Abstract
A novel biomaterial formed by the immobilization of the Aminopeptidase from Aeromonas Proteolytica (AAP) on synthetic Mg2+ and Al3+ ion-containing layered double hydroxide (LDH) particles was prepared. Immobilization of AAP on the LDH particles in a buffered, aqueous mixture is rapid such that the maximum loading capacity, 1 × 10-9 moles of AAP/mg LDH, is achieved in a few minutes. X-ray powder diffraction of LDH samples before and after treatment with AAP indicates that the enzyme does not intercalate between the layers of LDH, but instead binds to the surface. Treatment of AAP/LDH with various amounts of salt in a buffered mixture demonstrates that between 15 and 20% of AAP can be removed from the LDH by washing the composite material in 0.2 M NaCl. However, the residual AAP remains bound to the LDH even at 1 M salt concentrations. A suspension of the AAP/LDH biomaterial in 10 mM Tricine buffered, aqueous solution (pH 8.0 and 25 ° C) catalyzes the hydrolysis of l-leucine-p-nitroanilide demonstrating that immobilized AAP remains available to substrate and retains its catalytic activity. Recycling experiments reveal that the AAP/LDH particles can be recovered and reused multiple times without appreciable loss of activity. This work provides the foundation for the development of materials that will function in the degradation or detection of peptide hormones or neurotoxins.
Keywords: LDH, Enzyme Immobilization, Aminopeptidase
Introduction
The ability to exploit the selectivity and reactivity of enzymes in synthetic reactions or biochemical transformations is highly desirable. However, serious practical issues arise due to the difficulty of separating enzymes from reaction mixtures,1,2 and the lack of solubility or denaturation of enzymes in organic solvents employed in synthetic reactions.3,4 One way to overcome these obstacles is through the immobilization of enzymes on an inert material producing a stable bio-composite that is catalytically active, easily separated from a reaction mixture, and recyclable. These materials show great promise as chemical/biochemical sensors, separation media, or catalysts for laboratory and/or industrial transformations. Successful enzyme-containing bio-composites have been prepared using silica glasses derived through sol-gel processing,5 clays,6 and other inorganic host materials.7 A promising class of host materials for this application are layered double hydroxides (LDHs), which are readily prepared from inexpensive starting materials, provide large, two-dimensional surfaces for the adsorption of enzymes, and are considered more biocompatible and nontoxic than other inorganic materials.8 Though a number of LDH bio-composites have been reported previously,8,9 studies have focused mainly on chemical sensing with these materials, while their application in catalysis has received much less attention. Examples of the latter include the immobilization of laccase9a and lipase9b on LDH materials with retention of catalytic activity.
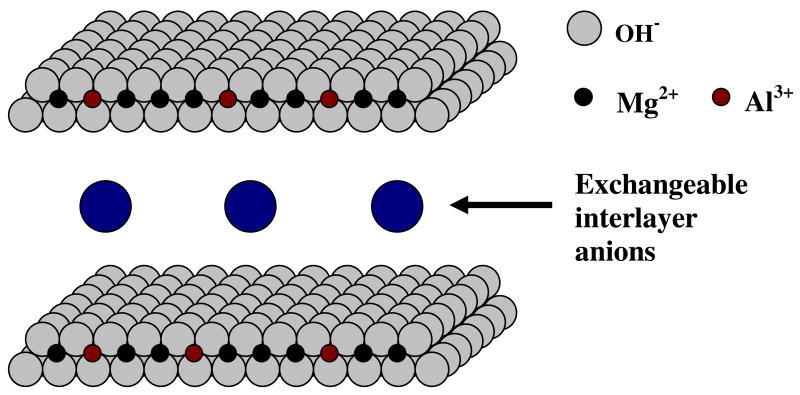
LDH materials have a hydrotalcite-like structure with the general formula [MII(1-x)MIIIx(OH)2]x+[An-]x/n• z H2O, where MII is a divalent cation, MIII is a trivalent cation, and An- is an anion.10 LDH contains brucite-like (Mg(OH)2) layers, in which some of the divalent cations have been substituted with trivalent cations, resulting in the formation of positively charged sheets (Figure 1). The positive charge of these sheets is offset by the presence of exchangeable, hydrated anions that exist in the interlayer space between sheets. The net result is that LDH materials are anion exchangers in contrast to most other clays that exchange cations. The fact that the octahedral sheets of LDH carry a positive charge is an important characteristic for the binding of enzymes, as a common enzymatic purification technique is the use of anion exchange chromatography, suggesting that any enzyme with a large dielectric constant should bind to LDH materials.
Figure 1.
Structure of Mg2+/Al3+ LDH, [Mg2+(1-x)Al3+x(OH)2]x+[Cl-]x.
Herein we report the immobilization of the aminopeptidase from Aeromonas proteolytica (AAP) on Mg2+ and Al3+ ion-containing LDH particles. AAP is ideally suited for bio-composite studies since it can be obtained in large quantities (>100 mgs), is a remarkably thermostable, 29.5 kDa monomer, and has a dielectric constant of ∼3.5.11 AAP is a metallohydrolase that contains a dinuclear Zn(II) active sites and catalyzes the hydrolysis of N-terminal amino acids from polypeptide chains. 12-18 AAP is of particular interest as it is involved in neuropeptide and peptidal hormone detection or degradation processes. Our data suggest that AAP binds tightly to the surface of LDH particles producing a bio-composite that is catalytically active, and can be recovered and reused multiple times without appreciable loss of activity. Therefore, LDH provides a suitable vehicle for the production of a novel, bio-catalytic material for N-terminal protein, hormone or neuropeptide processing.19
Experimental Section
Materials
The chemicals used in this study were of reagent grade or higher, obtained from commercial sources, and were used without further purification. Cultures of AAP were grown according to the previously published procedure.20 Purified enzyme was stored at 77 K until needed. The water used was filtered and deionized using a Barnstead NANOpure water purification system.
Preparation of Mg2+/Al3+/Cl- LDH
Mg2+/Al3+/Cl- LDH was prepared according to a co-precipitation reaction as previously reported.10 A solution of 10 g of MgCl2•6H2O (0.050 moles) and 6.7 g of AlCl3•6H2O (0.025 moles) in 200 mL of deionized water was slowly added (1-2 mL per minute) to 50 mL of 0.10 M NaOH (pH 10.0) at 25°C. The pH of the reaction mixture was monitored and maintained at 10.0 by the simultaneous addition of 2 M NaOH. When the addition was complete, the mixture was heated to 70°C and allowed to stir for 24 hrs. Care was taken to ensure that the solutions employed in this reaction were de-gassed and that the reaction mixture remained under a continuous flow of nitrogen. The product was isolated by centrifugation, washed repeatedly with de-ionized water, dried, and ground into a fine powder.
AAP Immobilization on LDH
AAP enzyme was immobilized on LDH by stirring them together in 10 mM tricine buffer containing 0.1 mM ZnSO4, pH 8.0. In a typical experiment, 30 mg of LDH was suspended in 20 mL of buffer and allowed to stir for 30 minutes. Various amounts of AAP (0.02-0.6 mg) were then added and the mixture was stirred for an additional 15 minutes. The bio-composite was separated from the solution by centrifugation at 12,000 rpm for 15 minutes using a Sorvall Stratos Biofuge with a #3335 rotor unless otherwise noted, and washed first with buffer containing 0.2 M KCl, and then twice with buffer containing no salt (enzyme was not present in the final washes as indicated by a lack of absorbance at 280 nm and enzyme activity). The resulting AAP/LDH biomaterial was re-suspended in 10 mL buffer to give 1.5 mg/mL stocks and stored at 4 °C.
AAP/LDH Bio-Composite Activity
The activity of the AAP/LDH bio-composite, hereafter AAP/LDH, was examined by reacting a suspension of this material in 10 mM tricine buffer containing 0.1 mM ZnSO4 at pH 8.0, with the substrate l-leucine-p-nitroanilide (LPNA) and compared to native AAP under identical conditions. In a typical reaction, approximately 0.75 mg of AAP/LDH (corresponding to an AAP concentration of 3.8 × 10-8 M) was suspended in 20 mL of buffer and allowed to stir for a period of 15 minutes. Following suspension of the bio-composite, a specific volume of substrate stock solution (10 mM LPNA) was added to give a final concentration of substrate in the range of 0.05-5 mM. A 3-mL sample was immediately removed from the reaction mixture and filtered through a 0.45 μm nylon, syringe filter to remove the bio-composite and stop the reaction. This procedure was repeated every minute for ∼5 minutes. The absorbance at 405 nm of the supernatants from each sample, relative to the buffer, was then recorded using an HP 8453 diode array UV-Vis spectrophotometer. Absorbance at 405 nm for each time point was corrected for background by subtracting the absorbance of a solution of buffer with an equivalent concentration of un-reacted LPNA. Absorbance values were then converted into concentration of 4-nitroaniline using an extinction coefficient of 10,800 M-1cm-1.21
Effect of Salt Concentration
A series of mixtures were prepared by suspending 1.5 mg of AAP/LDH in 20 mL of 10 mM tricine buffer containing 0.1mM ZnSO4 at pH 8.0 with varying NaCl concentration in the range of 0-1.0 M. Suspensions were stirred for ∼30 minutes. The AAP/LDH samples were separated from the salt solutions by centrifugation at 12,000 rpm for 15 minutes. These samples were re-suspended in 20 mL buffer without NaCl and stirred for ∼30 minutes. To these suspensions, 200 μL of 10 mM LPNA stock solution was added to each sample to give a final substrate concentration of 0.1 mM. Reactions were carried out for ∼3.5 minutes. Samples were immediately filtered through a 0.45 μm nylon, syringe filter to stop the reaction. Absorbance at 405 nm was recorded for the supernatant of each sample and corrected for background as described above in AAP/LDH bio-composite activity.
Recycling Experiments
To a reaction mixture of 0.75 mg AAP/LDH, 200 μL of 10 mM LPNA was added. After stirring the reaction mixture for ∼3.5 minutes, a 3 mL volume was removed and immediately centrifuged at ∼14,000 rpm for 1 minute, using a Spectrafuge 16 M microcentrifuge. The supernatant was then filtered through a 0.45 μm nylon, syringe filter. Absorbance at 405 nm of the supernatant was recorded and corrected for background as described in AAP/LDH bio-composite activity. The remainder of the reaction mixture was then centrifuged at ∼12,000 rpm. Pellets from this centrifugation step and the microcentrifugation step were combined, re-suspended in 20 mL buffer, and washed for 30 minutes. Following this wash, the AAP/LDH biomaterial was recovered by centrifugation and re-suspended in 20 mL buffer. The reaction with LPNA described above was then repeated. This process of reacting, recovering, washing, and re-suspending the AAP/LDH material was repeated 4 additional times resulting in 6 separate cycles with the same sample of AAP/LDH.
X-ray diffraction experiments
X-ray diffraction was performed on a Phillips Compact X-ray Diffractometer System (PW 1840) operating at a voltage of 45 KeV and a current of 35 mA. Fe filtered CoKα radiation was used with a wavelength of 1.790 Å. All XRD patterns were recorded with a step size of 0.010° (2θ) at 2.00 s per step and a receiving slit of 0.3 mm.
Results and Discussion
Synthesis of LDH
The traditional co-precipitation method to synthesize LDH was employed using a 2:1 ratio of Mg2+ to Al3+.10 Elemental analysis of this material provides a Mg2+/Al3+ ratio of 2.10 indicating that the approximate LDH composition is [Mg2.10Al(OH)6.20]Cl•xH2O.22 However, an IR spectrum (KBr) reveals a weak band at 1370 cm-1 suggesting a small amount of carbonate substitution for chloride ions. The XRD pattern of LDH (Figure 2) is typical of layered materials with narrow, symmetric (00l) lines at low 2θ values, corresponding to the basal spacing and higher order reflections, and weaker, less symmetric lines at higher 2θ values.23 The two peaks observed in the LDH pattern at 13.3° and 26.9° 2θ correspond to the (003) and (006) reflections and provide a basal spacing of 7.7 Å, matching the value reported previously for this material.10 Given that the width of a brucite layer is 4.77 Å,24 we estimate the gallery height of LDH to be 2.9 Å, consistent with the presence of hydrated chloride and carbonate ions. While the intensity of the diffraction peaks suggests that the LDH is highly crystalline, the line widths indicate that this material is comprised of small crystallites. The average crystallite size of the LDH was calculated from the (003) peak using the Debye-Scherrer equation,
Figure 2.
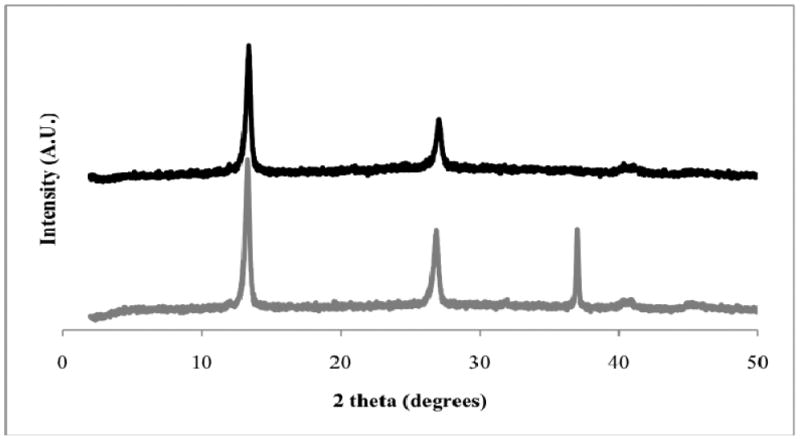
Powder XRD patterns of LDH (bottom) and AAP/LDH (top). Note that the line at 37° 2θ for the LDH sample is an artifact of the aluminum sample holder.
| (1) |
where L is the average crystallite width, λ is the wavelength of radiation used, β(θ) is the full width at half maximum (FWHM), and θ is the Bragg diffraction angle, providing a value of L = 235 Å or 23.5 nm.23 The relatively small size of these particles is clearly advantageous, providing a large surface area for enzyme immobilization and thereby enhancing catalytic activity.
Immobilization of AAP on LDH
Immobilization of AAP on LDH particles is achieved by stirring the enzyme together with a suspension of the synthetic material in a buffered, aqueous mixture for ∼15 minutes. The bio-composite is then recovered by centrifugation, treated with 0.2 M KCl, and washed several times with buffer. Complete immobilization is achieved within the 15-minute time period as indicated by a lack of absorbance at 280 nm for the wash solutions. The AAP/LDH biomaterial must be stored in a buffer solution since isolating and air-drying the results in the complete loss of catalytic activity. This is likely due to irreversible enzyme structural changes during the dehydration process suggesting that AAP is bound primarily to the surface of the LDH. We have also found it necessary to include 0.1 mM ZnSO4 in storage and reaction buffers since the AAP/LDH biomaterial gradually loses catalytic activity in the absence of Zn2+. When AAP/LDH is stored in Zn2+-containing buffer and kept refrigerated, the bio-composite retains full activity for over several months.
The XRD pattern of AAP/LDH is identical to that of LDH (Figure 2) demonstrating that the basal spacing and gallery height of LDH do not change upon AAP binding. Therefore AAP does not appear to intercalate into the gallery region of the LDH particles, but instead binds to the surface. This is not surprising given that the dimensions of AAP are approximately 35 × 40 × 48 Å resulting in a particle diameter that is not much smaller than the width of the LDH particles.25 This also suggests that the interaction of AAP with LDH is not dependent on the nature of the gallery anions. Therefore, the small amount of carbonate impurity that was detected in the LDH should not affect AAP loading or activity. Moreover, surface binding is advantageous since a significant portion of bound AAP remains available to substrate and retains its activity.
Reactivity of AAP/LDH
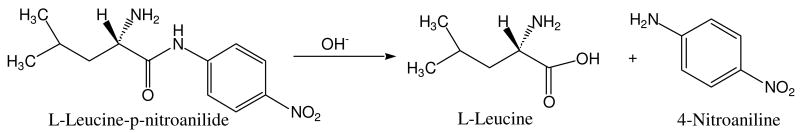
Hydrolysis of the colorless peptide substrate LPNA produces 4-nitroaniline (see Scheme 1) that is readily detected by its strong absorbance at 405 nm, providing a convenient parameter with which to monitor AAP catalytic activity. Figure 3 shows the production of 4-nitroaniline vs. time for reactions of LPNA with LDH, AAP, and the AAP/LDH biomaterial. The amount of AAP used in the solution control experiment was equivalent to the amount entrapped in the AAP/LDH bio-composite (as determined by a loading capacity experiment vide infra). LDH itself does not facilitate hydrolysis of this substrate but based on the initial rates of LPNA hydrolysis, AAP bound to the surface of LDH retains approximately 20% of the activity of AAP in solution. The loss in AAP activity observed for the AAP/LDH biomaterial is likely due to the inaccessibility of the dinuclear Zn(II) active site to substrate since the enzyme is randomly adhered to LDH clay surface. Nonetheless, a substantial portion of the entrapped AAP in the AAP/LDH biomaterial remains active and catalyzes the rapid hydrolysis of LPNA. Moreover, the fact that the 4-nitroaniline is measured in solution suggests that the bio-composite allows product to be readily released back into the solution and the reaction is limited only by substrate diffusion.
Scheme 1.
Hydrolysis of LPNA to produce L-leucine and 4-nitroaniline.
Figure 3.
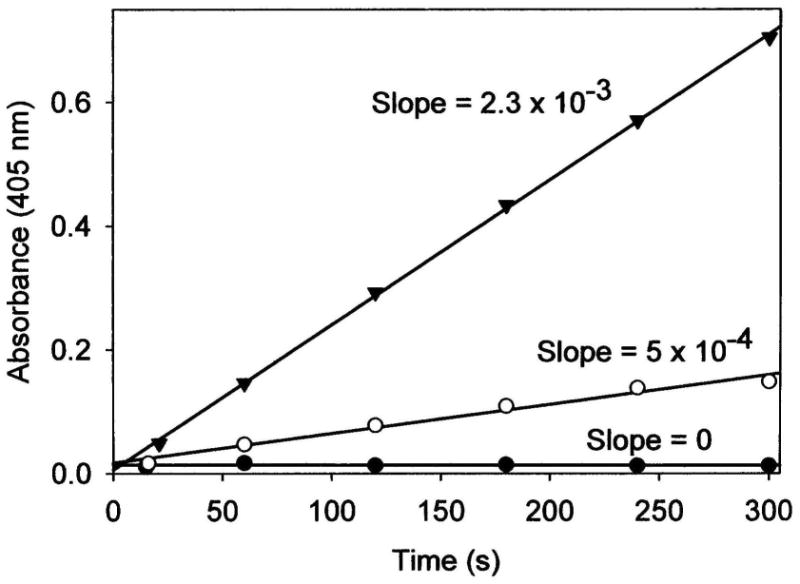
Production of 4-nitroaniline versus time for the reaction of 200 μM LPNA with 0.75 mg of LDH (solid circles), 0.75 mg AAP/LDH (open circles), or 3.8 × 10-8 M AAP (solid triangles). Reactions were carried out in 10 mM tricine buffer, 0.1mM ZnSO4, pH 8.0.
Effect of Salt Concentration
To further examine the nature of the interaction between AAP and LDH, we investigated the effect of washing the AAP/LDH bio-composite with buffer containing various concentrations of NaCl since the AAP-LDH interaction is electrostatic in nature. Following an ∼30 minute wash in buffer containing various concentrations of NaCl, AAP/LDH samples were recovered by centrifugation, re-suspended in buffer without salt, and tested for activity using LPNA as the substrate. A plot of absorbance of 4-nitroaniline produced after ∼4 minutes vs. [NaCl] is shown in Figure 4. These data reveal a 15 to 20% drop in AAP activity but only at salt concentrations 0.2 M or higher. At NaCl concentrations above 0.2 M, hydrolytic activity of AAP in the AAP/LDH biomaterial remains constant. These data suggest that AAP associates with LDH in two distinct ways. A potentially weak association will likely occur along edge sites and/or other regions of LDH particles that do not permit large scale electrostatic or hydrogen bonding interactions to take place. These weaker binding sites are disrupted by high salt concentrations. Similar salt concentrations (∼ 0.3 M) promote the dissociation of AAP from anion exchange media during purification of the enzyme.20 The second mode of association appears to be much stronger and likely results from more extensive interactions of AAP along the faces of LDH particles. In this mode of association, AAP remains bound to the LDH even at a salt concentration as high as 1 M. Based on these data, a 0.2 M KCl wash was routinely done to ensure that weakly-bound enzyme is removed prior to characterization and reactivity studies.
Figure 4.
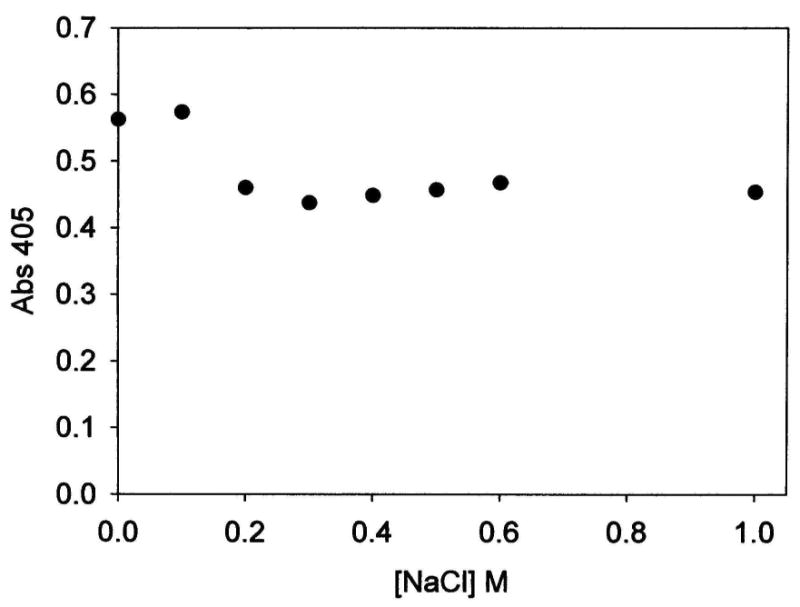
Activity of 1.5 mg AAP/LDH samples, treated with various concentrations of salt, as a function of the absorbance of 4-nitroaniline produced in a 4 minute reaction of each sample with 100 μM LPNA, versus the concentration of NaCl solution used to treat the particular sample of AAP/LDH. Reactions were carried out in 10 mM tricine buffer, 0.1mM ZnSO4, pH 8.0.
AAP Binding to LDH
The AAP loading capacity of LDH was determined by treating 15 mg samples of LDH particles with various concentrations of AAP (0 to 3 × 10-6 M) in buffer. AAP activity was determined for these AAP/LDH samples using LPNA as the substrate and ∼4 minute reaction times. The activity of these samples initially rises sharply due to increasing concentration of AAP, but levels off at approximately 1.5 × 10-6 M AAP. These data indicate that 15mg LDH can bind ∼1.5 × 10-8 moles AAP which corresponds to a loading capacity of ∼1 × 10-9 moles AAP/mg LDH. Furthermore, the binding is quantitative at this low concentration of enzyme (i.e. no free enzyme can be detected in the buffer following treatment) indicating that the association between the AAP and LDH is very strong and essentially irreversible under the conditions of our experiments. This is further corroborated by the recycling experiment described below.
AAP/LDH Recycling
To investigate the effect of recovering and recycling the AAP/LDH bio-composite on the catalytic activity of this material, we carried out an experiment in which a sample of this bio-composite is subject to multiple exposures of LPNA substrate. In between exposures, the AAP/LDH was recovered by centrifugation and washed with buffer. Figure 6 shows a plot of relative activity vs. cycle number demonstrating less than a 10% loss in activity after 5 cycles. This small decline in activity is most likely due to the loss of small amounts of material that occurs during the centrifugation and washing steps of the experiment rather than degradation of the bio-composite. These data reveal how robust the AAP/LDH bio-composite material is and verify the stability of the material.
Figure 6.
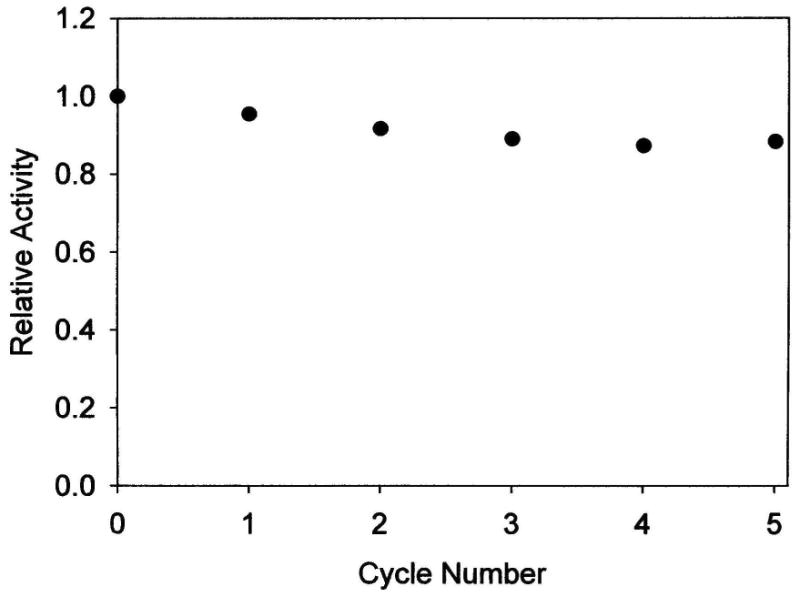
Plot of relative activity versus cycle number for a sample of AAP/LDH subjected to multiple treatments with 100 μM LPNA. The AAP/LDH sample was recovered between treatments by centrifugation and washed with 10 mM tricine buffer, 0.1mM ZnSO4, pH 8.0.
Conclusion
The immobilization of AAP on the surface of synthetic Mg2+ and Al3+-ion containing LDH particles produces a bio-composite that retains activity of the native enzyme and is capable of catalyzing the hydrolysis of LPNA substrate. Importantly, AAP/LDH biomaterials can be recovered from a reaction mixture by centrifugation, washed, and re-used repeatedly without appreciable loss of catalytic activity. The association of AAP with LDH is strong and essentially irreversible under the reaction conditions used. This study demonstrates the general utility of LDH as a host material for the production of catalytic, recyclable, bio-composite materials. This work also provides a foundation for the development of materials that will function in the degradation or detection of peptide hormones or neurotoxins.
Figure 5.
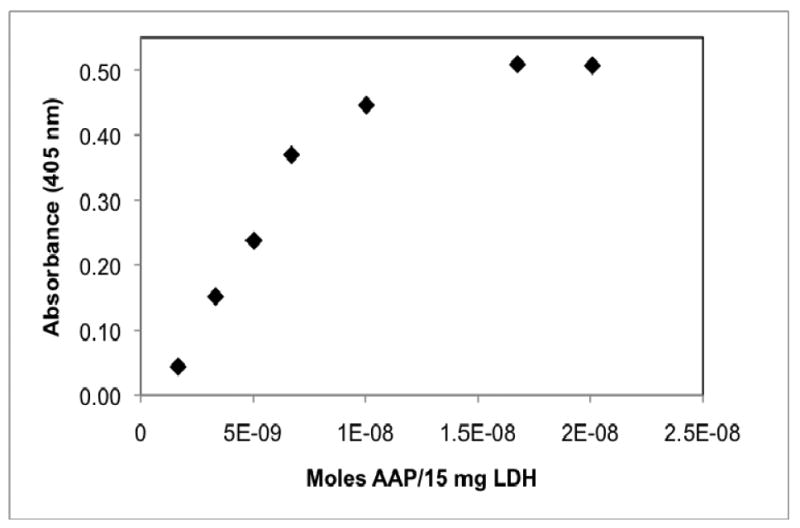
Activity of AAP/LDH samples produced by the treatment of 15 mg LDH with various number of moles of AAP. Activity is expressed as the absorbance of 4-nitroaniline produced in a 4 minute time period. Reaction mixtures were prepared by combining 1.5 mg AAP/LDH and 100 μM LPNA in 10 mM tricine buffer, 0.1mM ZnSO4, pH 8.0.
Acknowledgments
Acknowledgment is made to the donors of The Petroleum Research Fund, administered by the ACS, for partial support of this research. This work was also supported by the National Science Foundation (CHE-0652981, RCH) and the National Institutes of Health (R15 AI085559-01A1, RCH). The authors wish to thank Dr. Michael E. Hagerman for recording the powder XRD patterns and insightful discussions.
References
- 1.Mylerova V, Martinkova L. Cur Org Chem. 2003;7:1–17. [Google Scholar]
- 2.Banerjee A, Sharma R, Banerjee UC. Appl Microbiol Biotechnol. 2002;60:33–44. doi: 10.1007/s00253-002-1062-0. [DOI] [PubMed] [Google Scholar]
- 3.Arnold FH. Protein Eng. 1988;2:21–25. doi: 10.1093/protein/2.1.21. [DOI] [PubMed] [Google Scholar]
- 4.Griebenow K, Klibanov AM. J Am Chem Soc. 1996;118:11695–11700. [Google Scholar]
- 5.See, for example: O'Neill H, Greenbaum E. Chem Mater. 2005;17:2654–2661.Frenkel-Mullerad H, Avnir D. J Am Chem Soc. 2005;127:8077–8081. doi: 10.1021/ja0507719.Smith K, Silvernail NJ, Rodgers KR, Elgren TE, Castro M, Parker RM. J Am Chem Soc. 2002;124:4247–4252. doi: 10.1021/ja012215u.Ellerby LM, Nishida CR, Nishida F, Yamanaka SA, Dunn B, Valentine JS, Zink JI. Science. 1992;255:1113–1115. doi: 10.1126/science.1312257.Dave BC, Dunn B, Valentine JS, Zink JI. Anal Chem. 1994;66:11120A–11127A.
- 6.See, for example: Zen JM, Kumar AS. Anal Chem. 2004;76:205A–211A. doi: 10.1021/ac041566z.Mousty C. Appl Clay Sci. 2004;27:159–177.de Melo JV, Cosnier S, Mousty C, Martelet C, Jaffrezic-Renault N. Anal Chem. 2002;74:4037–4043. doi: 10.1021/ac025627+.
- 7.See for example: Chen GJ, Yen MC, Lin JJ, Chiu HC. Bioconj Chem. 2008;19:138–144. doi: 10.1021/bc700224q.Yu A, Liang Z, Caruso F. Chem Mater. 2005;17:171–175.Naik RR, Tomczak MM, Luckarift HR, Spain JC, Stone MO. Chem Commun. 2004:1684–1685. doi: 10.1039/b404586f.Park S, Chung TD, Kang SK, Jeong RA, Boo H, Kim HC. Anal Sci. 2004;20:1635–1638. doi: 10.2116/analsci.20.1635.
- 8.An Z, Lu S, He J, Wang Y. Langmuir. 2009;25:10704–10710. doi: 10.1021/la901205c. [DOI] [PubMed] [Google Scholar]
- 9.See, for example: Córdova DIC, Borges RM, Arizage GGC, Wypych F, Krieger N. Quím Nova. 2009;32:1495–1499.Rahman MB, Basri M, Hussein MZ, Rahman RN, Zainol DH, Salleh AB. Appl Biochem Biotechnol. 2004;118:313–320. doi: 10.1385/abab:118:1-3:313.Shan D, Mousty C, Cosnier S. Anal Chem. 2004;76:178–183. doi: 10.1021/ac034713m.Shan D, Cosnier S, Mousty C. Anal Chem. 2003;75:3872–3879. doi: 10.1021/ac030030v.Choy JH, Kwak SY, Jeong YJ, Park JS. Angew Chem Int Ed. 2000;39:4042–4045. doi: 10.1002/1521-3773(20001117)39:22<4041::aid-anie4041>3.0.co;2-c.
- 10.Constantino VRL, Pinnavaia TJ. Inorg Chem. 1995;34:883–892. [Google Scholar]
- 11.Prescott JM, Wilkes SH. Methods Enzymol. 1976;45:530–543. doi: 10.1016/s0076-6879(76)45047-4. [DOI] [PubMed] [Google Scholar]
- 12.Lipscomb WN, Sträter N. Chem Rev. 1996;96:2375–2433. doi: 10.1021/cr950042j. [DOI] [PubMed] [Google Scholar]
- 13.Wilcox DE. Chem Rev. 1996;96:2435–2458. doi: 10.1021/cr950043b. [DOI] [PubMed] [Google Scholar]
- 14.Dismukes GC. Chem Rev. 1996;96:2909–2926. doi: 10.1021/cr950053c. [DOI] [PubMed] [Google Scholar]
- 15.Hausinger RP. Biochemistry of Nickel. Vol. 12. Plenum Press; New York: 1993. [Google Scholar]
- 16.Chin J. Acc Chem Res. 1991;24:145–152. [Google Scholar]
- 17.Lai K, Dave KI, Wild JR. J Biol Chem. 1994;269:16579. [PubMed] [Google Scholar]
- 18.Menger FM, Gan LH, Johnson E, Durst DH. J Am Chem Soc. 1987;109:2800–2803. [Google Scholar]
- 19.Arnau J, Lauritzen C, Petersen GE, Pedersen J. Protein Expres Purif. 2006;48:1–13. doi: 10.1016/j.pep.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Chen G, Edwards T, D'souza VM, Holz RC. Biochemistry. 1997;36:4278–4286. doi: 10.1021/bi9618676. [DOI] [PubMed] [Google Scholar]
- 21.Baker JO, Wilkes SH, Bayliss ME, Prescott JM. Biochemistry. 1983;22:2098–2103. doi: 10.1021/bi00278a009. [DOI] [PubMed] [Google Scholar]
- 22.Elemental analysis of the Mg2+/Al3+/Cl- LDH by Quantitative Technologies, Inc. using ICP resulted in 19.22 % Mg and 10.18 % Al.
- 23.Millange F, Walton RI, O'Hare D. J Mater Chem. 2000;10:1713–1720. [Google Scholar]
- 24.Tagaya H, Sato S, Morioka H, Kadokawa J, Karasu M, Chiba K. Chem Mater. 1993;5:1431–1433. [Google Scholar]
- 25.Chevrier B, Schalk C, D'Orchymont H, Rondeau JM, Moras D, Tarnus C. Structure. 1994;2:283–291. doi: 10.1016/s0969-2126(00)00030-7. [DOI] [PubMed] [Google Scholar]