-related functional MRI, H215O- and FDG-PET. Int J Biomed Imaging. 2006 doi: 10.1155/IJBI/2006/79862. doi: 10.1155/IJBI/2006/79862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottaghy FM, Gangitano M, Krause BJ, Pascual-Leone A. Chronometry of parietal and prefrontal activations in verbal working memory revealed by transcranial magnetic stimulation. Neuroimage. 2003;18:565–575. doi: 10.1016/s1053-8119(03)00010-7. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM, Gangitano M, Sparing R, Pascual-Leone A. Segregation of areas related to visual working memory in the prefrontal cortex revealed by rTMS. Cereb Cortex. 2002;12:369–375. doi: 10.1093/cercor/12.4.369. [DOI] [PubMed] [Google Scholar]
- Nixon P, Lazarova J, Hodinott-Hill I, Gough P, Passingham R. The inferior frontal gyrus and phonological processing: an investigation using rTMS. J Cogn Neurosci. 2004;16:289–300. doi: 10.1162/089892904322984571. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Turriziani P, Carlesimo GA, Koch G, Tomaiuolo F, Panella M, Caltagirone C. Parieto-frontal interactions in visual-object and visual-spatial working memory: evidence from transcranial magnetic stimulation. Cereb Cortex. 2001;11:606–618. doi: 10.1093/cercor/11.7.606. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Gates JR, Dhuna A. Induction of speech arrest and counting errors with rapid-rate transcranial magnetic stimulation. Neurology. 1991;41:697–702. doi: 10.1212/wnl.41.5.697. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Rubio B, Pallardo F, Catala MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233–237. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Lehner C, Mentschel C, Munchau A, Conrad B, Siebner HR. 5-Hz repetitive transcranial magnetic stimulation of the human primary motor cortex reduces intracortical paired-pulse inhibition. Neurosci Lett. 2000;296:21–24. doi: 10.1016/s0304-3940(00)01616-5. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Meyer E, Evans AC. Functional activation of human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci USA. 1993;90:878–882. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragert P, Dinse HR, Pleger B, Wilimzig C, Frombach E, Schwenkreis P, Tegenthoff M. Combination of 5 Hz repetitive transcranial stimulation (rTMS) and tactile coactivation boosts tactile discrimination in humans. Neurosci Lett. 2003;348:105–108. doi: 10.1016/s0304-3940(03)00745-6. [DOI] [PubMed] [Google Scholar]
- Rakitin BC, Zarahn E, Hilton HJ, Abela D, Flynn J, Tatarina O, Basner R, Brown T, Stern Y. An event-related fMRI study of the effects of sleep deprivation on short-term memory performance. Cogn Neurosci Soc Abstr. 2004 F116. [Google Scholar]
- Rypma B, Berger JS, D'Esposito M. The influence of working memory demand and subject performance on prefrontal cortical activity. J Cogn Neurosci. 2002;14:721–731. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Scalise A, Desiato MT, Gigli GL, Romigi A, Tombini M, Marciani MG, Izzi F, Placidi F. Increasing cortical excitability: a possible explanation for the proconvulsant role of sleep deprivation. Sleep. 2006;29:1595–1598. doi: 10.1093/sleep/29.12.1595. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Peller M, Willoch F, Minoshima S, Boecker H, Auer C, Drzezga A, Conrad B, Bartenstein P. Lasting cortical activation after repetitive TMS of the motor cortex: a glucose metabolic study. Neurology. 2000;54:956–963. doi: 10.1212/wnl.54.4.956. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J International Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1969;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Terao Y, Yoshikazu U, Suzuki M, Sakai K, Hanajima R, Gemba-Shimizu K, Kanazawa I. Shortening of simple reaction time by peripheral electrical and submotor-threshold magnetic cortical stimulation. Exp Brain Res. 1997;115:541–545. doi: 10.1007/pl00005724. [DOI] [PubMed] [Google Scholar]
- Topper R, Mottaghy FM, Brugmann M, Noth J, Huber W. Facilitation of picture naming by focal transcranial magnetic stimulation of Wernicke's area. Exp Brain Res. 1998;121:371–378. doi: 10.1007/s002210050471. [DOI] [PubMed] [Google Scholar]
- Walsh V, Ellison A, Battelli L, Cowey A. Task specific impairments and enhancements induced by magnetic stimulation of human visual area V5. Proc R Soc Lond B Biol Sci. 1998;265:537–543. doi: 10.1098/rspb.1998.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]