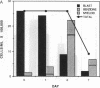
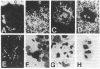
Abstract
The signals and cellular interactions required for hematopoietic stem-cell commitment to the T lineage are unknown, yet are central to understanding the early stages of normal T-cell development. To study the differentiative capacity of T-cell precursors, we isolated CD4-, CD8-, surface(s) CD3- thymocytes from postnatal human thymuses and determined their capacity to differentiate into lymphoid and nonlymphoid lineages in vitro. We found that CD4-, CD8-, sCD3- thymocytes, which differentiated in the presence of T-cell conditioned medium plus interleukin 2 into T cells expressing the gamma delta receptor for antigen, were capable of differentiating into myeloid or erythroid lineages in the presence of either 5637 bladder carcinoma cell line conditioned medium plus recombinant human erythropoietin or human thymic epithelial cell conditioned medium. Thymic epithelial cell conditioned medium was as effective as 5637 supernatant plus erythropoietin in inducing myeloerythroid differentiation in the CD4-, CD8-, sCD3- thymocytes. Sixty-eight +/- 14% of CD4-, CD8-, sCD3- thymocytes underwent nonlymphoid differentiation within 4 days in culture with 5637 supernatant plus erythropoietin. Twenty-six +/- 4% of freshly isolated CD4-, CD8-, sCD3- cells were CD34+, and clonal granulocyte/macrophage, granulocyte/erythrocyte/monocyte/megakaryocyte, and T-cell progenitors were found in both CD34+ and CD34- subsets of CD4-, CD8-, sCD3- thymocytes. Thus, cells within the human CD4-, CD8-, sCD3- thymocyte subset can give rise to gamma delta+ T cells as well as to cells of myeloerythroid lineages. Moreover, CD34+, CD4-, CD8-, sCD3- cells can give rise to clonal T-cell progenitors as well as to clonal myeloid progenitors.
Full text
PDF

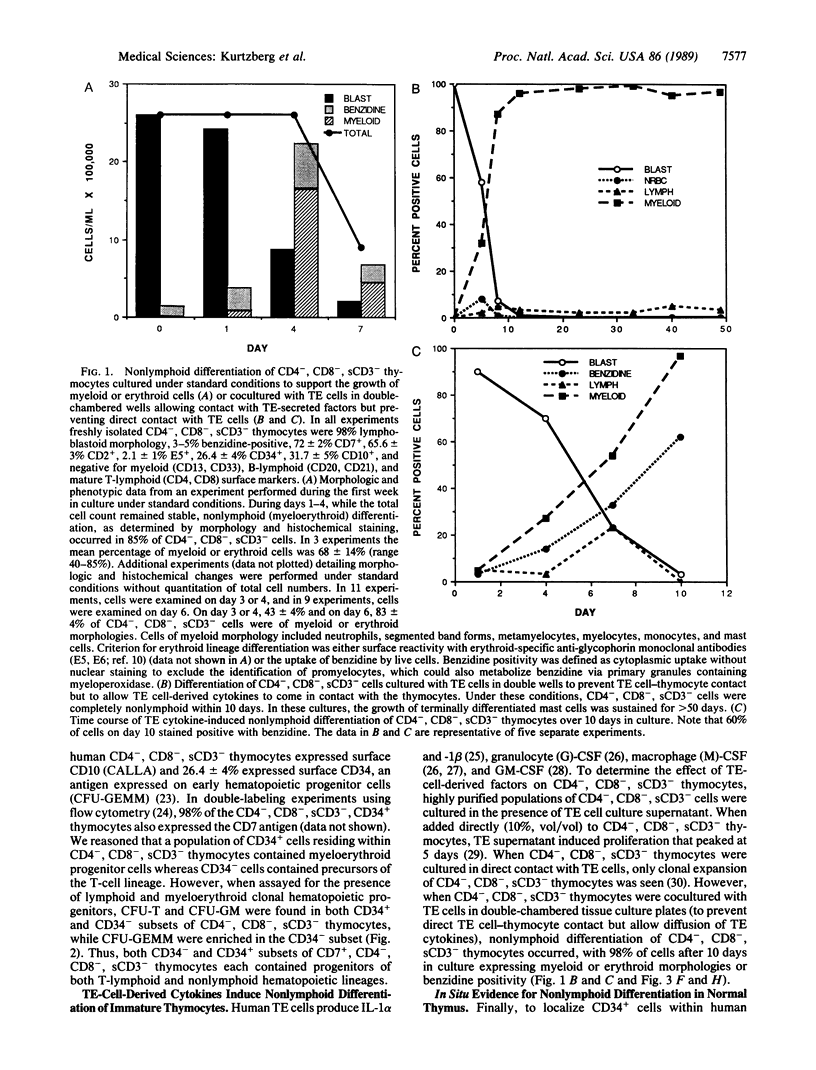
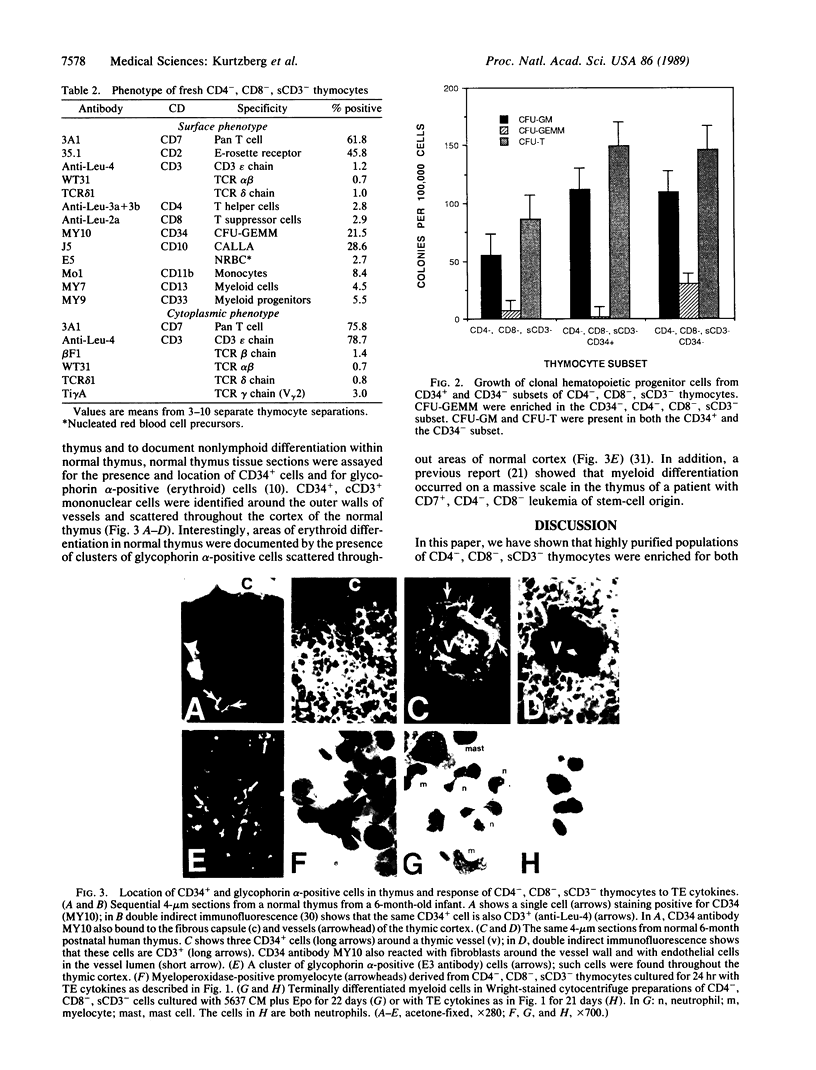

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Band H., Hochstenbach F., McLean J., Hata S., Krangel M. S., Brenner M. B. Immunochemical proof that a novel rearranging gene encodes the T cell receptor delta subunit. Science. 1987 Oct 30;238(4827):682–684. doi: 10.1126/science.3672118. [DOI] [PubMed] [Google Scholar]
- Barg M., Mandel T. E., Johnson G. R. Haemopoietic stem cells in the foetal mouse thymus. Aust J Exp Biol Med Sci. 1978 Apr;56(2):195–200. doi: 10.1038/icb.1978.21. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Scheft H., Warnke R. A., Jones N., Strominger J. L. Characterization and expression of the human alpha beta T cell receptor by using a framework monoclonal antibody. J Immunol. 1987 Mar 1;138(5):1502–1509. [PubMed] [Google Scholar]
- Campana D., Janossy G., Coustan-Smith E., Amlot P. L., Tian W. T., Ip S., Wong L. The expression of T cell receptor-associated proteins during T cell ontogeny in man. J Immunol. 1989 Jan 1;142(1):57–66. [PubMed] [Google Scholar]
- Civin C. I., Strauss L. C., Brovall C., Fackler M. J., Schwartz J. F., Shaper J. H. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984 Jul;133(1):157–165. [PubMed] [Google Scholar]
- Denning S. M., Kurtzberg J., Le P. T., Tuck D. T., Singer K. H., Haynes B. F. Human thymic epithelial cells directly induce activation of autologous immature thymocytes. Proc Natl Acad Sci U S A. 1988 May;85(9):3125–3129. doi: 10.1073/pnas.85.9.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning S. M., Kurtzberg J., Leslie D. S., Haynes B. F. Human postnatal CD4- CD8- CD3- thymic T cell precursors differentiate in vitro into T cell receptor delta-bearing cells. J Immunol. 1989 May 1;142(9):2988–2997. [PubMed] [Google Scholar]
- Denning S. M., Tuck D. T., Singer K. H., Haynes B. F. Human thymic epithelial cells function as accessory cells for autologous mature thymocyte activation. J Immunol. 1987 Feb 1;138(3):680–686. [PubMed] [Google Scholar]
- Griffin J. D., Ritz J., Nadler L. M., Schlossman S. F. Expression of myeloid differentiation antigens on normal and malignant myeloid cells. J Clin Invest. 1981 Oct;68(4):932–941. doi: 10.1172/JCI110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Denning S. M., Singer K. H., Kurtzberg J. Ontogeny of T-cell precursors: a model for the initial stages of human T-cell development. Immunol Today. 1989 Mar;10(3):87–91. doi: 10.1016/0167-5699(89)90232-6. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Eisenbarth G. S., Fauci A. S. Human lymphocyte antigens: production of a monoclonal antibody that defines functional thymus-derived lymphocyte subsets. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5829–5833. doi: 10.1073/pnas.76.11.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Martin M. E., Kay H. H., Kurtzberg J. Early events in human T cell ontogeny. Phenotypic characterization and immunohistologic localization of T cell precursors in early human fetal tissues. J Exp Med. 1988 Sep 1;168(3):1061–1080. doi: 10.1084/jem.168.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Scearce R. M., Lobach D. F., Hensley L. L. Phenotypic characterization and ontogeny of mesodermal-derived and endocrine epithelial components of the human thymic microenvironment. J Exp Med. 1984 Apr 1;159(4):1149–1168. doi: 10.1084/jem.159.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Singer K. H., Denning S. M., Martin M. E. Analysis of expression of CD2, CD3, and T cell antigen receptor molecules during early human fetal thymic development. J Immunol. 1988 Dec 1;141(11):3776–3784. [PubMed] [Google Scholar]
- Hershfield M. S., Kurtzberg J., Harden E., Moore J. O., Whang-Peng J., Haynes B. F. Conversion of a stem cell leukemia from a T-lymphoid to a myeloid phenotype induced by the adenosine deaminase inhibitor 2'-deoxycoformycin. Proc Natl Acad Sci U S A. 1984 Jan;81(1):253–257. doi: 10.1073/pnas.81.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitsukawa S., Faure F., Lipinski M., Triebel F., Hercend T. A novel subset of human lymphocytes with a T cell receptor-gamma complex. J Exp Med. 1987 Oct 1;166(4):1192–1197. doi: 10.1084/jem.166.4.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston R., Jenkinson E. J., Owen J. J. A single stem cell can recolonize an embryonic thymus, producing phenotypically distinct T-cell populations. 1985 Oct 31-Nov 6Nature. 317(6040):811–813. doi: 10.1038/317811a0. [DOI] [PubMed] [Google Scholar]
- Kurtzberg J., Bigner S. H., Hershfield M. S. Establishment of the DU.528 human lymphohemopoietic stem cell line. J Exp Med. 1985 Nov 1;162(5):1561–1578. doi: 10.1084/jem.162.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzberg J., Waldmann T. A., Davey M. P., Bigner S. H., Moore J. O., Hershfield M. S., Haynes B. F. CD7+, CD4-, CD8- acute leukemia: a syndrome of malignant pluripotent lymphohematopoietic cells. Blood. 1989 Feb;73(2):381–390. [PubMed] [Google Scholar]
- Le P. T., Kurtzberg J., Brandt S. J., Niedel J. E., Haynes B. F., Singer K. H. Human thymic epithelial cells produce granulocyte and macrophage colony-stimulating factors. J Immunol. 1988 Aug 15;141(4):1211–1217. [PubMed] [Google Scholar]
- Le P. T., Tuck D. T., Dinarello C. A., Haynes B. F., Singer K. H. Human thymic epithelial cells produce interleukin 1. J Immunol. 1987 Apr 15;138(8):2520–2526. [PubMed] [Google Scholar]
- Mage M., Mathieson B., Sharrow S., McHugh L., Hämmerling U., Kanellopoulos-Langevin C., Brideau D., Jr, Thomas C. A., 3rd Preparative nonlytic separation of Lyt2+ and Lyt2- T lymphocytes, functional analyses of the separated cells and demonstration of synergy in graft-vs.-host reaction of Lyt2+ and Lyt2- cells. Eur J Immunol. 1981 Mar;11(3):228–235. doi: 10.1002/eji.1830110312. [DOI] [PubMed] [Google Scholar]
- Martin P. J., Longton G., Ledbetter J. A., Newman W., Braun M. P., Beatty P. G., Hansen J. A. Identification and functional characterization of two distinct epitopes on the human T cell surface protein Tp50. J Immunol. 1983 Jul;131(1):180–185. [PubMed] [Google Scholar]
- McCune J. M., Namikawa R., Kaneshima H., Shultz L. D., Lieberman M., Weissman I. L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988 Sep 23;241(4873):1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- Mizutani S., Watt S. M., Robertson D., Hussein S., Healy L. E., Furley A. J., Greaves M. F. Cloning of human thymic subcapsular cortex epithelial cells with T-lymphocyte binding sites and hemopoietic growth factor activity. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4999–5003. doi: 10.1073/pnas.84.14.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Pelkonen J. Prethymic and intrathymic mouse T-cell progenitors. Growth requirements and analysis of the expression of genes encoding TCR/T3 components and other T-cell-specific molecules. Immunol Rev. 1988 Aug;104:5–27. doi: 10.1111/j.1600-065x.1988.tb00757.x. [DOI] [PubMed] [Google Scholar]
- Papiernik M., Lepault F., Pontoux C. Synergistic effect of colony-stimulating factors and IL-2 on prothymocyte proliferation linked to the maturation of macrophage/dendritic cells within L3T4-Lyt-2-Ia-Mac- cells. J Immunol. 1988 Mar 1;140(5):1431–1434. [PubMed] [Google Scholar]
- Scollay R., Wilson A., D'Amico A., Kelly K., Egerton M., Pearse M., Wu L., Shortman K. Developmental status and reconstitution potential of subpopulations of murine thymocytes. Immunol Rev. 1988 Aug;104:81–120. doi: 10.1111/j.1600-065x.1988.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Singer K. H., Harden E. A., Robertson A. L., Lobach D. F., Haynes B. F. In vitro growth and phenotypic characterization of mesodermal-derived and epithelial components of normal and abnormal human thymus. Hum Immunol. 1985 Jul;13(3):161–176. doi: 10.1016/0198-8859(85)90009-6. [DOI] [PubMed] [Google Scholar]
- Spangrude G. J., Heimfeld S., Weissman I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988 Jul 1;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Taylor C. R., Skinner J. M. Evidence for significant hematopoiesis in the human thymus. Blood. 1976 Feb;47(2):305–313. [PubMed] [Google Scholar]
- Telen M. J., Scearce R. M., Haynes B. F. Human erythrocyte antigens. III. Characterization of a panel of murine monoclonal antibodies that react with human erythrocyte and erythroid precursor membranes. Vox Sang. 1987;52(3):236–243. doi: 10.1111/j.1423-0410.1987.tb03035.x. [DOI] [PubMed] [Google Scholar]
- Thiel E., Kranz B. R., Raghavachar A., Bartram C. R., Löffler H., Messerer D., Ganser A., Ludwig W. D., Büchner T., Hoelzer D. Prethymic phenotype and genotype of pre-T (CD7+/ER-)-cell leukemia and its clinical significance within adult acute lymphoblastic leukemia. Blood. 1989 Apr;73(5):1247–1258. [PubMed] [Google Scholar]
- Toribio M. L., Alonso J. M., Bárcena A., Gutiérrez J. C., de la Hera A., Marcos M. A., Márquez C., Martínez C. Human T-cell precursors: involvement of the IL-2 pathway in the generation of mature T cells. Immunol Rev. 1988 Aug;104:55–79. doi: 10.1111/j.1600-065x.1988.tb00759.x. [DOI] [PubMed] [Google Scholar]
- Welte K., Platzer E., Lu L., Gabrilove J. L., Levi E., Mertelsmann R., Moore M. A. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1526–1530. doi: 10.1073/pnas.82.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]