Abstract
Thiazolidinediones (TZDs) may prevent or attenuate CNS injury due to an ischemic event. We performed meta-analysis of experimental studies in which a TZD (either rosiglitazone or pioglitazone) was administered in a rodent model of focal or global cerebral ischemia. Infarct volume was the primary endpoint for analysis of drug efficacy, and neurological outcome was also assessed. We identified 31 studies through the use of PubMed and Embase, 22 of which met our pre-specified inclusion criteria and were analyzed with the Cochrane Review Manager software. Treatment with TZDs decreased infarct volume and improved neurological outcome regardless of study quality, dose timing, or ischemia model (transient or permanent). Rosiglitazone and pioglitazone were similarly effective in reducing infarct volume and protecting neurologic function. Importantly, the collective data suggest that pretreatment with a TZD is not required for neuroprotection, although additional studies are clearly needed to define the breadth of the therapeutic window. The data warrant further studies into the potential acute use of TZDs for ischemic stroke therapy in the general population.
Keywords: ischemic stroke, meta-analysis, pioglitazone, rosiglitazone, systematic review, thiazolidinedione
Thiazolidinediones (TZDs), including rosiglitazone and pioglitazone, are FDA-approved insulin-sensitizing agents used in the treatment of type 2 diabetes (Barnett, 2009). These agents were developed as agonists of peroxisome proliferator-activated receptor gamma (PPARγ), a transcription factor involved in regulating cell differentiation and metabolism (Michalik et al, 2006). Patients with diabetes are at high risk of macrovascular events and epidemiological data indicate that pioglitazone reduces the number of macrovascular events including stroke in type 2 diabetic patients (Dormandy et al, 2005). In contrast, similar data on chronic administration of rosiglitazone suggest a modest increase in cardiovascular risk (Nissen and Wolski, 2007; Graham et al, 2010) sufficient to prompt additional safety warnings that are the topic of ongoing controversy1. TZDs have also been observed to inhibit inflammation due to ischemia and decrease various inflammatory markers independently of their effects on glycemic control (Luo et al, 2006; Sundararajan et al, 2005). In vitro studies have shown that neuronal PPARγ can contribute to neuroprotection and that TZDs can prevent neuronal apoptosis induced by LPS (Luna-Medina et al. 2005). In response to oxidative injury, pioglitazone decreases COX-2 expression and prevents neuronal death (Zhao 2006). PPARγ ligands reduce the secretion of neurotoxic molecules (i.e. NO, TNF-α, IL-6) that follows macrophage and microglia activation due to ischemia (Lee 2000, Luna-medina 2005). The in vitro neuroprotective effects of TZDs are linked to increased PPARγ DNA binding and PPARγ antagonists reverse these effects (Zhao 2006b).
Pioglitazone and rosiglitazone are currently being investigated in ongoing as well as closed clinical trials for neuroprotection in patients with type 2 diabetes or metabolic syndrome. In these studies, stroke and cognition are among the endpoint measures (see http://clinicaltrials.gov: clinical trial numbers NCT00095654, Dream Trial Investigators, 2008; NCT00636766, Kadaglou et al, 2010; NCT00212290, NCT00736996; NCT00879970; NCT00242593). In addition, there is an ongoing study of the neuroprotective efficacy of pioglitazone in non-diabetic patients in hemorrhagic stroke (NCT00827892). There is an increased incidence of Alzheimer's Disease in individuals with type 2 diabetes (Craft 2006). This observation has led to pilot studies for the use of pioglitazone in patients with type 2 diabetes and Alzheimer's Disease or mild cognitive impairment (Hanyu et al, 2009). Additional trials are investigating pioglitazone administration in normoglycemic patients with mild cognitive impairment (NCT00242593) and in depressive disorders in individuals with or without diabetes (McIntyre et al, 2007; NCT01109030; NCT00671515, NCT00835120, Kemp et al, 2009; NCT00242619, Rasgon et al, 2010). The anti-inflammatory properties of pioglitazone prompted a pilot study that has reported promising results for its use in multiple sclerosis patients (Shukla et al, 2010; NCT00242177). Further, pioglitazone is currently being tested in a small cohort of Friedreich's Ataxia patients (NCT00811681). However, a trial in amyotrophic lateral sclerosis (NCT00690118) was halted early due to a lack of efficacy at interim analysis.
Thus, the breadth of indications in which TZDs are being tested in humans has broadened beyond insulin resistant, hyperlipidemic conditions. The purpose of this review is to evaluate the preclinical data on the use of rosiglitazone and pioglitazone in stroke models to collectively assess and compare their ability to reduce infarct volume and improve neurological outcome in normoglycemic animals. In particular, we analyze the available preclinical data on the ability of TZDs to provide protection without pretreatment, in order to more aptly address the potential for their acute use in stroke therapy.
Experimental procedures
Study Identification
To identify preclinical studies investigating the use of thiazolidinediones in focal or global cerebral ischemia, literature searches were conducted in PubMed and Embase for all published articles up until July 1st, 2010. Keywords included combinations of thiazolidinedione (TZD), rosiglitazone or pioglitazone with stroke, ischemic brain injury, cerebral ischemia, or middle carotid artery occlusion (MCAO). Reference lists from the resulting publications and reviews were used to identify further relevant publications. To prevent bias, inclusion criteria were pre-specified as the following: (i) experimental ischemic stroke was induced in rodents by transient stroke (transient MCAO or embolic stroke), permanent MCAO, or transient global ischemia (bilateral CAO), (ii) rosiglitazone or pioglitazone was administered, (iii) there was no administration of other agents with potentially neuroprotective effects, (iv) infarct volume was assessed, and (v) a control group was included in the study design. Unlike infarct volume, assessment of neurological outcome was not required for inclusion in this study, but was evaluated as an outcome measure in studies also reporting infarct volume. We chose to only evaluate studies of rosiglitazone and pioglitazone, as these are currently the only FDA-approved thiazolidinediones on the market.
Data extraction
Data on infarct size and neurological outcomes from vehicle- and TZD-treated animals were extracted from all included studies. The effects of rosiglitazone and pioglitazone treatment on infarct volume and neurological deficits were compared to control groups of animals treated with vehicle prior to, during, or after induction of cerebral ischemia. Extracted data included the mean infarct size (expressed as mm3 or percent of normal brain) or mean neurological score. In addition to the mean values, the standard deviation and the number of animals per treatment group were also extracted. Many studies had multiple groups to facilitate dose responses or time course evaluations; the data from these experiments were extracted from each group as individual values. Data presented graphically rather than reported in the text were obtained from enlarged versions of the graphs. In all included studies, edema was measured and was reported as not significant or was used to correct for infarct volume. Data for neurological outcome were extracted from various neurological tests including: (i) neurological score (subjective sensory motor assessment), (ii) modified limb placement test, (iii) rotarod latency, (iv) tightrope test for forelimb and hindlimb grasping, (v) paw placement cylinder test, (vi) wire hang test, (vii) adhesive tab removal, and (viii) incline test, as documented in Table I. If ischemic duration was insufficient to induce a loss in neurologic function in a particular test, the data were not included. Data were not extracted if mean neurologic scores were not reported (i.e. if only median and confidence intervals were given).
Table I. Characteristics of studies included in the review.
| Study | Parameters assessed1 | TZD (dose) | First dose timing | Species2 | Model3 | Route of drug administration4 |
|---|---|---|---|---|---|---|
| Allahtavakoli et al., 2006 | infarct volume, % (48h) | Rosi (0.033, 0.1, 0.3, 1mg/kg) | -1h, 0h,or+4h | Male WR | embolic stroke | i.p. |
| Allahtavakoli et al., 2007 | infarct volume, % (48h) | Rosi (0.1 mg/kg) | Oh | Male WR | embolic stroke | i.p. |
| Allahtavakoli et al., 2009 | infarct volume, % (72h) | Rosi (5 mg/kg) | +24h | Male WR | embolic stroke | i.p. |
| Chu et al.,2006 | infarct volume, % (24h); MLPT (1,7,14,21,28,35d) | Rosi (0.3, 3 mg/kg) | -7d | Male SD | MCAO (t) 90 min | p.o. |
| Fatehi-Hassanabad & Tasker, 2010 | infarct volume, % (1,3,7,14d); TT (6,24,72h), PPT & RR (6h,1,3,7,14d) | Rosi (5, 10 mg/kg) | -10min | Male CD1 mice | BCAO (t) 45 min | i.p. |
| Glatz et al, 2010 | infarct volume, mm3 (24,48h); SSMA (24,48h) | Pio (3nmol/hour) | -6d | Male WR | MCAO (t) 90 min | i.c.v. |
| Ji et al., 2009 | infarct volume, mm3(72h) | Pio (20 mg/kg) | Oh or+1d | 129/SV mice | MCAO (t) | i.p. |
| Lee et al., 2009 | infarct volume, mm3(24h) | Pio (40 mg/kg/day) | -3d | Male C57BL/6 mice | MCAO (t) | p.o. |
| Luo et at., 2006 | infarct volume, mm3 (2,7d); SSMA & CT (7d) | Rosi (0.75 -12 mg/kg) | -1h | Male C57BL/6 mice | MCAO (t) 90 min | i.p. |
| Nategh et al. 2010 | infarct volume, %(24h) | Rosi (1mg/kg) | Oh | Male WR | embolic stroke | i.p. |
| Pereira et al.,2006 | infarct volume, mm3 (48h); SSMA (48h) | Rosi (1-3 mg/kg) | +10min or+2h | Male FR | MCAO (p) | i.p. |
| Schmerbach et al., 2008 | infarct volume, mm3 (48h); SSMA (24,48h) | Pio (2,20 mg/kg/day) | -5d | Male WR | MCAO (t) 90 min | p.o. |
| Schock et al., 2008 | infarct volume, % (72h) | Rosi (6mg/kg) | Oh | Male mice | MCAO (t) 60 min | i.p. |
| Shimazu et al., 2004 | infarct volume, mm3 (24h); SSMA-U (24h) | Pio (20 mg/kg/day) | -72h | Male SD | MCAO (t) 90 min, MCAO (p) | feed |
| Sobrado et al., 2009 | infarct volume, mm3 (48h & 7d); SSMA (48h), WH (48h) | Rosi (1,3 mg/kg) | +10min | Male FR, B6;129F2/J mice | MCAO (p) | i.p. |
| Sundararajan et al., 2005 | infarct volume, % (24h & 22d); SSMA (1-22d), ATR (1-22d), IT(1-22d) | Pio (1mg/kg) | -24h | Male WR Male SD, SHR, C57BL/6, SV129, db/db, S2/SvPasCrl | MCAO (t) 2h | i.p. |
| Tureyen et al., 2007 | infarct volume, mm3(72h),SSMA (72h) | Rosi, Pio (0.5-6 mg/kg) | -4h through+6h | mice | MCAO (t) 2h | i.p. |
| Victor et al., 2006 | infarct volume, %(24h) | Pio, Rosi (1 & 0.1 mg/kg) | -24h | Male WR | MCAO (t) 2h | i.p. |
| Wang et al., 2009 | ||||||
| Experiment 1 | infarct volume, % (48h); SSMA-U | Rosi (2,10 mg/kg/day) | -2wk | Male WR | embolic stroke | p.o. |
| Experiment 2 | infarct volume, % (48h) | Rosi (2 mg/kg/day) | +1,3, or 6h | Male WR | embolic stroke | p.o. |
| Wu et al., 2008 | ||||||
| Experiment 1 | infarct volume, mm3(24h) | Rosi (0.2-500 ng) | Oh | Long Evans | BCAO (t) 30 min | i.c.v. |
| Experiment 2 and 3 | infarct volume, mm3(24h) | Rosi (50ng) | +2h | Long Evans | BCAO (t) 30 min | i.c.v. |
| Zhao et al., 2005 | infarct volume, mm3 (48h); SSMA (24, 48h) | Pio (3nmol/hour) | -5d | Male WR | MCAO (t) 90 min | i.c.v. |
| Zhao et al., 2006 | infarct volume, mm3(48hr) | Pio (3nmol/hour) | -5d | Male WR | MCAO (t) 90 min | i.c.v. |
MLPT= modified limb placement test; TT = tightrope test for forelimb & hindlimb grasping; PPT= paw placement cylinder test; RR = rotarod latency; CT= Corner Test (no. of left turns); SSMA= subjective sensory motor assessment; SSMA-U = SSMA data was presented, but mean values were not extractable for meta-analysis; WH = wire hang test (latency to fall); ATR = adhesive tab removal; IT = Incline test
WR=Wistar rat; SD=Sprague Dawley rat; SHR=Spontaneously hypertensive rat
MCAO=middle cerebral artery occlusion; BCAO=bilateral carotid artery occlusion; (t)=transient; (p)=permanent
i.p.=intraperitoneaI injection; p.o.=by mouth (oral gavage); i.c.v.= intracerebroventricular injection (by Alzet pump); feed=drug formulated into peanut butter
An eight-point scale was used to determine the quality of each of the included studies based on their stated methodology (Gibson et al, 2008). Written evidence was required in order for a study to receive one point in each of the following categories: randomization of treatment groups, assessment of physiological measurements, dose-response evaluation, time course for treatment, investigator blinding, outcome measurements at days 1-3, outcome measurements beyond day 3, and measurement of both infarct size and neurological outcome.
Data analysis
Cochrane Review Manager (version 5) and the statistical package therein (fixed effect model) was used to analyze the extracted data. A one-way ANOVA with a post hoc Bonferroni test was used to determine statistically significant effects among the routes of drug administration. The standardized mean difference was calculated and applied to assess the effect of rosiglitazone and pioglitazone on infarct volume or neurological outcome compared to vehicle treated controls. By this method, the difference between each control and treatment group is divided by the standard deviation of the data. This accounts for the fact that measurements may have been taken differently in each experiment (i.e. mm3 vs. percent for infarct volume and neurological score vs. other tests for neurological outcome) and that different animal models may have been employed. Statistical heterogeneity was accounted for through the use of the DerSimonian and Laird (1986) pooling model of random effects. The experiments were grouped and analyzed based on (i) outcome (infarct volume or neurological deficits), (ii) methodological quality, (iii) type of TZD administered (rosiglitazone or pioglitazone), (iv) timing of first dose (prior to or following ischemia), or (v) type of model (transient or permanent). For those tests in which an increase in nominal value represented an improvement in outcome (for instance, in the wire hang test), the inverse of the extracted data was used for data comparisons.
Results
Design of Studies
Based on our search criteria, we identified 31 studies that investigated the use of TZDs in an animal model of cerebral ischemia. The earliest study was published in 2004 (Shimazu). Seven of these studies were excluded because they did not measure infarct volume and one study was excluded because the injury model was traumatic brain injury rather than focal or global ischemia (Yi et al, 2008). Out of the final 22 included studies (Table I; Allahtavakoli et al 2006, 2007, 2009; Fatehi-Hassanabad & Tasker, 2010; Glatz et al, 2010; Ji et al, 2009; Lee et al, 2009; Luo et al, 2006; Nategh et al 2010; Pereira et al, 2006; Schmerbach et al, 2008; Schock et al, 2008; Shimazu et al, 2004; Sobrado et al, 2009; Sundurarajan et al, 2005; Tureyen et al, 2007; Victor et al, 2006; Wang et al 2009; Wu et al, 2008; Zhao et al, 2005; and Zhao et al, 2006), 15 employed the use of an animal model of transient MCAO, 5 performed studies of embolic stroke (Allahtavakoli et al 2006, 2007, 2009; Chu et al, 2006, Nategh et al 2010; and Wang et al 2009) and 2 used transient bilateral carotid artery occlusion (Fatehi-Hassanabad and Tasker, 2010; Wu et al, 2008).
All studies compared administration of either rosiglitazone or pioglitazone to a vehicle-treated group of animals and measured mean infarct volume in mm3 or as a percent of either total brain volume or the control hemisphere. The included studies came from 19 independent research groups and data were analyzed from 1348 experimental subjects for infarct volume measurements. Ten of the included studies, from nine independent groups, included measurements of neurological function in 939 experimental subjects. Experimental models for assessment of neurological outcome included subjective neurological scoring as well as objective tests for motor and sensory performance (Table I).
Male rat models (Wistar, Sprague-Dawley, Fisher, Long Evans) were used in 17 of the studies while the remaining 5 used male mouse models (C57BL/6, CD1, 129/SV), though primary data on infarct volume and neurological outcome may not have been measured or reported from all of the included animal models (Table 1). In 18 of the studies the TZD was administered either orally or intraperitoneally. Four studies administered the TZD intracerebroventricularly using Alzet pumps while one mixed the drug into peanut butter (Shimazu et al, 2004). The timing of the first dose ranged from 2 weeks prior to ischemic insult to 1 day following injury. Fourteen studies began administration of the drug prior to injury while 10 started treatment with the TZD either at the time of ischemia or thereafter. Rosiglitazone was administered in 13 of the studies and pioglitazone was used in the remaining 9 studies (Table I).
Overall Efficacy of Drug Treatment
There was a significant protective effect of TZD treatment on infarct size (1.54, 1.40 to 1.68, p<0.00001) and neurological outcome (1.19, 1.04 to 1.34, p<0.00001) across all models (Figure 1). The nature of this statistical approach is to compare the standardized mean difference between drug treatment and vehicle across all studies. A standardized mean difference of zero represents a lack of drug effect, whereas a positive value indicates a beneficial effect, and a negative mean difference reflects a detrimental effect of the drug.
Fig. 1.
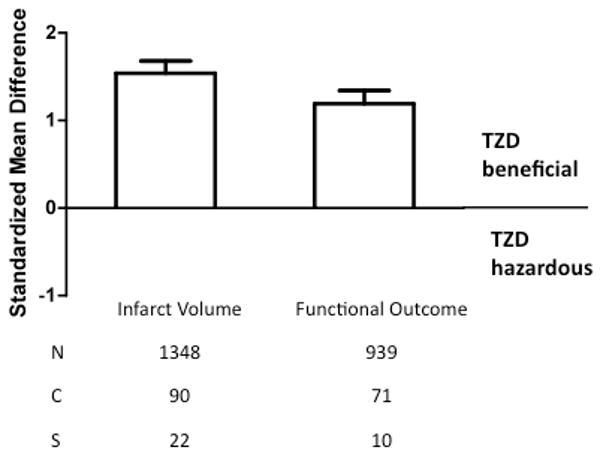
Standardized mean difference and 95% confidence interval by outcome measure. A beneficial effect of TZD (rosiglitazone or pioglitazone) was detected on infarct size and neurologic function (p<0.00001) as reported in the publications listed in Table I. N=number of animals; C=number of comparisons; S=number of published studies.
Reported study quality
The quality scores ranged from 1-8 with a mean of 4.3. Analysis of studies based upon individual quality scores was not very meaningful as scores unevenly distributed over the scoring range; for instance, no studies had a quality score of 3 or 7. Therefore, data from experiments with a study quality of 1-4 were grouped together and compared to those with a study quality of 5-8. Infarct volume was reduced to a similar degree regardless of study quality (χ2=1.91, df=1, p=0.17). However, studies with a quality rating of 1-4 were less likely to show efficacy of TZD treatment for improvements in neurological outcome than those with a study quality of 5-8 (χ2=14.01, df=1, p<0.0002).
Benefits of rosiglitazone vs. pioglitazone
Rosiglitazone and pioglitazone were analyzed separately for their effects on infarct volume and neurological deficits (Fig. 3). Rosiglitazone significantly decreased lesion volume (effect size=1.55, C.I.=1.39 to 1.72, p<0.00001) and improved neurological outcome (1.19, 0.99 to 1.38, p<0.00001). Pioglitazone also reduced lesion size (1.50, 1.24 to 1.76, p<0.00001) and improved neurological outcome (1.2, 0.95 to 1.44, p<0.00001). There was no difference in the effectiveness of the two different drugs with regard to either infarct volume (χ2=0.12, df=1, p=0.73) or neurological deficits (χ2=0.00, df=1, p=0.95).
Fig. 3.
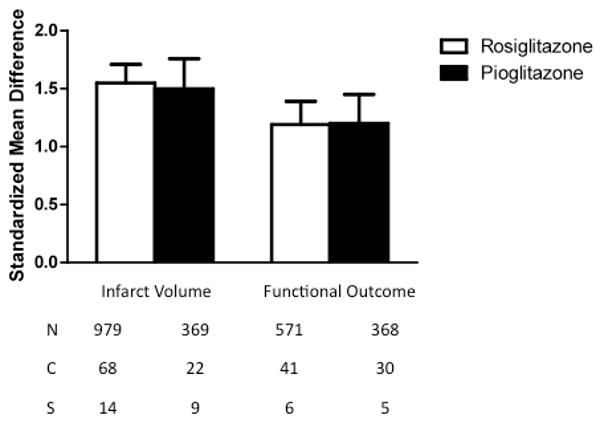
Standardized mean difference and 95% confidence interval by outcome measure with subcomparison of the two thiazolidinediones under consideration, rosiglitazone versus pioglitazone. The drugs were similarly protective to both infarct size and neurologic function (p<0.00001). N=number of animals; C=number of comparisons; S=number of published studies.
Efficacy of TZD depending on first dose timing
TZD treatment was effective at reducing lesion volume and improving neurological outcomes when dosed prior to or after the induction of focal or global ischemia (Fig. 4). When dosed before ischemic injury, TZDs decreased infarct size (1.56, 1.36 to 1.76, p<0.00001) and improved neurological outcomes (1.12, 0.96 to 1.29, p<0.00001). Administering TZDs at or after the induction of ischemic stroke was also effective in reducing lesion volume (1.52, 1.32 to 1.72, p<0.00001) and improving neurological outcomes (1.73, 1.26 to 2.19, p<0.00001). There was no difference in lesion volume reduction due to dose timing (χ2=0.08, df=1, p=0.78). Treatment with a TZD after the onset of ischemic injury resulted in a greater improvement in neurological outcomes than did dosing prior to ischemia (χ2=5.82, df=1, p=0.02).
Fig. 4.
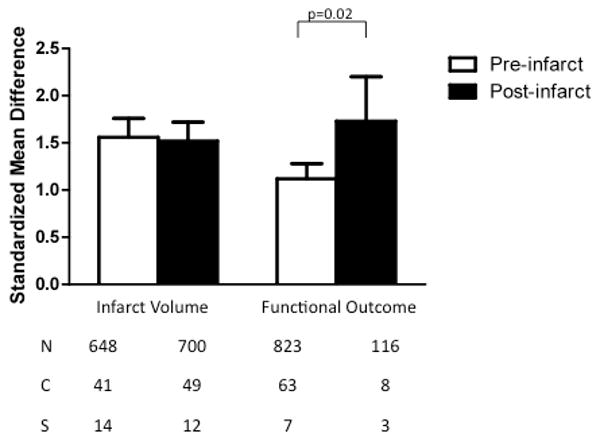
Standardized mean difference and 95% confidence interval by outcome measure with subcomparison of dose timing, either pre-treatment or treatment at the time of initiation of ischemia or any time thereafter, as indicated in Table I. Infarct size was independent of dose timing (p<0.00001). However, drug administration at or following the time of ischemia was more effective than with pre-treatment (p=0.02). N=number of animals; C=number of comparisons; S=number of published studies.
Effect of TZD on infarct size depending on drug and timing of first dose
Though there were no statistically significant differences in anatomical outcome measures based on the timing of the first dose or the type of TZD administered (rosiglitazone or pioglitazone), there was a difference in effect when the data was analyzed separately for each of the two drugs based on dose timing (Fig. 5). The effects of rosiglitazone on the reduction of lesion size were similar whether it was administered before or after ischemia (χ2=1.33, df=1, p=0.25). Pioglitazone was more effective at reducing lesion volume when administered before the induction of focal or global ischemia (χ2=5.93, df=1, p=0.01).
Fig. 5.
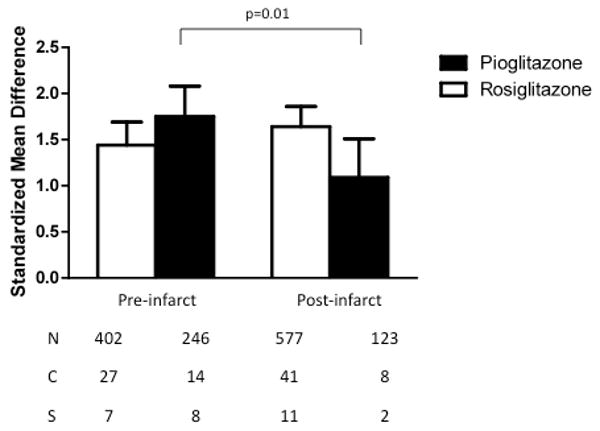
Standardized mean difference and 95% confidence interval for effects of rosiglitazone or pioglitazone on infarct size with subcomparison of dose timing, either pre-treatment or treatment at the time of initiation of ischemia or any time thereafter, as indicated in Table I. Although rosiglitazone was similarly protective with pre-versus post-treatment, pioglitazone was more effective when administered before rather than after the induction of either focal or global ischemia (p=0.01). N=number of animals; C=number of comparisons; S=number of published studies.
Drug effects based on model
All of the included studies employed a model of either transient or permanent ischemia. TZD treatment in transient ischemia models led to improvements in lesion size (1.51, 1.36 to 1.65, p<0.00001) and neurological outcome (1.17, 1.01 to 1.34, p<0.00001) (Fig. 6). The administration of rosiglitazone or pioglitazone was also effective in permanent ischemia models at reducing infarct volume (2.00, 1.40 to 2.60, p<0.00001) and neurological impairments (1.36, 0.84 to 1.87, p<0.00001). There were no significant differences in either outcome measure comparing permanent versus transient ischemia models (infarct volume: χ2=2.43, df=1, p=0.12; neurological outcome: χ2=2.09, df=1, p=0.15).
Fig. 6.
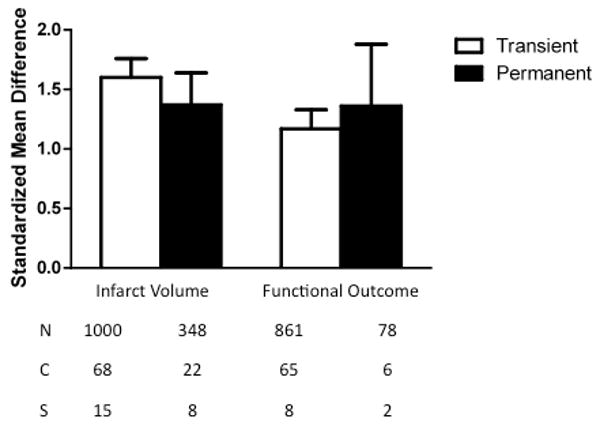
Standardized mean difference and 95% confidence interval by outcome measure with subcomparison of transient versus permanent ischemia model. Infarct size and neurologic function were similarly effective in both types of ischemia models (p<0.00001). N=number of animals; C=number of comparisons; S=number of published studies.
Effect size according to route of drug administration
The effects of TZDs on anatomic and neurological outcomes significantly differed based on the route of drug administration (Fig. 7). Dosing the TZD by oral gavage tended to be less effective at reducing infarct volume than intraperitoneal (p<0.05), intracerebroventricular (p<0.05), or food-based administration (NS). Conversely, for neurologic outcome, the efficacy according to route of administration was highest with peroral, followed by intraperitoneal and intracerebroventricular delivery (p<0.05 between all groups).
Fig. 7.
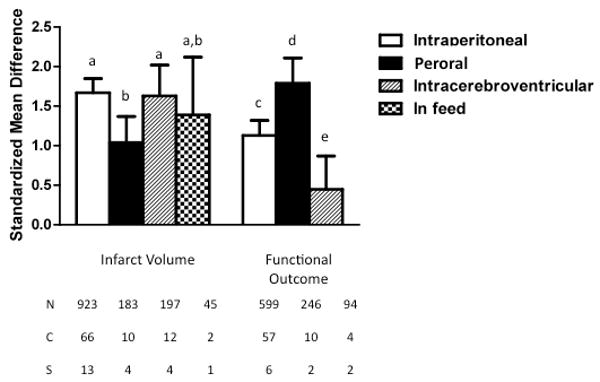
Standardized mean difference and 95% confidence interval by outcome measure with subcomparison of route of drug administration. Post-hoc analysis was performed by outcome measure, and bars with same letter were statistically similar to one another. Thus, administration by oral gavage (peroral) was less effective at decreasing infarct volume than i.p. or i.c.v. administration, and efficacy of TZD in preserving neurologic function was greatest with peroral administration, followed by i.p. administration, and was least effective with i.c.v. drug delivery. (p<0.05). N=number of animals; C=number of comparisons; S=number of published studies.
Discussion
The results of this meta-analysis suggest that rosiglitazone and pioglitazone are effective agents for reducing infarct volume and improving neurologic outcome in rodent models of stroke. In the available data, the two drugs were similarly neuroprotective, and more importantly, the protective effects were retained regardless of the timing of the first dose of drug (Table I).
Whereas the effects of TZD treatment on infarct volume were independent of first dose timing, the improvement in neurological outcome was greater when drug was given during or following the ischemic insult (Fig. 4). Although there may be multiple explanations, it is possible that TZDs independently affect sensory/motor coordination in a manner that is influenced by the timing of the dose and/or the cumulative dose. At the time of behavioral assessment, the cumulative dose in the periphery would likely be lower with post-ischemic administration that with pretreatment. The possibility that effects in the periphery are influencing performance in the behavioral tests is supported by the data in Figure 7 that documents the diminished benefit to neurologic outcome when drug is administered intracerebroventricularly.
An advantage of considering TZDs for acute stroke therapy is that these are currently clinically used drugs for which the pharmacokinetics and toxicity are generally well studied. With longer term use, there are issues with these compounds with regard to tolerability, and repeated use is associated with significant undesirable side effects, including weight gain and edema. These shortcomings would unlikely compromise the ability to utilize the compounds acutely for the treatment of stroke, as only short term dosing might be required. The issue with regard to potential cardiovascular risk of rosiglitazone might also be obviated, but this would require further consideration.
Mechanistically, the efficacy of TZDs in stroke models has been attributed to a variety of effects, including their broad anti-inflammatory properties involving induction of antioxidant enzymes, suppression of proinflammatory cytokines, iNOS, COX-2, and adhesion molecules, decreased macrophage infiltration, and promotion of neovascularization (Culman et al, 2007; Chu et al, 2006). There is significant evidence that protection involves activation of PPARγ, at least in part (Glatz et al, 2010; Lin et al, 2006; Luo et al, 2010; Pereira et al, 2006; Schock et al, 2008; Sobrado et al, 2009; Tureyen et al, 2007; Victor et al, 2006; Wu et al, 2009; Zhao et al, 2006), but there is also data suggesting that non-PPARγ-mediated mechanisms specifically at mitochondria may be involved in the mechanism of action of thiazolidinediones (Feinstein et al, 2005). Whether the higher affinity of rosiglitazone for PPARγ (Feinstein et al, 2005) is what allows for administration of generally lower concentrations than pioglitazone (Table I) remains unclear.
Although promising, the data indicate that additional studies are required to determine the efficacy of delayed administration of TZDs (i.e. 6 hrs or longer) and assess outcome measures at delayed time points (i.e. longer than 72 hours, as reported in Chu et al., 2006; Fatehi-Hassanabad & Tasker, 2010; Luo et at., 2006; Sundararajan et al., 2005). Additional studies should take into consideration other variables as suggested by Howells et al (2010) that may strengthen the rationale and guide plans for testing in the clinical setting. Some of these variables were broached in the current studies, such as testing in the context of co-morbidities, including hypertension and diabetes (Tureyen et al, 2007) or tPA treatment (Wang et al, 2009) and elevated temperature (Nategh et al, 2010). In each of these studies, TZD treatment was neuroprotective. Of note, most studies that tested delayed administration of TZD from 4 to 24 hours (Allahtavakoli et al 2006, 2009; Ji et al 2009; Tureyen et al, 2007; Wang et al, 2009) found protective effects of rosiglitazone. In the single study that tested delayed (24 hr) administration of pioglitazone with assessment at 6 weeks (Ji et al, 2009), treatment was no longer as effective as it had been at an earlier point of evaluation, but these observations need to be strengthened with further studies. Overall, current data are supportive that thiazolidinediones could be effective agents in the acute treatment of ischemic stroke.
Fig. 2.
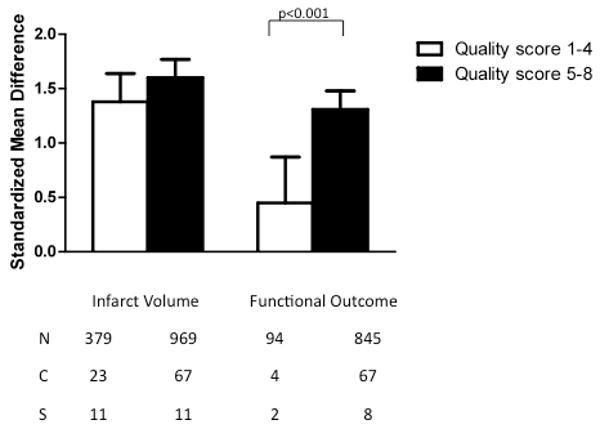
Standardized mean difference and 95% confidence interval by outcome measure with subcomparison by study quality score, assigned as described in the text. A beneficial effect was detected on both infarct size and neurologic function, although lower study quality was associated with a smaller effect size on neurologic outcome (p<0.002). N=number of animals; C=number of comparisons; S=number of published studies.
Acknowledgments
Support for this study is provided by the American Diabetes Association 0-08-RA-139, NIH-NIDDK 1R42DK081298, NIH-NIDDK 2P01 DK054441 to A.N.M.
Abbreviations used
- MCAO
middle carotid artery occlusion
- PPARγ
peroxisome proliferator-activated receptor gamma
- TZD
thiazolidinedione
Footnotes
References
- Allahtavakoli M, Shabanzadeh A, Roohbakhsh A, Pourshanazari A. Combination therapy of rosiglitazone, a peroxisome proliferator-activated receptor-gamma ligand, and NMDA receptor antagonist (MK-801) on experimental embolic stroke in rats. Basic Clin Pharmacol Toxicol. 2007;101:309–314. doi: 10.1111/j.1742-7843.2007.00127.x. [DOI] [PubMed] [Google Scholar]
- Allahtavakoli M, Shabanzadeh AP, Sadr SS, Parviz M, Djahanguiri B. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma ligand, reduces infarction volume and neurological deficits in an embolic model of stroke. Clin Exp Pharmacol Physiol. 2006;33:1052–1058. doi: 10.1111/j.1440-1681.2006.04486.x. [DOI] [PubMed] [Google Scholar]
- Allahtavakoli M, Moloudi R, Arababadi MK, Shamsizadeh A, Javanmardi K. Delayed post ischemic treatment with Rosiglitazone attenuates infarct volume, neurological deficits and neutrophilia after embolic stroke in rat. Brain Res. 2009;1271:121–127. doi: 10.1016/j.brainres.2009.03.040. [DOI] [PubMed] [Google Scholar]
- Barnett AH. Redefining the role of thiazolidinediones in the management of type 2 diabetes. Vasc Health Risk Manag. 2009;5:141–51. doi: 10.2147/vhrm.s4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Lee ST, Koo JS, Jung KH, Kim EH, Sinn DI, Kim JM, Ko SY, Kim SJ, Song EC, Kim M, Roh JK. Peroxisome proliferator-activated receptor-gamma-agonist, rosiglitazone, promotes angiogenesis after focal cerebral ischemia. Brain Res. 2006;1093:208–218. doi: 10.1016/j.brainres.2006.03.114. [DOI] [PubMed] [Google Scholar]
- Craft S. Insulin resistance syndrome and Alzheimer disease: pathophysiologic mechanisms and therapeutic implications. Alzheimer Dis Assoc Disord. 2006;20:298–301. doi: 10.1097/01.wad.0000213866.86934.7e. [DOI] [PubMed] [Google Scholar]
- Dormandy JA, Charbonnel B, Eckland DJ, et al. PROactive investigators Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomized controlled trial. Lancet. 2005 Oct 8;366(9493):1279–89. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- DREAM Trial Investigators. Dagenais GR, Gerstein HC, Holman R, Budaj A, Escalante A, Hedner T, Keltai M, Lonn E, McFarlane S, McQueen M, Teo K, Sheridan P, Bosch J, Pogue J, Yusuf S. Effects of ramipril and rosiglitazone on cardiovascular and renal outcomes in people with impaired glucose tolerance or impaired fasting glucose: results of the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial. Diabetes Care. 2008;31:1007–14. doi: 10.2337/dc07-1868. [DOI] [PubMed] [Google Scholar]
- Fatehi-Hassanabad Z, Tasker RA. Peroxisome Proliferator-Activated Receptor-gamma (PPAR-gamma) Activation Confers Functional Neuroprotection in Global Ischemia. Neurotox Res. 2010 doi: 10.1007/s12640-010-9201-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Feinstein DL, Spagnolo A, Akar C, Weinberg G, Murphy P, Gavrilyuk V, Dello Russo C. Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key? Biochem Pharmacol. 2005;70:177–188. doi: 10.1016/j.bcp.2005.03.033. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Gray LJ, Bath PMW, Murphy SP. Progesterone for the treatment of experimental brain injury; a systematic review. Brain. 2008;131:318–328. doi: 10.1093/brain/awm183. [DOI] [PubMed] [Google Scholar]
- Glatz T, Stock I, Nguyen-Ngoc M, Gohlke P, Herdegen T, Culman J, Zhao Y. Peroxisome-proliferator-activated receptors gamma and peroxisome-proliferator-activated receptors beta/delta and the regulation of interleukin 1 receptor antagonist expression by pioglitazone in ischaemic brain. J Hypertens. 28:1488–1497. doi: 10.1097/HJH.0b013e3283396e4e. [DOI] [PubMed] [Google Scholar]
- Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, Ali F, Sholley C, Worrall C, Kelman JA. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304:411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- Hanyu H, Sato T, Kiuchi A, Sakurai H, Iwamoto T. Pioglitazone improved cognition in a pilot study on patients with Alzheimer's disease and mild cognitive impairment with diabetes mellitus. J Am Geriatr Soc. 2009;57:177–179. doi: 10.1111/j.1532-5415.2009.02067.x. [DOI] [PubMed] [Google Scholar]
- Howells DW, Porritt MJ, Rewell SS, O'Collins V, Sena ES, van der Worp HB, Traystman RJ, Macleod MR. Different strokes for different folks: the rich diversity of animal models of focal cerebral ischemia. J Cereb Blood Flow Metab. 30:1412–1431. doi: 10.1038/jcbfm.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S, Kronenberg G, Balkaya M, Farber K, Gertz K, Kettenmann H, Endres M. Acute neuroprotection by pioglitazone after mild brain ischemia without effect on long-term outcome. Exp Neurol. 2009;216:321–328. doi: 10.1016/j.expneurol.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Kadoglou NP, Iliadis F, Sailer N, Athanasiadou Z, Vitta I, Kapelouzou A, Karayannacos PE, Liapis CD, Alevizos M, Angelopoulou N, Vrabas IS. Exercise training ameliorates the effects of rosiglitazone on traditional and novel cardiovascular risk factors in patients with type 2 diabetes mellitus. Metabolism. 2010;59:599–607. doi: 10.1016/j.metabol.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Kemp DE, Ismail-Beigi F, Calabrese JR. Antidepressant response associated with pioglitazone: support for an overlapping pathophysiology between major depression and metabolic syndrome. Am J Psychiatry. 2009;166:619. doi: 10.1176/appi.ajp.2008.08081195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Kim HY, Hong JS, Baek WK, Park JW. PPARgamma agonist pioglitazone reduces matrix metalloproteinase-9 activity and neuronal damage after focal cerebral ischemia. Biochem Biophys Res Commun. 2009;380:17–21. doi: 10.1016/j.bbrc.2008.12.181. [DOI] [PubMed] [Google Scholar]
- Luna-Medina R, Cortes-Canteli M, Alonso M, Santos A, Martinez Al, Perez-Castillo A. Regulation of inflammatory response in neural cells in vitro by thiadiazolidinones derivative through peroximsome proliferator-activated receptor gamma activation. J Biol Chem. 2005;280:21453–62. doi: 10.1074/jbc.M414390200. [DOI] [PubMed] [Google Scholar]
- Luo Y, Yin W, Signore AP, Zhang F, Hong Z, Wang S, Graham SH, Chen J. Neuroprotection against focal ischemic brain injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. J Neurochem. 2006;97:435–448. doi: 10.1111/j.1471-4159.2006.03758.x. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Soczynska JK, Woldeyohannes HO, Lewis GF, Leiter LA, MacQueen GM, Miranda A, Fulgosi D, Konarski JZ, Kennedy SH. Thiazolidinediones: novel treatments for cognitive deficits in mood disorders? Expert Opin Pharmacother. 2007;8:1615–1628. doi: 10.1517/14656566.8.11.1615. [DOI] [PubMed] [Google Scholar]
- Michalik L, Auwerx J, Berger JP, Chatterjee VK, Glass CK, Gonzalez FJ, Grimaldi PA, Kadowaki T, Lazar MA, O'Rahilly S, Palmer CN, Plutzky J, Reddy JK, Spiegelman BM, Staels B, Wahli W. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev. 2006;58:726–741. doi: 10.1124/pr.58.4.5. [DOI] [PubMed] [Google Scholar]
- Nategh M, Shaveisi K, Shabanzadeh AP, Sadr SS, Parviz M, Ghabaei M. Systemic Hyperthermia Masks the Neuroprotective Effects of MK-801, but not Rosiglitazone in Brain Ischaemia. Basic Clin Pharmacol Toxicol. doi: 10.1111/j.1742-7843.2010.00570.x. [DOI] [PubMed] [Google Scholar]
- Pereira MP, Hurtado O, Cardenas A, Bosca L, Castillo J, Davalos A, Vivancos J, Serena J, Lorenzo P, Lizasoain I, Moro MA. Rosiglitazone and 15-deoxy-Delta12,14-prostaglandin J2 cause potent neuroprotection after experimental stroke through noncompletely overlapping mechanisms. J Cereb Blood Flow Metab. 2006;26:218–229. doi: 10.1038/sj.jcbfm.9600182. [DOI] [PubMed] [Google Scholar]
- Rasgon NL, Kenna HA, Williams KE, Powers B, Wroolie T, Schatzberg AF. Rosiglitazone add-on in treatment of depressed patients with insulin resistance: a pilot study. ScientificWorldJournal. 2010;10:321–8. doi: 10.1100/tsw.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmerbach K, Schefe JH, Krikov M, Muller S, Villringer A, Kintscher U, Unger T, Thoene-Reineke C. Comparison between single and combined treatment with candesartan and pioglitazone following transient focal ischemia in rat brain. Brain Res. 2008;1208:225–233. doi: 10.1016/j.brainres.2008.02.032. [DOI] [PubMed] [Google Scholar]
- Schock SC, Xu J, Duquette PM, Qin Z, Lewandowski AJ, Rai PS, Thompson CS, Seifert EL, Harper ME, Chen HH. Rescue of neurons from ischemic injury by peroxisome proliferator-activated receptor-gamma requires a novel essential cofactor LMO4. J Neurosci. 2008;28:12433–12444. doi: 10.1523/JNEUROSCI.2897-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu T, Inoue I, Araki N, Asano Y, Sawada M, Furuya D, Nagoya H, Greenberg JH. A peroxisome proliferator-activated receptor-gamma agonist reduces infarct size in transient but not in permanent ischemia. Stroke. 2005;36:353–359. doi: 10.1161/01.STR.0000152271.21943.a2. [DOI] [PubMed] [Google Scholar]
- Shukla DK, Kaiser CC, Stebbins GT, Feinstein DL. Effects of pioglitazone on diffusion tensor imaging indices in multiple sclerosis patients. Neurosci Lett. 472:153–156. doi: 10.1016/j.neulet.2010.01.046. [DOI] [PubMed] [Google Scholar]
- Sobrado M, Pereira MP, Ballesteros I, Hurtado O, Fernandez-Lopez D, Pradillo JM, Caso JR, Vivancos J, Nombela F, Serena J, Lizasoain I, Moro MA. Synthesis of lipoxin A4 by 5-lipoxygenase mediates PPARgamma-dependent, neuroprotective effects of rosiglitazone in experimental stroke. J Neurosci. 2009;29:3875–3884. doi: 10.1523/JNEUROSCI.5529-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130:685–696. doi: 10.1016/j.neuroscience.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Kapadia R, Bowen KK, Satriotomo I, Liang J, Feinstein DL, Vemuganti R. Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J Neurochem. 2007;101:41–56. doi: 10.1111/j.1471-4159.2006.04376.x. [DOI] [PubMed] [Google Scholar]
- Victor NA, Wanderi EW, Gamboa J, Zhao X, Aronowski J, Deininger K, Lust WD, Landreth GE, Sundararajan S. Altered PPARgamma expression and activation after transient focal ischemia in rats. Eur J Neurosci. 2006;24:1653–1663. doi: 10.1111/j.1460-9568.2006.05037.x. [DOI] [PubMed] [Google Scholar]
- Wang CX, Ding X, Noor R, Pegg C, He C, Shuaib A. Rosiglitazone alone or in combination with tissue plasminogen activator improves ischemic brain injury in an embolic model in rats. J Cereb Blood Flow Metab. 2009;29:1683–1694. doi: 10.1038/jcbfm.2009.87. [DOI] [PubMed] [Google Scholar]
- Wu JS, Cheung WM, Tsai YS, Chen YT, Fong WH, Tsai HD, Chen YC, Liou JY, Shyue SK, Chen JJ, Chen YE, Maeda N, Wu KK, Lin TN. Ligand-activated peroxisome proliferator-activated receptor-gamma protects against ischemic cerebral infarction and neuronal apoptosis by 14-3-3 epsilon upregulation. Circulation. 2009;119:1124–1134. doi: 10.1161/CIRCULATIONAHA.108.812537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Patzer A, Gohlke P, Herdegen T, Culman J. The intracerebral application of the PPARgamma-ligand pioglitazone confers neuroprotection against focal ischaemia in the rat brain. Eur J Neurosci. 2005;22:278–282. doi: 10.1111/j.1460-9568.2005.04200.x. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Patzer A, Herdegen T, Gohlke P, Culman J. Activation of cerebral peroxisome proliferator-activated receptors gamma promotes neuroprotection by attenuation of neuronal cyclooxygenase-2 overexpression after focal cerebral ischemia in rats. Faseb J. 2006;20:1162–1175. doi: 10.1096/fj.05-5007com. [DOI] [PubMed] [Google Scholar]


