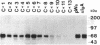Abstract
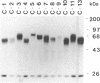
Serologic analysis of rabbit secretory IgA initially identified two subclasses of IgA, IgA-f and IgA-g. Recent molecular genetic studies have resulted in the identification and cloning of 13 genes encoding the constant region (C) of rabbit IgA heavy chains. Each of these 13 C alpha genes, C alpha 1-C alpha 13, was subcloned into an expression vector containing the VDJ (V, variable; D, diversity; J, joining) gene of a dansyl (DNS)-binding hybridoma antibody. The alpha heavy-chain constructs were transfected into SP2/0 cells producing murine light chains with specificity for DNS. Of the 13 resulting transfectomas, 12 were shown by ELISA to secrete DNS-binding chimeric rabbit-mouse IgA molecules. By immunoblot analysis, the 12 IgA-producing transfectomas were shown to secrete alpha chains ranging in size from 60 to 72 kDa. These data suggest that rabbit IgA may be composed of as many as 12 IgA isotypes. This is in marked contrast to mouse and human, in which only 1 and 2 IgA isotypes, respectively, are found. Serologic analyses, using anti-IgA-f and anti-IgA-g alloantisera, revealed that 11 of the 12 transfectoma IgAs reacted with anti-IgA-f and not with anti-IgA-g antibodies and that one reacted with anti-IgA-g and not with anti-IgA-f antibodies. Each of the IgA-producing transfectomas was cocultured with a Madin-Darby canine kidney cell line expressing the rabbit polymeric immunoglobulin receptor, and the transcytosed IgA antibodies were analyzed by immunoblots to determine whether they associated with secretory component (SC) through covalent or noncovalent interactions. Each of the 11 IgA-f isotypes was shown to bind SC by a disulfide linkage, whereas the single IgA-g isotype appeared to bind SC through noncovalent interactions only.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conway T. P., Dray S., Lichter E. A. Identification and genetic control of the f4 and f5 rabbit gamma-A immunoglobulin allotypes. Phenogroups at the f locus. J Immunol. 1969 Oct;103(4):662–667. [PubMed] [Google Scholar]
- DRAY S., YOUNG G. O., GERALD L. IMMUNOCHEMICAL IDENTIFICATION AND GENETICS OF RABBIT GAMMA-GLOBULIN ALLOTYPES. J Immunol. 1963 Sep;91:403–415. [PubMed] [Google Scholar]
- Dangl J. L., Wensel T. G., Morrison S. L., Stryer L., Herzenberg L. A., Oi V. T. Segmental flexibility and complement fixation of genetically engineered chimeric human, rabbit and mouse antibodies. EMBO J. 1988 Jul;7(7):1989–1994. doi: 10.1002/j.1460-2075.1988.tb03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray S., Kim B. S., Gilman-Sachs A. Allogroups of rabbit Ig heavy chains. Ann Immunol (Paris) 1974 Jan;125C(1-2):41–47. [PubMed] [Google Scholar]
- Gretch D. R., Suter M., Stinski M. F. The use of biotinylated monoclonal antibodies and streptavidin affinity chromatography to isolate herpesvirus hydrophobic proteins or glycoproteins. Anal Biochem. 1987 May 15;163(1):270–277. doi: 10.1016/0003-2697(87)90123-0. [DOI] [PubMed] [Google Scholar]
- Hanly W. C., Lichter E. A., Dray S., Knight K. L. Rabbit immunoglobulin A allotypic specificities. Localization to two papain fragments, fab 2 and fc 2 , of secretory immunoglobulin A. Biochemistry. 1973 Feb;12(4):733–741. doi: 10.1021/bi00728a025. [DOI] [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Kulhavy R., Tomana M., Butler W. T. IgA1 proteases from Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis, and Streptococcus sanguis: comparative immunochemical studies. J Immunol. 1980 Jun;124(6):2596–2600. [PubMed] [Google Scholar]
- Knight K. L., Burnett R. C., McNicholas J. M. Organization and polymorphism of rabbit immunoglobulin heavy chain genes. J Immunol. 1985 Feb;134(2):1245–1250. [PubMed] [Google Scholar]
- Knight K. L., Hanly W. C. Genetic control of alpha chains of rabbit IgA: allotypic specificities on the variable and the constant regions. Contemp Top Mol Immunol. 1975;4:55–88. doi: 10.1007/978-1-4615-8930-3_3. [DOI] [PubMed] [Google Scholar]
- Knight K. L., Lichter E. A., Hanly W. C. Papain cleavage of rabbit secretory immunoglobulins A. Differential sensitivity of f and g subclasses. Biochemistry. 1973 Aug 14;12(17):3197–3203. [PubMed] [Google Scholar]
- Knight K. L., Martens C. L., Stoklosa C. M., Schneiderman R. D. Genes encoding alpha-heavy chains of rabbit IgA: characterization of cDNA encoding IgA-g subclass alpha-chains. Nucleic Acids Res. 1984 Feb 10;12(3):1657–1670. doi: 10.1093/nar/12.3.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight K. L., Schneiderman R. D., Burnett R. C. Expression of rabbit IgA heavy chain genes in E. coli and in murine myeloma cells. Vet Immunol Immunopathol. 1987 Dec;17(1-4):279–289. doi: 10.1016/0165-2427(87)90147-4. [DOI] [PubMed] [Google Scholar]
- Knight K. L., Vetter M. L., Malek T. R. Distribution of covalently bound and non-covalently bound secretory component on sbuclasses of rabbit secretory IgA. J Immunol. 1975 Aug;115(2):595–598. [PubMed] [Google Scholar]
- Koshland M. E. The coming of age of the immunoglobulin J chain. Annu Rev Immunol. 1985;3:425–453. doi: 10.1146/annurev.iy.03.040185.002233. [DOI] [PubMed] [Google Scholar]
- Malek T. R., Hanly W. C., Knight K. L. Papain-resistant (f) and papain-sensitive (g) subclasses of rabbit sLgA. Allotypic specificities on different domains of the g subclass of alpha-chains (alpha g). Eur J Immunol. 1974 Oct;4(10):692–697. doi: 10.1002/eji.1830041011. [DOI] [PubMed] [Google Scholar]
- Malek T. R., Knight K. L. Digestion of the "proteolytic resistant" f-subclass of rabbit secretory IgA. Localization of f-subclass allotypic specificities to the Fc2alpha fragment. Immunochemistry. 1977 Jul;14(7):493–496. doi: 10.1016/0019-2791(77)90301-9. [DOI] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., Blobel G. Transcellular transport of polymeric immunoglobulin by secretory component: a model system for studying intracellular protein sorting. Ann N Y Acad Sci. 1983 Jun 30;409:441–451. doi: 10.1111/j.1749-6632.1983.tb26888.x. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., Deitcher D. L. Polymeric immunoglobulin receptor expressed in MDCK cells transcytoses IgA. Cell. 1986 Aug 15;46(4):613–621. doi: 10.1016/0092-8674(86)90887-1. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth K. L., Hanly W. C., Knight K. L. Serologic cross-reactions among rabbit secretory IgA molecules: evidence for multiple subclasses of secretory IgA-f molecules. Mol Immunol. 1983 Sep;20(9):989–999. doi: 10.1016/0161-5890(83)90040-8. [DOI] [PubMed] [Google Scholar]
- Plaut A. G., Wistar R., Jr, Capra J. D. Differential susceptibility of human IgA immunoglobulins to streptococcal IgA protease. J Clin Invest. 1974 Dec;54(6):1295–1300. doi: 10.1172/JCI107875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng J., Knight K. L. Differential susceptibility of rabbit sIgA subclasses to trypsin cleavage: characterization of the fragments obtained from the g subclass. J Immunol. 1975 Aug;115(2):454–461. [PubMed] [Google Scholar]
- Weicker J., Underdown B. J. A study of the association of human secretory component with IgA and IgM proteins. J Immunol. 1975 Apr;114(4):1337–1344. [PubMed] [Google Scholar]
- Wofsy L., Burr B. The use of affinity chromatography for the specific purification of antibodies and antigens. J Immunol. 1969 Aug;103(2):380–382. [PubMed] [Google Scholar]