Summary
Maternal smoking during pregnancy increases the incidence and severity of respiratory infections in neonates. Surfactant proteins A and D (SP-A and SP-D, respectively) are components of pulmonary innate immunity and have an important role in defense against inhaled pathogens. The purpose of this study was to determine if nicotine exposure during the third trimester of pregnancy alters the expression of SP-A and SP-D of fetal lung epithelia. Pregnant ewes were assigned to four groups; a nicotine-exposed full-term and pre-term group, and control full-term and pre-term group. Lung tissue was collected for Western blot and IHC analysis of SP-A level, Western blot analysis of SP-D level and qPCR analysis of SP-A and SP-D mRNA expression. Exposure to nicotine significantly decreased SP-A gene expression (P = 0.01) and SP-A protein level in pre-term lambs. This finding suggests that maternal nicotine exposure during the last trimester of pregnancy alters a key component of lung innate immunity in offspring.
Keywords: nicotine, lung, fetal, lung surfactant proteins
INTRODUCTION
The lungs are constantly exposed to potentially pathogenic microorganisms present in the inspired air. Microbial agents trigger responses of the innate immune system of lung epithelia which reduces microbial colonization and infection. In addition, an effective innate immune response by epithelia minimizes the adaptive immune responses, thereby reducing leukocytic infiltration and allowing efficient pulmonary function and gaseous exchange.
The lung innate immune system includes three major components; physical barrier, cellular responses, and secretory products. The physical barrier includes respiratory tract epithelia and constant ciliary movement in order to propel invading particles out of the respiratory tract.1 The alveolar macrophages, intravascular macrophages, and other cells with phagocytic and pro-inflammatory properties are cellular components which function to prevent pulmonary infection.2 The secretory component includes anti-microbial peptides, proteins, and other molecules secreted by respiratory epithelia which line the pulmonary airways.3 Surfactant proteins A and SP-D are key elements of the secretory lung innate immune defense. They are synthesized and secreted by alveolar type II cells (ATII) and non-ciliated bronchial and bronchiolar epithelial cells (Clara cells).4 Surfactant protein A and SP-D are involved in host defense against various bacterial and viral pathogens. The secretion of SP-A and SP-D is regulated by developmental stage,5 hormones and potentially, by environmental factors such as cigarette smoke.
Previous studies have shown that maternal smoking during pregnancy is associated with many adverse health effects in the developing fetus which may be manifested during postnatal life.6 Undoubtedly, maternal smoking during pregnancy is a major cause of pre-mature birth7 and low birth weight for gestational age.8 There is a relatively more recent body of work, suggesting that cigarette smoke is immunosuppressive and pre-disposes infants to respiratory infections.6 Although cigarette smoke contains thousands of different chemicals, it is suspected that nicotine is a major immunomodulatory component since it readily crosses the placenta and accumulates in the fetal compartment.9
It is our hypothesis that in addition to pre-mature birth, cigarette smoking during pregnancy may have a significant regulatory role on lung surfactant protein and consequential increase in susceptibility to various respiratory infections in newborn infants.
MATERIALS AND METHODS
Experimental Design
Animal use and experimental procedures were approved by Iowa State University’s Animal Care and Use Committee according to the requirements of the NIH guide for the Care and Use of Laboratory Animals. The lateral neck region was clipped and fitted with a transdermal nicotine patch (21 mg, Nicoderm CQ, GlaxoSmithKline, Brentford, Middlesex, UK) in 16, mixed breed (1/2 Rambouillet, 1/4 Dorset, and 1/4 Finnish sheep), date-mated, pregnant ewes ~6 weeks prior to lambing dates. The ewes were randomly assigned to two groups, nicotine exposed and a control group. Within these groups, ewes were further randomly assigned to a full-term group in which the lambs were naturally delivered and a pre-term group in which the lambs were delivered pre-maturely via abdominal incision and uterotomy (day 138 of gestation, term = 147). Prior to uterotomy, the ewes were anesthetized with tiletamine and zolazepam (Telazol, 1–2 mg/kg, I.M; Fort Dodge, IA) and upon uterotomy; the ewes were euthanized by administration of sodium pentobarbital (Beuthanasia-D, 2 ml/10 pounds, Schering-Plough Animal Health Corp., Union, NJ). The ewes received either nicotine or supportive bandage alone. A total of 24 lambs were obtained and assigned to experimental groups. Full-term control (FTC) and pre-term control (PTC) groups had six lambs each (n = 6), full-term nicotine-exposed group (FTN) had five lambs (n = 5) and pre-term nicotine-exposed group (PTN) had seven lambs (n = 7).
Nicotine Administration Protocol
Approximately 20 cm2 of the lateral neck region was shaved and cleaned. After the neck was dry, a 21 mg nicotine patch was placed on the skin and gentle pressure was applied over the patch for 5 min. The patch was secured by additional bandaging. Blood samples (10 ml) were drawn from the jugular vein 6, 12, and 24 hr after patch placement and assessed for cotinine plasma levels (CPL) by HPLC analysis. The procedure was repeated on a daily basis during the last 6 weeks of pregnancy.
Tissue Collection
Lung tissue was collected within 24 hr of natural lambing or uterotomy. The samples from the most lateral portions of the right, cranial lobes were collected into cryovials, snap frozen in liquid nitrogen, and stored at −80°C until protein and RNA extraction for Western blot and qPCR analysis. The remaining tissue from the same lung lobes were fixed in 10% buffered formalin for immunohistochemistry (IHC).
qPCR Analysis
Methodology for qPCR analysis of SP-A and SP-D mRNA levels has been described previously in detail.10,11 Briefly, total RNAwas isolated from the fresh, frozen lung tissue and assessed for quantity and purity by spectrophotometry. This procedure was followed by DNase treatment (TURBO DNA-free kit, Ambion, Austin, TX). Prior to qPCR analysis, a test plate was run to determine which RNA dilution ranges gave the best signal for SP-A and SP-D targets. The DNase treated RNA isolates were diluted 1:10 with nuclease-free water, then diluted to their ideal ranges on a per-sample, per-target basis, and further placed in equal amount, in duplicate into 96-well qPCR reaction plates (Applied Biosystems Incorporated, Forest City, CA). The plates were run in the GeneAmp 5700 Sequence Detection System (Applied Biosystems Incorporated) for detection and relative quantification of SP-A and SP-D mRNA targets. Results were gathered by the GeneAmp 5700 SDS software and processed using custom Excel files. In addition, the real-time mRNA data was normalized to ovine ribosomal protein S15 mRNA.
IHC Analysis
Surfactant protein A immunohistochemical staining have been described previously in detail by our laboratory.12 Briefly, upon collection, the lung tissue was fixed in 10% buffered formalin for 24 hr. Prior to IHC run, 3 μm thick tissue sections were placed on glass slides. The glass slides were deparaffinized and rehydrated followed by antigen retrieval, a protein blocking procedure, SP-A specific primary antibody incubation (Mouse IgM anti-human SP-A, Chemicon International, Inc., Temecula, CA), secondary antibody application (biotinylated Rat anti-Mouse Isotype Rat (LOU) IgG2a, B.D. Pharmingen, San Diego, CA), streptavidin-conjugated horseradish peroxidase incubation (BioGenex, San Ramon, CA) and Nova Red substrate (Vector, Burlingame, CA) development. All slides were counter-stained with Shandon’s - strength hematoxylin (Shandon-Lipshaw, Pittsburgh, PA), dehydrated through a series of graded solvents, and cover-slipped using Permount (Fisher, Hanover, IL) according to standard IHC procedure. For IHC scoring, five fields of each lung section were assessed for intensity and distribution of SP-A-stained epithelial cells in the terminal bronchioles and bronchiolar/alveolar junctions. Scoring was based on a pre-determined scale; 0 = no staining of cells; 1 = <30% of epithelial cells stained with minimal detectable intracytoplasmic staining; 2 = 30–60% of epithelial cells stained with minimal detectable intra-cytoplasmic staining; 3 = 30–60% of epithelial cells stained with >50% of cytoplasm stained; 4 = >60% of epithelial cells stained with >50% of cytoplasm stained.
Western Blot Analysis
Fresh, frozen lung tissues (0.1 g) collected from the lateral portion of the right, cranial lung lobe were homogenized in 2 ml T-PER® Tissue Protein Extraction Reagent (Pierce, Rockford, IL) containing Complete TM Protease Inhibitor Cocktail (Santa Cruz Biotechnology, Santa Cruz, CA). The tissue homogenates were centrifuged at 13, 000 rpm for 5 min at 4°C and supernatant (protein extract) was collected. The sodium dodecyl sulfate (SDS) sample buffer was added and samples were boiled for 5 min. The protein extracts were loaded onto polyacrylamide gel (PAG, 8–16%, Precise TM Protein gels, Thermo Scientific, Rockford, IL) for protein separation and then transferred to polyvinylidene fluoride transfer membrane (PVDF transfer membrane, Millipore, Bedford, MA). The membrane was blocked with 5% non-fat dry milk in 1 × TBS, after which the membrane was incubated overnight at 4°C with the primary polyclonal rabbit anti-bovine SP-A antibodies (Chemicon International, Inc.) or primary polyclonal rabbit anti-human SP-D antibodies (Santa Cruz Biotechnology). Both antibodies were diluted in 1×TBS, 0.05% Tween and 5% non-fat dry milk at 1:1,000. After rinsing three times for 5 min with 1×TBS, 0.05% Tween the membranes were further incubated for 90 min at room temperature with secondary Alexa Fluor 488 goat anti-rabbit normal IgG antibodies (Invitrogen, Carlsbad, CA) diluted in 1× TBS, 0.05% Tween at 1:2,000. To demonstrate equal loading of samples, a duplicate membrane was probed with mono-clonal mouse anti-β-actin antibodies (Sigma, St. Louis, MO) diluted in 1×TBS, 0.05% Tween at 1:5,000 and secondary antibody goat anti-mouse Alexa fluor 488 at 1:2,000. The fluorescence was detected by Typhoon imaging system (GE Healthcare, Baie d’Urfe, Quebec, Canada) using excitation wavelength 488 and 520 bp 40 emission filter.
Statistical Analysis
For qPCR and IHC data, summary statistics (mean and standard error of the mean) were calculated for each experimental group. Student’s t-tests (GraphPad Sigma software, San Diego, CA) were also performed on normalized qPCR and IHC data. The acceptable level of significance was P <0.05.
RESULTS
Sheep Cotinine Plasma Levels (CPL)
The cotinine in the maternal plasma was measured 6, 12, and 24 hr after patch placement. Six hours after patch placement, CPL ranged from 30 to 79 μg/ml with a mean of 49.8 ± 6.7 μg/ml. Twelve hours after patch placement, CPL ranged from undetectable (in 1 ewe) to 73 μg/ml with a mean of 45.4 ± 7.22 μg/ml. Twenty-four hours after patch placement, CPL ranged from undetectable (in 1 ewe) to 38 μg/ml with a mean of 30.0 ± 2.59 μg/ml (Table 1).
TABLE 1.
The Cotinine Plasma Levels (CPL) Measured in the Maternal Plasma 6, 12, and 24 hr After the Patch Placement in 6 ewes
| Ewe 1 | Ewe 2 | Ewe 3 | Ewe 4 | Ewe 5 | Ewe 6 | |
|---|---|---|---|---|---|---|
| 6 hr | 79 | 47 | 52 | 51 | 40 | 30 |
| 12 hr | 73 | 45 | 41 | 33 | 35 | <25 |
| 24 hr | 38 | 28 | 34 | 25 | 25 | <25 |
The CPL is expressed in μg/ml.
qPCR Results
Pregnant ewes exposed to nicotine during the last 6 weeks of gestation had significantly reduced (P = 0.01) SP-A mRNA expression in lungs of lambs to PTC lambs not exposed to nicotine. Although maternal exposure to nicotine exhibited a tendency toward decreased SP-D mRNA expression in PTN lambs when compared to PTC lambs, this difference was not statistically significant (P = 0.09). The results are presented in Figure 1. There were no significant changes in both, SP-A and SP-D mRNA expression between FTC and FTN lambs.
Fig. 1.
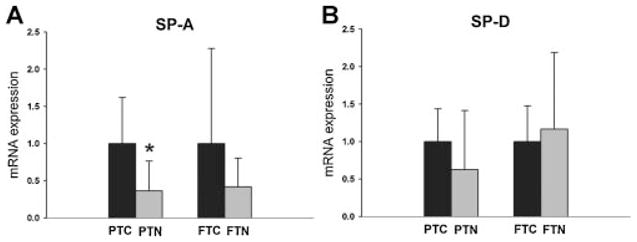
The relative SP-A mRNA and SP-D mRNA expression in PTC and FTC lambs as well as PTN and FTN lambs. The FTC and PTC values are equal to one. The PTN and FTN values are presented relative to the corresponding controls. The SP-A mRNA expression is significantly reduced (P = 0.01) in PTN lambs (n = 7 lambs) when compared to PTC lambs (n = 6 lambs). There is no significant change in SP-A mRNA expression between FTN and FTC lambs. Although maternal nicotine administration had a tendency to decrease the SP-D mRNA expression in the PTN lambs (n = 7 lambs) when compared to the PTC (n = 6 lambs), this difference was not statistically significant (P = 0.09). There is no significant change in SP-D mRNA expression between FTN and FTC lambs.
IHC Analysis Results
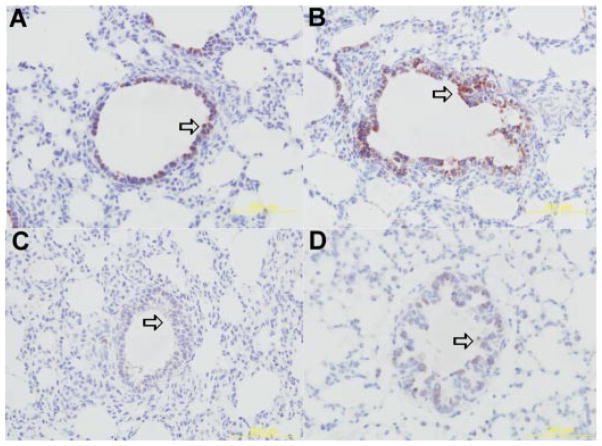
The SP-A antigen was detected within the cytoplasm of terminal bronchiolar and bronchiolar/alveolar junction epithelial cells in all four groups of lambs (Fig. 2). This cellular localization of SP-A is similar to previous studies.12 The mean score for each group was; PTC, 1.9 ±0.15; FTC, 2.88 ±0.39; PTN, 2.09 ±0.21; and FTN, 3.32 ±0.15. There was no statistical significance between FTC and FTN groups (P = 0.1) as well as PTC and PTN (P =0.08) groups.
Fig. 2.
Representative immunohistochemical detection of SP-A protein in bronchial/bronchiolar epithelium of FTC-(A), FTN-(B), PTC-(C), and PTN-(D) lambs. Arrows depict epithelial cells with SP-A protein expression. There was no statistically significant difference in SP-A level between PTN and PTC group. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Western Blot Results
The Western blot analysis detected a single ~35 kDa band that corresponds to the expected size of SP-A protein and ~43 kDa band that corresponds to the expected size of SP-D protein. The SP-A and SP-D proteins were detected in all four groups of lambs; PTC and PTN, FTC and FTN lambs. The SP-A bands were significantly less detectable in the PTN group when compared to PTC group (Fig. 3). This indicates decreased SP-A protein level in nicotine exposed, pre-term lambs when compared to the control group. The SP-A bands were similar in size in FTN group when compared with FTC group (Fig. 3). There was no difference in size of SP-D bands in PTN group when compared to PTC group or FTN group when compared to FTC group (Fig. 4).
Fig. 3.
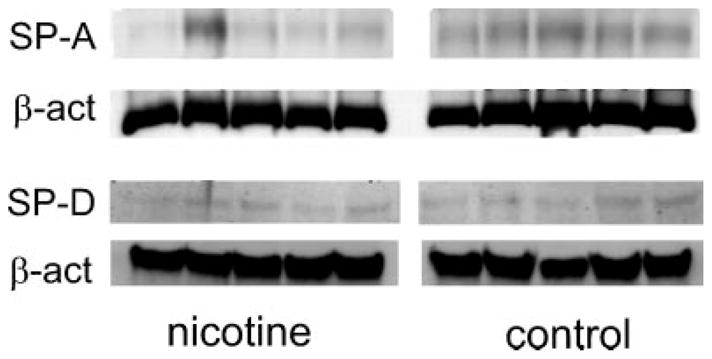
Representative Western blot detection of the SP-A and SP-D proteins in the lung tissue of the PTC and PTN lambs. The SP-A protein bands in PTN group are less detectable when compared to PTC group. There is no difference in SP-D protein bands between PTN and PTC group.
Fig. 4.
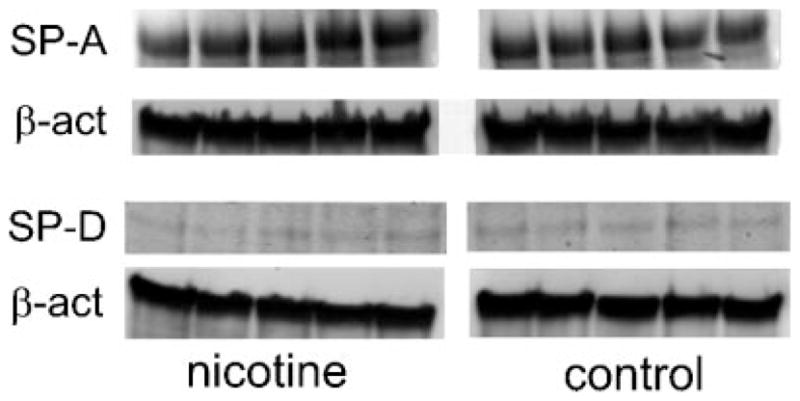
Representative Western blot detection of the SP-A and SP-D proteins in the lung tissue of the FTC and FTN lambs. There are no differences in SP-A and SP-D protein bands between FTN and FTC groups.
DISCUSSION
We found that maternal nicotine exposure during pregnancy significantly reduces SP-A gene expression and protein level in pre-term born lambs, while has no effect in naturally delivered, full-term lambs. It is likely that the additional gestational time allows more complete transcriptional activation or enhanced cellular differentiation and proliferation of SP-A producing cells in full-term lambs. Nicotine exposure did not alter SP-D gene expression and protein level in pre-term or full-term born lambs. The qPCR results show wide standard errors of mean. This may be explained by the fact that sheep used in this experiment are outbred. Individual genetic variability in these animals tends to be wide in contrast to commonly used experimental animals such are mice and rats, which are inbred and have less genetic variation within the same strains. Although the wide errors of mean may question the validity of qPCR data, the protein levels determined by Western blot analysis closely parallel the qPCR findings which confirm biological significance of changes determined at mRNA levels. The IHC analysis did not show any statistically significant difference in SP-A protein level in PTN lambs when compared to PTC group; the significant difference was not present between FTN and FTC groups. This finding does not closely match the Western blot results in which the SP-A protein level was reduced in nicotine-exposed lambs born pre-maturely compared to the controls. This may be explained by the fact that the IHC scoring system includes only proteins located within the intracellular compartment (intracytoplasmic SP-A) and does not take into account proteins located within the extracellular compartment (SP-A secreted into intraalveolar space). In contrast, Western blot analysis measures protein located in both, intra-cellular and extracellular compartments and therefore represents more sensitive and more reliable method of lung surfactant protein level determination.
Surfactant proteins are considered to be the key aspect of secretory lung innate immunity, and nicotine-induced suppressive effects may explain, in part, the increased susceptibility of pre-term born, nicotine-exposed neonates to lower respiratory infections. Although lung epithelia secrete many anti-microbial peptides, proteins, and molecules, SP-A and SP-D are among the most extensively studied pulmonary host defense/innate proteins. They are constitutively expressed and secreted by ATII cells and Clara cells and belong to a subgroup of collectin-containing mammalian C-type lectins, called collectins.13 SP-A and SP-D can bind, aggregate and opsonize many microorganisms including Gram-negative and Gram-positive bacteria, enveloped viruses like Influenza AVirus (IAV), RSV, non-enveloped viruses like Rotavirus and fungal organisms in order to protect lung from potential infection.13 In animal studies, the deficiency of SP-A, SP-D, or both were associated with increased susceptibility to pulmonary Pseudomonas aeruginosa,14 Streptococcal infections.15 Elevated levels of SP-A and SP-D mRNA in the lungs of neonatal lambs are associated with clearance of Parainfluenza virus-3 (PIV-3).13 Also, reduced clearance of RSV in lambs is associated with pre-maturity and inadequate expression of pulmonary collectins12 while SP-A has been shown to enhance RSV clearance in mice.16 In addition, when SP-A and SP-D were administered to the lung in a murine model of invasive pulmonary aspergillosis, the mortality rate of infected animals was significantly reduced.13 In rats, administration of SP-A reduces the oxidative damage of ventilated lungs.17
Published data on the influence of smoking and nicotine on ATII cells, lung surfactant, and surfactant proteins are conflicting. There are several reports clearly indicating that cigarette smoking or nicotine alone affects alveolar ATII cells and therefore, possibly lung surfactant metabolism. In a study published by Maritz and Thomas,18 intrauterine exposure of rat pups to nicotine resulted in increased proliferation of ATII cells. Nicotine-exposed ATII cells exhibited obvious mitochondrial swelling and damage which may have disrupted adequate cellular metabolism, including metabolism of lung surfactant and surfactant proteins. In the most recent study published by Rehan et al.,19 nicotine exposure accelerated ATII cell proliferation, differentiation and metabolism in rat embryonic lung and stimulated synthesis of surfactant lipids. However, in both of these studies the influence of nicotine on SP-A and SP-D was not investigated. A study conducted in healthy adult smokers showed reduced content of SP-A and SP-D in BAL fluid when compared to non-smokers.20 A similar study conducted in rats confirmed this finding since rats chronically exposed to cigarette smoke had significantly reduced SP-A and SP-C levels in BAL fluid.21 Several studies conducted in rats showed that maternal nicotine or cigarette smoke exposure alters the expression of surfactant proteins in neonatal pups. In one study, maternal nicotine exposure increased SP-A and SP-D expression in neonatal pups on postnatal day 7 and decreased SP-A and SP-D expression on postnatal day 14.22 Yet, in another study conducted in rats, maternal side-stream cigarette smoke exposure showed reduced levels of SP-A in BAL of pups on postnatal day 1 and increased levels of SP-A in BAL on postnatal day 21.23 However, there are reports that suggest that maternal smoking does not influence lung surfactant proteins of exposed neonates. For example, neonates from mothers who smoked during pregnancy did not show reduced levels of SP-A according to amniotic fluid measurements.24 There is also a report indicating that smoking in healthy individuals increases levels of SP-A when measured in serum.25 In addition, there is scientific evidence that pre-natal nicotine exposure has permanent detrimental effect on later, postnatal lung development and function. It has been shown that in utero exposure to nicotine influences the ‘‘programmed’’ lung aging and maintenance thus accelerates aging and pre-disposes lung to parenchymal damage.18
In addition, there is controversy related to the safety of nicotine patch use during pregnancy. Although the nicotine patch is highly recommended as nicotine replacement therapy in pregnant women25 during the smoking cessation, to our knowledge there are no studies conducted to evaluate the effect of nicotine patch on developing fetus. In this study, we have shown that nicotine delivered to the mother by nicotine patch does have an undesirable effect on fetal lung epithelial development.
Differences of the above studies with the lamb model are likely related to differences in animal lung maturation at the various stages of lung development, duration of nicotine or cigarette smoke exposure, as well as in the techniques used to determine surfactant protein levels. Although cigarette smoking during pregnancy remains a significant cause of neonatal mortality and morbidity, previous studies frequently utilized rodents as animal models which do not adequately parallel fetal lung development in humans. This is especially relevant in alveolar and pulmonary surfactant development research area. At birth, the lung of rodents is in saccular stage and alveoli with lung surfactant develop during first 3 weeks of postnatal life.26 In contrast, gestating ewes and their offspring are well-suited for these studies since: (1) alveolar development in lambs occurs pre-natally as in humans26 (2) lambs can be derived pre-term and survive, unlike many other animal models,12 (3) ovine respiratory epithelia of airways, distal bronchioles and alveoli are similar to those of human lung including similar numbers of Clara cells (which can reach 50% of the epithelium of mice), and the ovine lung has submucosal glands not present in some animal models,10,27 (4) expression of SP-A and SP-D and other innate immune genes of ovine lung are well-characterized and are very similar to human orthologs,10 and (5) pre-term lambs are susceptible to RSV, a known pathogen of pre-mature infants, and maternal cigarette smoking is a risk for pre-term birth. Finally, sheep have long been used for respiratory studies related to human disease as well as for studying the effects of nicotine on pulmonary function.
Nicotine is the major cigarette constituent that crosses the placenta and accumulates in the fetal compartment to influence fetal lung growth and development.9,28
The transdermal patch as a nicotine delivery method was used for several reasons. First, when fitted to the prepared skin area, the patch slowly and constantly releases nicotine for at least a 12 hr time period, which closely resembles the smoking habit in humans. In contrast, other nicotine delivery techniques such as intravenous administration and nicotine releasing subcutaneous pumps are invasive and may result in excessive nicotine blood levels which are difficult to control. Second, according to several published studies, nicotine metabolism in pregnant women is significantly accelerated compared to non-pregnant females and males.29 Also, pregnant women tend to quit or at least significantly reduce the number of cigarettes smoked per day. According to a study published by Peacock et al.,30 pregnant women who smoked during the last trimester of pregnancy most frequently had CPL ranging from 15–75 ng/ml. Similar levels were present in maternal blood in this study. In addition, depending on cigarette brand and strength, this plasma cotinine level would be equivalent to 2–6 cigarettes smoked per day. Lastly, although administering nicotine during entire length of gestation would more closely mimic the human behavior, we have chosen to administer nicotine during the last 6 weeks of ovine gestation since this time frame corresponds to the time frame of pulmonary surfactant development in fetal lambs.31
In conclusion, nicotine exposure during the last trimester of pregnancy in sheep is associated with a significant reduction in SP-A gene expression and protein level in lambs born pre-term. This finding may have clinical significance and will allow us to further investigate RSV infection as a means to address the hypothesis that nicotine exposure during pregnancy may increase RSV susceptibility in human infants. To our knowledge, there are no publications describing similar animal model in lung development research area. In this work we have described an innovative animal model that may be used by broad research community focused on neonatal nicotine-related pathological processes such as respiratory distress syndrome, impaired intrauterine lung growth, and other pulmonary abnormalities during postnatal life.
Acknowledgments
This work was funded, in part, by JG Salsbury Endowment and NIH NIAID grant R01AI062787. The authors would like to thank other individuals involved with the project including Maria Dimitrova, Rachel J. Derscheid, Pam Knake, Francis Zacharakis-Jutz, and Beth Hafkey.
Grant sponsor: JG Salsbury Endowment. Grant sponsor: NIH NIAID; Grant number: R01AI062787.
ABBREVIATIONS
- SP-A
surfactant protein A
- SP-D
surfactant protein D
- FTC
full-term control group
- PTC
pre-term control group
- FTN
full-term nicotine-exposed group
- PTN
pre-term nicotine-exposed group
- qPCR
fluorogenic real-time quantitative polymerase chain reaction
- IHC
immunohistochemistry
- ATII
alveolar type II cell
- RSV
respiratory syncytial virus
- CPL
cotinine plasma level
- SDS
sodium dodecyl sulfate
- PAG
polyacrylamide gel
- PVDF transfer membrane
polyvinylidene fluoride transfer membrane
Footnotes
This paper was presented in part as a poster at 2008 American College of Veterinary Pathologists annual meeting, San Antonio, Texas.
References
- 1.Thompson AB, Robbins RA, Romberger DJ, Sisson JH, Spurzem JR, Teschler H, Rennard SI. Immunological functions of the pulmonary epithelium. Eur Respir J. 1995;8:127–149. doi: 10.1183/09031936.95.08010127. [DOI] [PubMed] [Google Scholar]
- 2.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 3.Brogden KA, Ackermann M, McCray PB, Jr, Tack BF. Antimicrobial peptides in animals and their role in host defences. Int J Antimicrob Agents. 2003;22:465–478. doi: 10.1016/s0924-8579(03)00180-8. [DOI] [PubMed] [Google Scholar]
- 4.Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, Bernal AL, Reid KB, Madan T, Chakraborty T. Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol. 2006;43:1293–1315. doi: 10.1016/j.molimm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 5.King RJ, Ruch J, Gikas EG, Platzker AC, Creasy RK. Appearance of paoproteins of pulmonary surfactant in human amniotic fluid. J Appl Physiol. 1975;39:735–741. doi: 10.1152/jappl.1975.39.5.735. [DOI] [PubMed] [Google Scholar]
- 6.DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics. 2004;113:1007–1015. [PubMed] [Google Scholar]
- 7.Kyrklund-Blomberg NB, Granath F, Cnattingius S. Maternal smoking and causes of very preterm birth. Acta Obstet Gynecol Scand. 2005;84:572–577. doi: 10.1111/j.0001-6349.2005.00848.x. [DOI] [PubMed] [Google Scholar]
- 8.Ward C, Lewis S, Coleman T. Prevalence of maternal smoking and environmental tobacco smoke exposure during pregnancy and impact on birth weight: retrospective study using Millennium Cohort. BMC Public Health. 2007;7:81. doi: 10.1186/1471-2458-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pastrakuljic A, Schwartz R, Simone C, Derewlany LO, Knie B, Koren G. Transplacental transfer and biotransformation studies of nicotine in the human placental cotyledon perfused in vitro. Life Sci. 1998;63:2333–2342. doi: 10.1016/s0024-3205(98)00522-0. [DOI] [PubMed] [Google Scholar]
- 10.Kawashima K, Meyerholz DK, Gallup JM, Grubor B, Lazic T, Lehmkuhl HD, Ackermann MR. Differential expression of ovine innate immune genes by preterm and neonatal lung epithelia infected with respiratory syncytial virus. Viral Immunol. 2006;19:316–323. doi: 10.1089/vim.2006.19.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallup JM, Ackermann MR. Addressing fluorogenic real-time qPCR inhibition using the novel custom Excel file system ‘FocusField2-6GallupqPCRSet-upTool-001’ to attain consistently high fidelity qPCR reactions. Biol Proced Online. 2006;8:87–152. doi: 10.1251/bpo122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyerholz DK, Grubor B, Fach SJ, Sacco RE, Lehmkuhl HD, Gallup JM, Ackermann MR. Reduced clearance of respiratory syncytial virus infection in a preterm lamb model. Microbes Infect. 2004;6:1312–1319. doi: 10.1016/j.micinf.2004.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grubor B, Meyerholz DK, Ackermann MR. Collectins and cationic antimicrobial peptides of the respiratory epithelia. Vet Pathol. 2006;43:595–612. doi: 10.1354/vp.43-5-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeVine AM, Kurak KE, Bruno MD, Stark JM, Whitsett JA, Korfhagen TR. Surfactant protein-A-deficient mice are susceptible to Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol. 1998;19:700–708. doi: 10.1165/ajrcmb.19.4.3254. [DOI] [PubMed] [Google Scholar]
- 15.LeVine AM, Bruno MD, Huelsman KM, Ross GF, Whitsett JA, Korfhagen TR. Surfactant protein A-deficient mice are susceptible to group B streptococcal infection. J Immunol. 1997;158:4336–4340. [PubMed] [Google Scholar]
- 16.LeVine AM, Gwozdz J, Stark J, Bruno M, Whitsett J, Korfhagen T. Surfactant protein-A enhances respiratory syncytial virus clearance in vivo. J Clin Invest. 1999;103:1015–1021. doi: 10.1172/JCI5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey TC, Maruscak AA, Petersen A, White S, Lewis JF, Veldhuizen RA. Physiological effects of oxidized exogenous surfactant in vivo: effects of high tidal volume and surfactant protein A. Am J Physiol Lung Cell Mol Physiol. 2006;291:L703–L709. doi: 10.1152/ajplung.00538.2005. [DOI] [PubMed] [Google Scholar]
- 18.Maritz GS, Thomas RA. Maternal nicotine exposure: response of type II pneumocytes of neonatal rat pups. Cell Biol Int. 1995;19:323–331. doi: 10.1006/cbir.1995.1075. [DOI] [PubMed] [Google Scholar]
- 19.Rehan VK, Wang Y, Sugano S, Santos J, Patel S, Sakurai R, Boros LW, Lee WP, Torday JS. In utero nicotine exposure alters fetal rat lung alveolar type II cell proliferation, differentiation, and metabolism. Am J Physiol Lung Cell Mol Physiol. 2007;292:L323–L333. doi: 10.1152/ajplung.00071.2006. [DOI] [PubMed] [Google Scholar]
- 20.Honda Y, Takahashi H, Kuroki Y, Akino T, Abe S. Decreased contents of surfactant proteins A and D in BAL fluids of healthy smokers. Chest. 1996;109:1006–1009. doi: 10.1378/chest.109.4.1006. [DOI] [PubMed] [Google Scholar]
- 21.Subramaniam S, Whitsett JA, Hull W, Gairola CG. Alteration of pulmonary surfactant proteins in rats chronically exposed to cigarette smoke. Toxicol Appl Pharmacol. 1996;140:274–280. doi: 10.1006/taap.1996.0222. [DOI] [PubMed] [Google Scholar]
- 22.Chen CM, Wang LF, Yeh TF. Effects of maternal nicotine exposure on lung surfactant system in rats. Pediatr Pulmonol. 2005;39:97–102. doi: 10.1002/ppul.20122. [DOI] [PubMed] [Google Scholar]
- 23.Subramaniam S, Srinivasan S, Bummer PM, Gairola CG. Perinatal sidestream cigarette smoke exposure and the developing pulmonary surfactant system in rats. Hum Exp Toxicol. 1999;18:206–211. doi: 10.1191/096032799678839923. [DOI] [PubMed] [Google Scholar]
- 24.Hermans C, Libotte V, Robin M, Clippe A, Wattiez R, Falmagne P, Langhendries JP, Bernard A. Maternal tobacco smoking and lung epithelium-specific proteins in amniotic fluid. Pediatr Res. 2001;50:487–494. doi: 10.1203/00006450-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Kida K, Oda H, Yamano Y, Kagawa J. Effects of cigarette smoking on the serum concentration of lung surfactant protein A (SP-A) Eur Respir J. 1997;10:2124–2126. doi: 10.1183/09031936.97.10092124. [DOI] [PubMed] [Google Scholar]
- 26.Harding RPK, Plopper CG, editors. The lung development, aging and the environment. London: Elsevier Acadenic Press; 2004. p. 403. [Google Scholar]
- 27.Meyerholz DK, Grubor B, Lazic T, Gallup JM, de Macedo MM, McCray PB, Jr, Ackermann MR. Monocytic/macrophagic pneumonitis after intrabronchial deposition of vascular endothelial growth factor in neonatal lambs. Vet Pathol. 2006;43:689–694. doi: 10.1354/vp.43-5-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nekhayeva IA, Nanovskaya TN, Pentel PR, Keyler DE, Hankins GD, Ahmed MS. Effects of nicotine-specific antibodies, Nic311 and Nic-IgG, on the transfer of nicotine across the human placenta. Biochem Pharmacol. 2005;70:1664–1672. doi: 10.1016/j.bcp.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 29.Hegaard HK, Kjaergaard H, Moller LF, Wachmann H, Ottesen B. Determination of a saliva cotinine cut-off to distinguish pregnant smokers from pregnant non-smokers. Acta Obstet Gynecol Scand. 2007;86:401–406. doi: 10.1080/00016340601147517. [DOI] [PubMed] [Google Scholar]
- 30.Peacock JL, Cook DG, Carey IM, Jarvis MJ, Bryant AE, Anderson HR, Bland JM. Maternal cotinine level during pregnancy and birthweight for gestational age. Int J Epidemiol. 1998;27:647–656. doi: 10.1093/ije/27.4.647. [DOI] [PubMed] [Google Scholar]
- 31.Mescher EJ, Platzker AC, Ballard PL, Kitterman JA, Clements JA, Tooley WH. Ontogeny of tracheal fluid, pulmonary surfactant, and plasma corticoids in the fetal lamb. J Appl Physiol. 1975;39:1017–1021. doi: 10.1152/jappl.1975.39.6.1017. [DOI] [PubMed] [Google Scholar]