Abstract
Objectives. To determine the incidence of herpes zoster (HZ) in GCA.
Methods. Utilizing the resources of the Rochester Epidemiology Project, all incident cases of GCA diagnosed between 1 January 1950 and 31 December 2004 were identified. For each GCA patient, two subjects without GCA of the same gender and similar age and length of medical history were randomly selected from the population. Patients were followed until death, last contact or 31 December 2006.
Results. The study population included 204 GCA patients and 407 non-GCA subjects. The GCA cohort had 163 (79%) women and 41 (21%) men, with a mean age of 76.0 (8.2) years. The non-GCA cohort had 325 (80%) women and 82 (20%) men, with a mean age of 75.6 (8.4) years. During follow-up, 21 GCA patients and 38 non-GCA subjects developed HZ. There was no difference in the development of HZ in GCA patients compared with non-GCA patients [hazard ratio (HR): 1.22; 95% CI 0.71, 2.08; adjusted for age, sex and calendar year]. No GCA patient and one non-GCA subject developed HZ within 6 months of index date. The frequency of post-herpetic neuralgia was similar between both groups (P = 0.64).
Conclusions. Patients with GCA do not appear to be at increased risk of HZ compared with the general population, even during the first 6 months of therapy when glucocorticoid doses are usually highest.
Keywords: Giant cell arteritis, Herpes zoster, Glucocorticoids
Introduction
Herpes zoster (HZ) is caused by the reactivation of varicella zoster virus (VZV). Cell-mediated immunity appears to be important in maintaining VZV in the latent form [1]. Patients who are immunosuppressed and the elderly are at increased risk of HZ, as are patients with autoimmune diseases, including SLE and RA [2–9].
GCA is a granulomatous vasculitis affecting the aorta and its primary branches. Because of their older age (mean age at diagnosis, 74.8 years) [10] and treatment with CSs, patients with GCA may be at increased risk of HZ. However, the incidence of HZ in GCA remains unknown. The purpose of this study was to calculate and compare the cumulative incidence of HZ in a population-based cohort of patients with GCA with age- and gender-matched non-GCA patients from the same geographical region. We hypothesized that GCA patients are at increased risk of HZ due to glucocorticoid use.
Patients and methods
This study utilized the resources of the Rochester Epidemiology Project (REP) to identify an incident cohort of GCA patients as previously described [10, 11]. The REP is a unique linkage system, which allows ready access to the medical records of all health care providers for the population of Olmsted County, MN, USA [12]. Detailed indices containing all clinical and pathological diagnoses and surgical procedures have been recorded since 1909 and diagnoses assigned at each visit are indexed continuously.
This study was approved by the institutional review boards at Mayo Clinic and Olmsted Medical Center. All study subjects provided authorization for a review of their medical records.
GCA cohort
Using the REP, an incident cohort of all Olmsted County, MN, USA residents diagnosed with GCA between 1 January 1950 and 31 December 2004 has been established [10, 11]. All patients met the 1990 ACR classification criteria for GCA [13].
Referent cohort
For each GCA patient, two subjects without GCA of the same sex and similar age (±1 year) and length of the medical history were randomly selected from the Olmsted County population. Each non-GCA subject was assigned an index date corresponding to the date of diagnosis of GCA of the matched GCA patient.
Case definitions
HZ was defined as a painful, vesicular rash in a dermatomal distribution witnessed by a health care provider. Post-herpetic neuralgia (PHN) was defined as neuropathic pain lasting longer than 1 month after disappearance of the rash.
Case ascertainment and follow-up
Patients in both cohorts were followed by review of their medical records. Diagnostic codes from the International Classification of Diseases, ninth revision (ICD-9) for HZ, shingles and HZ ganglionitis, were used to screen patients from both groups. The medical records of all identified subjects were then systematically reviewed by two investigators (V.S.S. and T.A.K.) and the diagnosis of HZ confirmed. Information about the date of diagnosis, prior history of HZ, glucocorticoid dose at the time of diagnosis of HZ, development of PHN and duration of pain were abstracted. Patients in both cohorts were followed until death, last contact or 31 December 2004.
Statistical analysis
Descriptive statistics were used to summarize the data. The proportions of patients with history of HZ in the GCA and non-GCA cohorts were compared using a chi-square test. The cumulative incidence of HZ during follow-up was estimated using Kaplan–Meier methods. Cox proportional hazard models were used to examine the influence of GCA on the development of HZ after adjusting for age, sex and calendar year of index date. Standardized incidence ratios (SIRs) were used to compare the observed rate of development of HZ in both the GCA and the non-GCA cohorts with the expected rate of development. The expected number of cases of HZ was derived by applying age- and sex-specific incidence rates of HZ from the local population to the age- and sex-specific person-years of follow-up in each cohort. By assuming that the expected rates are fixed and the observed fractures follow a Poisson’s distribution [14], 95% CIs for the SIRs were calculated.
Results
The GCA cohort included in this study consisted of 204 patients, 163 (80%) women and 41 (20%) men, with a mean age of 76.0 (8.2) years. The non-GCA cohort had 407 subjects, 325 (80%) women and 82 (20%) men, with a mean age of 75.6 (8.4) years. Median follow-up was 7.7 years (total 1856 person-years) in the GCA cohort and 8.1 years (total 3890 person-years) for the referent cohort. At index date, 26 (13%) GCA patients and 48 (12%) non-GCA patients had a history of HZ (P = 0.73). After GCA diagnosis/index date, 21 GCA patients and 41 non-GCA patients developed HZ. Excluding patients with a history of HZ, 21 GCA patients and 38 non-GCA subjects developed the first episode of HZ. Mean age at diagnosis of HZ was 80.4 years for GCA patients and 81.3 years for non-GCA subjects. GCA patients were not at increased risk of developing HZ compared with non-GCA subjects [hazard ratio (HR): 1.22; 95% CI 0.71, 2.08; adjusted for age, sex and calendar year]. Table 1 summarizes the incidence of HZ in GCA and non-GCA patients by gender, age and calendar year of the study.
Table 1.
Incidence of HZ in subjects with GCA and non-GCA subjects
| Subgroup | GCA, n (rate per 100 person-years) | Non-GCA, n (rate per 100 person-years) | Rate ratio (95% CI) |
|---|---|---|---|
| Female | 19 (1.38) | 29 (0.98) | 1.42 (0.78, 2.48) |
| Male | 2 (0.67) | 9 (1.29) | 0.61 (0.10, 2.03) |
| Aged 50–64 years | 1 (1.19) | 1 (0.46) | 2.60 (0.17, 40.1) |
| Aged 65–79 years | 10 (1.40) | 15 (0.92) | 1.54 (0.67, 3.33) |
| Aged ≥80 years | 10 (1.13) | 22 (1.21) | 0.96 (0.43, 1.92) |
| 1950–1984 | 6 (1.09) | 14 (1.17) | 0.98 (0.34, 2.33) |
| 1985–2004 | 15 (1.32) | 24 (0.98) | 1.37 (0.70, 2.56) |
No GCA patients and one non-GCA subject developed HZ within 6 months of index date. One GCA patient and five non-GCA subjects developed HZ within 1 year of index date.
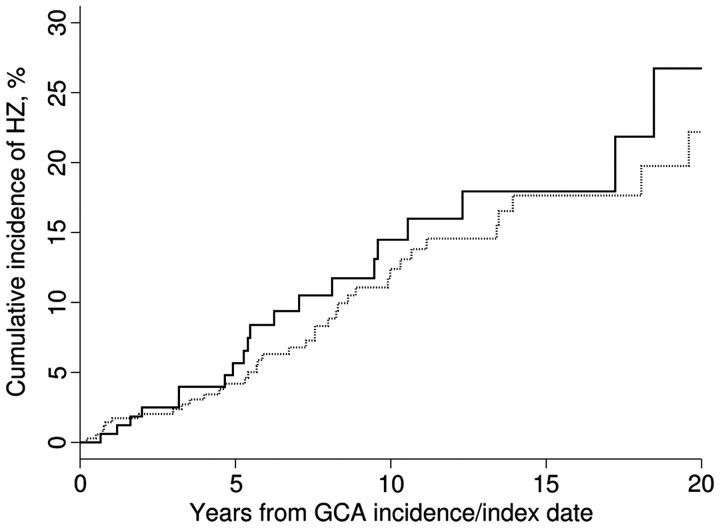
The 2-, 10- and 20-year cumulative incidences of HZ were 2.5% (1.2%), 14.5% (3.5%) and 26.7% (7.0%) among GCA patients, and 2.0% (0.8%), 12.4% (2.3%) and 22.2% (4.2%) among the non-GCA patients (Fig. 1). Ten of the 21 GCA subjects who developed HZ were on glucocorticoid therapy at the time of diagnosis of HZ. Mean dose of glucocorticoids at diagnosis of HZ was 3.7 mg/day. Only three subjects (1.5%) in the GCA cohort were on steroid-sparing agents. None of these patients developed HZ.
Fig. 1.
Cumulative incidence of HZ in subjects with GCA (—) and non-GCA subjects (…).
Four (19%) GCA patients and nine (24%) non-GCA subjects were diagnosed with PHN (P = 0.64). The median duration of pain from PHN was similar—4 months in GCA patients (range 2–36 months) and 2 months in non-GCA subjects (range 1–16 months) (rank-sum P = 0.50). None of the patients who developed HZ were hospitalized. One GCA patient and one non-GCA subject had disseminated HZ.
Finally, we compared the observed rates of HZ in this study with rates previously reported for this population [15]. Our observed rates were similar to the expected rates for the GCA cohort (SIR 1.35; 95% CI 0.83, 2.06) and the non-GCA cohort (SIR 1.14; 95% CI 0.90, 1.56).
Discussion
To our knowledge, this is the first population-based study evaluating the risk of HZ in patients with GCA. Contrary to our hypothesis, patients with GCA in our study were not at increased risk of developing HZ compared with referent subjects. Our findings are in contrast to patients with other autoimmune diseases including RA, SLE and ANCA-associated necrotizing granulomatous vasculitis (AAV), who are at increased risk of HZ [3–7, 9, 16, 17]. It is unknown whether the increased risk in these conditions is related to the underlying disease or the immunosuppressive therapy or both [8]. Although most cases of HZ occurring in the community occur in immunocompetent hosts [15], patients with underlying conditions such as HIV, cancer, immunosuppression or other cell-mediated immunosuppressive disorders are at increased risk of HZ [18]. Furthermore, the incidence of HZ increases with age. It has been proposed that this is due to age-related decline in cell immunity (immunosenescence) or from waning virus-specific immunity over time [18].
The risk of HZ has been evaluated in AAV [16, 17]. Up to 17% of infectious complications in patients with AAV are due to HZ recurrences [16]. Median time to the development of HZ was 5 years in this study with two subjects developing HZ after treatment for AAV had been completed for 1–3 years [16]. In another study evaluating HZ in patients with AAV, 10% of the cohort developed HZ. The incidence of HZ was significantly increased in this study compared with estimates for the general population [17]. Renal dysfunction and female gender were strong risk factors for HZ in this study population. It may be that the lack of increased risk of HZ in GCA patients is because steroid-sparing agents typically added in management of other diseases such as SLE, RA and AAV are not commonly used for GCA. In this cohort, only three subjects were on steroid-sparing agents and none of them developed HZ.
We evaluated the risk of HZ in the first 6 months after diagnosis when glucocorticoid doses are typically the highest and found no increase in HZ in GCA even during this time period. A similar finding has been reported in a recent study that evaluated the incidence and timing of HZ in patients with AAV [17]. Wung et al. [17] found that although patients with AAV were at increased risk of developing HZ, the median time for the development of HZ was 16.5 (9.4) months. Only 4 of the 19 episodes of HZ occurred during the first 6 months when immunosuppression was the greatest [17]. We did not find any increased risk in GCA patients compared with referent subjects. Additionally, only half the GCA subjects were on prednisone at the time of HZ diagnosis (mean dose 3.7 mg/day).
Finally, the frequency and duration of PHN in the current study was similar in patients with GCA compared with patients in the referent cohort. In this study, 19% of GCA patients and 24% of non-GCA patients with HZ developed PHN. This percentage is similar to the rates of PHN reported previously for this population [15]. The duration of PHN was also similar in both groups. Furthermore, no differences were noted between the two groups in terms of severity of HZ as measured by rates of hospitalization or disseminated HZ. Firm conclusions regarding severity are limited by the small numbers of patients with this complication.
Limitations of this study include the retrospective design. Since patients were not followed prospectively, it is possible that cases of HZ were not detected since only cases coming to medical attention would be captured. Our data abstraction was limited to only information already available in the medical records. Therefore, complications such as PHN for which a medical encounter was not generated, would be underestimated. The abstractors (V.S.S. and T.A.K.) were not blinded to the two groups and, therefore, there is potential for ascertainment bias. However, all patients were screened using ICD-9 codes and all identified subjects’ charts were carefully reviewed to confirm the diagnosis of interest in both patient groups. The Olmsted County population is of predominantly Northern European ancestry, but this background is typical of patients with GCA elsewhere. Finally, we did not abstract information on CS therapy and were, therefore, unable to evaluate the association between starting dose or cumulative CS doses and risk of HZ in GCA patients. Instead, we evaluated the risk of HZ in the first 6 months after diagnosis, since this is when glucocorticoid doses are typically the highest. In two population-based studies using this GCA cohort, the median starting dose of prednisone was 60 mg/day [19, 20]. In the study evaluating glucocorticoid treatment in this cohort, the median duration of treatment to reach a daily dose of prednisone 5 mg was 7.5 months. In 87 patients (70%) who managed to permanently discontinue glucocorticoids, the median treatment duration was 21.6 months (range 2.3–122 months). Furthermore, the median cumulative dose of prednisone from initiation to time of permanent remission for this cohort is 6.47 g [19].
The strengths of this study include its population-based design. We included a referent cohort, which was age- and gender-matched for comparison. This is important since age is a risk factor for both HZ and GCA. The ability to access medical records from all providers in Olmsted County is an additional strength of this study. All cases of HZ were first identified using ICD-9 codes but then verified by chart review. This ensures accuracy in measurement of our outcome. Median length of follow-up after GCA diagnosis/index date in this study was ∼7 years and was, therefore, sufficient to ascertain the outcome of interest. The observed rates in this study were similar to those reported for this population, which further strengthens our observations.
This study provides important information on the incidence of HZ in GCA. Use of novel therapies for GCA may alter the risk of developing HZ in patients with GCA. At present, GCA patients do not appear at increased risk of HZ after diagnosis. The risk of HZ is not increased even during the first 6 months when glucocorticoid doses are typically highest. The severity of HZ and complication of PHN is similar in subjects with GCA and referent patients.

Acknowledgements
Funding: This study was made possible by the Rochester Epidemiology Project (Grant # R01-AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Abendroth A, Arvin AM. Immune evasion as a pathogenic mechanism of varicella zoster virus. Semin Immunol. 2001;13:27–39. doi: 10.1006/smim.2001.0293. [DOI] [PubMed] [Google Scholar]
- 2.Strangfeld A, Listing J, Herzer P, et al. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agents. JAMA. 2009;301:737–44. doi: 10.1001/jama.2009.146. [DOI] [PubMed] [Google Scholar]
- 3.Smitten AL, Choi HK, Hochberg MC, et al. The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum. 2007;57:1431–8. doi: 10.1002/art.23112. [DOI] [PubMed] [Google Scholar]
- 4.Pope JE, Krizova A, Ouimet JM, Goodwin JL, Lankin M. Close association of herpes zoster reactivation and systemic lupus erythematosus (SLE) diagnosis: case-control study of patients with SLE or noninflammatory musculoskeletal disorders. J Rheumatol. 2004;31:274–9. [PubMed] [Google Scholar]
- 5.Nagasawa K, Yamauchi Y, Tada Y, Kusaba T, Niho Y, Yoshikawa H. High incidence of herpes zoster in patients with systemic lupus erythematosus: an immunological analysis. Ann Rheum Dis. 1990;49:630–3. doi: 10.1136/ard.49.8.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald JR, Zeringue AL, Caplan L, et al. Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin Infect Dis. 2009;48:1364–71. doi: 10.1086/598331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahl LE. Herpes zoster infections in systemic lupus erythematosus: risk factors and outcome. J Rheumatol. 1994;21:84–6. [PubMed] [Google Scholar]
- 8.Chakravarty EF. Viral infection and reactivation in autoimmune disease. Arthritis Rheum. 2008;58:2949–57. doi: 10.1002/art.23883. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe F, Michaud K, Chakravarty EF. Rates and predictors of herpes zoster in patients with rheumatoid arthritis and non-inflammatory musculoskeletal disorders. Rheumatology. 2006;45:1370–5. doi: 10.1093/rheumatology/kel328. [DOI] [PubMed] [Google Scholar]
- 10.Salvarani C, Crowson CS, O’Fallon WM, Hunder GG, Gabriel SE. Reappraisal of the epidemiology of giant cell arteritis in Olmsted County, Minnesota, over a fifty-year period. Arthritis Rheum. 2004;51:264–8. doi: 10.1002/art.20227. [DOI] [PubMed] [Google Scholar]
- 11.Kermani TA, Schafer VS, Crowson CS, et al. Increase in age at onset of giant cell arteritis: a population-based study. Ann Rheum Dis. 2009;69:780–1. doi: 10.1136/ard.2009.111005. [DOI] [PubMed] [Google Scholar]
- 12.Maradit Kremers H, Crowson CS, Gabriel SE. Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases. Rheum Dis Clin North Am. 2004;30:819–34. doi: 10.1016/j.rdc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–8. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 14.Cox D. Some simple approximate tests for Poisson variates. Biometrika. 1953;40:354. [Google Scholar]
- 15.Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82:1341–9. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- 16.Charlier C, Henegar C, Launay O, et al. Risk factors for major infections in Wegener granulomatosis: analysis of 113 patients. Ann Rheum Dis. 2009;68:658–63. doi: 10.1136/ard.2008.088302. [DOI] [PubMed] [Google Scholar]
- 17.Wung PK, Holbrook JT, Hoffman GS, et al. Herpes zoster in immunocompromised patients: incidence, timing, and risk factors. Am J Med. 2005;118:1416. doi: 10.1016/j.amjmed.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis. 2004;4:26–33. doi: 10.1016/s1473-3099(03)00857-0. [DOI] [PubMed] [Google Scholar]
- 19.Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum. 2003;49:703–8. doi: 10.1002/art.11388. [DOI] [PubMed] [Google Scholar]
- 20.Nuenninghoff DM, Hunder GG, Christianson TJ, McClelland RL, Matteson EL. Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum. 2003;48:3522–31. doi: 10.1002/art.11353. [DOI] [PubMed] [Google Scholar]