Abstract
The synergistic actions of atorvastatin (ATST) with γ-tocotrienol (γ-TT) and celecoxib (CXIB) were studied in human colon cancer cell lines HT29 and HCT116. The synergistic inhibition of cell growth by ATST and γ-TT was demonstrated by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay and isobologram analysis. δ-TT exhibited a similar inhibitory action when combined with ATST. Mevalonate and geranylgeranyl pyrophosphate eliminated most of the growth inhibitory effect of ATST, but only marginally decreased that of γ-TT; whereas farnesyl pyrophosphate and squalene exhibited little effect on the inhibitory action of ATST and γ-TT, indicating protein geranylgeranylation, but not farnesylation are involved in the inhibition of colon cancer cell growth. Both mevalonate and squalene restored the cellular cholesterol level that was reduced by ATST treatment, but only mevalonate eliminated the cell growth inhibitory effect, suggesting that the cholesterol level in cells does not play an essential role in inhibiting cancer cell growth. Protein level of HMG-CoA reductase increased after ATST treatment, and the presence of γ-TT attenuated the elevated level of HMG-CoA reductase. ATST also decreased membrane-bound RhoA, possibly due to a reduced level of protein geranylgeranylation; addition of γ-TT enhanced this effect. The mediation of HMG-CoA reductase and RhoA provides a possible mechanism for the synergistic action of ATST and γ-TT. The triple combination of ATST, γ-TT, and CXIB showed a synergistic inhibition of cancer cell growth in MTT assays. The synergistic action of these three compounds was also illustrated by their induction of G0/G1 phase cell cycle arrest and apoptosis.
Keywords: atorvastatin tocotrienol, triple combination, synergy, HMG-CoA reductase
INTRODUCTION
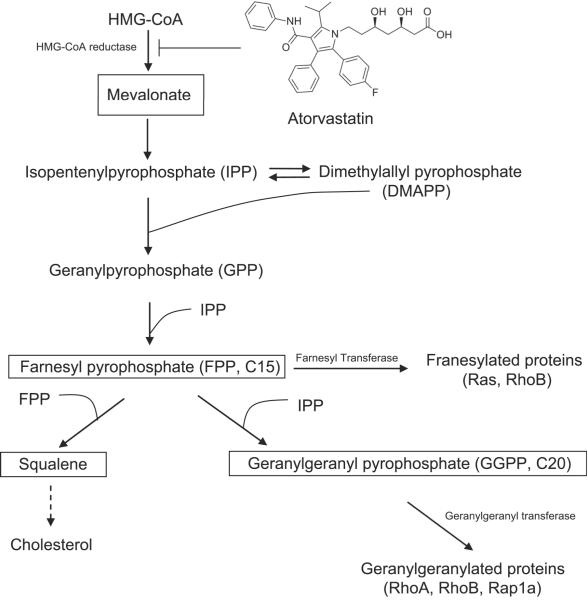
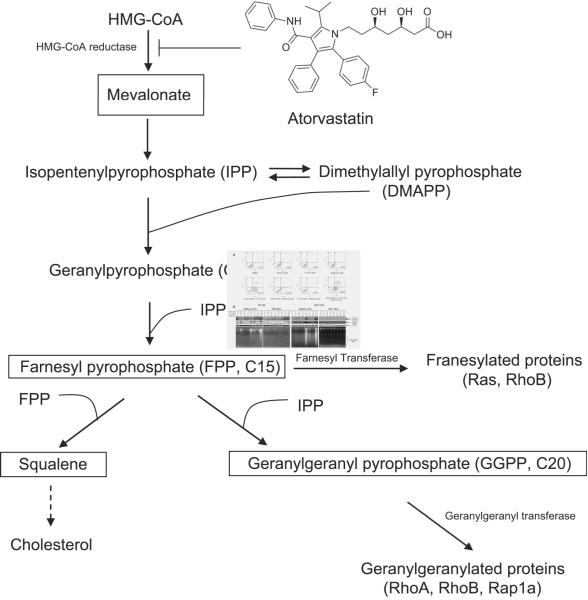
Atorvastatin (ATST, commercial name Lipitor or Torvast) belongs to the statin family, a class of compounds used to reduce the levels of cholesterol and low density lipoproteins. The efficacy of statins in lowering the risk of cardiovascular disease and the relatively low toxicity of statins has led to their widespread use. Statins function by inhibiting the activity of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in cholesterol biosynthesis (Fig. 1).1 The product of HMG-CoA reductase, mevalonate, is the precursor for many important intermediates in a pathway commonly known as the “mevalonate cascade”. Statins inhibit the formation of many intermediate products such as farnesyl pyrophosphate and geranylgeranyl pyrophosphate, which isoprenylate various proteins, including small G-proteins such as the Ras/Rho super family proteins. The isoprenylation of these proteins is essential for them to anchor to cell membranes and thus become functional.2 The cancer prevention activity reported for statins is believed to be due to their inhibition of the isoprenylation of G-proteins and the subsequent alteration of downstream signaling pathways.3, 4 In addition, statins can also affect several HMG-CoA reductase-independent targets. For example, simvastatin inhibits the RANKL (the receptor activator of NF-kB ligand)-induced NF-kB activation pathway. This inhibition leads to the suppression of osteoclastogenesis induced by RANKL and by tumor cells.5 Lovastatin has been shown to bind directly to an intergrin called lymphocyte-function-associated antigen 1 (LFA1) and inhibit the interaction of LFA1 with intracellular-adhesion molecule 1 (ICAM1), thus preventing cell-adhesion, invasion and inflammation.6 Statins also inhibit the protein degradation machinery, specifically the proteasomes.7 Inhibition of proteasome activity is likely to be related to the effect of statins on G1 phase cell cycle arrest. In general, statins exert their cancer prevention effects by regulating several disease-associated cellular events including inflammation, immunomodulation, angiogenesis, apoptosis, and proliferation.8
Fig. 1.
A diagram of the mevalonate cascade and the structure of the HMG-CoA reductase inhibitor ATST used in this study (modified from Holstein et al.1).
Tocotrienols (TTs), together with tocopherols (TPs), are members of the vitamin E family. The difference between TTs and TPs is that TPs contain a 16-carbon saturated phytyl side chain, whereas TTs possess an unsaturated isoprenoid side chain. Based on the number and location of the methyl groups on their chromanol rings, they present as α-, β-, γ- and δ- TPs and α-, β-, γ- and δ-TTs.9 Emerging evidences suggest that TTs exhibit more potent anti-cancer effects than TPs.9 TTs affect various molecular targets and signaling pathways. For example, treatment of human colon carcinoma RKO cells with a tocotrienol rich fraction (TRF) of palm oil results in an alteration of Bax/Bcl2 ratio and consequent apoptosis; TRF transcriptionally up-regulates p21Cip1/Waf1 in a p53-dependent, but cell cycle-regulation-independent manner.10 γ-TT inhibits cancer cell proliferation by decreasing NF-κB and Akt activity and by inhibiting NF-κB signaling pathways.11 TTs also effectively reduce the expression of adhesive molecules and prevents monocyte-endothelial cell interaction.11 Most importantly, γ- and δ-TTs attenuate the level of HMG-CoA reductase under a sterol-depleted condition by suppressing its mRNA transcription and stimulating its ubiquitination and degradation.12, 13
Celecoxib (CXIB, commercial name Celebrex), belongs to the family of nonsteroidal anti-inflammatory drugs (NSAIDs). It is a specific inhibitor of cyclooxygenase-2 (COX-2). Aberrant functioning of COX-2 has been associated with carcinogenesis by promoting cell survival, angiogenesis, and metastasis14-16 and it has been demonstrated that CXIB could prevent carcinogenesis via COX-2 dependent and independent mechanisms.17 CXIB induces cell cycle arrest at the G1 phase in multiple cancer cell lines by increasing the level of cyclin-dependent kinase inhibitors and decreasing the level of cyclins. CXIB also causes cell growth inhibition through Akt-mediated inactivation of p21Cip/Waf1 and p27Kip1.18, 19 CXIB promotes cancer cell apoptosis by inhibition of the transcription activity of NF-κB.20 APC/β-catenin pathway, an essential pathway in the development of various cancers, is also inhibited by CXIB.21 CXIB rapidly decreases the DNA-binding activity of β-catenin, which is followed by extensive degradation of β-catenin through a caspase- and proteasome–dependent mechanism.
Despite the anti-cancer activity of these three compounds, the use of only a single compound requires a relatively high dose, which is usually associated with adverse effects. For example, long-term use of CXIB is associated with severe gastrointestinal and cardiovascular adverse effects.22-24 One promising approach to solve this problem is to explore the synergistic action using the combination of agents, which can obtain the same efficacy as the single agent while the dose of each agent used is much lower. Previously, we studied the synergistic action of ATST and CXIB.25 In this study, we investigated the possible synergistic action of ATST when combined with γ-TT against human colon cancer cell lines HT29 and HCT116. Possible mechanisms of the synergy related to HMG-CoA reductase level, the mevalonate cascade, and RhoA protein geranylgeranylation were investigated. Based on our previous and current data, ATST is the key component in both of the double combination studies (ATST+CXIB and ATST+γ-TT) against colon cancer cell lines. Therefore, we further pursued a triple combination of ATST, γ-TT, and CXIB in terms of cell growth inhibition, cell cycle arrest, and apoptosis. The synergy of ATST, γ-TT, and CXIB further reduced the effective concentration of each individual agent, demonstrating that combination treatments may have clinical applications.
MATERIAL AND METHODS
Cell culture and cell growth inhibitory assay
All chemicals used in this article were from Sigma-Aldrich (St. Louis, MI) unless otherwise specified. Human colon cancer cell lines HCT116 and HT29 were obtained from the American Type Cell Collection (ATCC, Manassas, VA), and were maintained in McCoy’s 5A medium (ATCC) supplemented with 5% heat-inactivated FBS, 100 units/ml of penicillin, and 0.1 mg/ml of streptomycin (Mediatech, Herndon, VA) at 37 °C with 5% CO2 and 95% relative water humidity. Both cell lines were used between 3 and 30 passages. Atorvastatin and celecoxib were obtained from LKT Laboratories (St. Paul, MN). TTs and TPs were purchased from Palm Oil Company (Malaysia). ATST, TTs, and CXIB were dissolved in DMSO, while mevalonate and squalene were dissolved in ethanol. The final amount of DMSO or ethanol used was equal or less than 0.1% of the cell growth medium volume. For MTT assays, HCT116 (2,500 cells/well) and HT29 (5,000 cells/well) were seeded in 96-well plates. After 24 h, cells in each well were treated with different concentrations of ATST, TT, CXIB, and their combinations in 200 μl of cell growth medium containing 5% FBS and antibiotics. At 24, 48, and 72 h after treatment, the cell growth medium was replaced by 100 μL fresh medium containing 50 μg of MTT. After incubation at 37 °C for 1 h, MTT-containing medium was removed and the reduced formazan dye was solubilized by adding 100 μl of DMSO to each well. After gentle mixing, the absorbance was measured at 550 nm using a micro-plate reader (TECAN, Phenix Research Products, Candler, NC).
Cell cycle analysis
HCT116 (8 × 104 cells/well) and HT29 (1 × 105 cells/well) cells were seeded in 6-well plates. After 24 h, cells were treated with different concentrations of ATST, γ-TT, CXIB, and their combinations. After another 24 h, medium containing floating cells was collected and combined with adherent cells that were detached by brief trypsinization (0.25% trypsin-EDTA, Mediatech). Cell pellets were washed with 1 ml of ice-cold PBS, resuspended in 0.5 ml of 70% ethanol, and kept at −20 °C overnight. The next day, cells were pelleted by centrifugation, resuspended in 0.5 ml of PBS containing 50 μg RNase A and 5 μg propidium iodide (PI), and incubated in dark at room temperature for 30 min. Cells were fully resuspended and cell cycle was analyzed using the Beckman Coulter Cytomics FC500 flow cytometer at the Analytical Cytometry/Image Analysis Core Facility in Rutgers University. The data were processed using AXP acquisition and analysis software.
Flow cytometry of Annexin-V and propidium iodide co-stained cells
Apoptotic cells were quantified by Annexin V/PI double staining followed by flow cytometry. Annexin V/PI staining was performed using the Apo-detect kit (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. In brief, cells were harvested as described in the cell cycle analysis section 48 h after treatment and washed with ice-cold 1xPBS. Next, 7 × 104 cells were resuspended in 200 μl of binding buffer containing Annexin V, and incubated in dark at room temperature for 10 min. The cells were pelleted by centrifugation and resuspended in 200 μl of binding buffer containing 5 μg/ml PI for 5 min in dark at room temperature. Early apoptotic cells were detected as Annexin V positive and PI negative using Beckman Coulter Cytomics FC500 flow cytometer. The data were processed using AXP acquisition and analysis software.
DNA fragmentation assay
Cells (1.5 × 106/dish) were seeded in 100 × 20 mm tissue culture dishes, treated for 48 h, and harvested as described in the cell cycle analysis section. The cell pellets were resuspended in DNA lysis buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM MaCl2, and 0.5% Triton X-100) and put on ice for 30 min. The supernatant containing fragmented DNA was subjected to centrifugation (14,000 × g, at 4 °C for 15 min) and incubated with RNase A (final concentration 0.2 mg/ml) at room temperature overnight and then with proteinase K (final concentration 0.1 mg/ml) at 37 °C for 2 h. The resulting supernatant was extracted with equal volumes of phenol:chloroform:isoamyl alcohol (25:24:1, v/v/v) twice. The aqueous phase was collected, and the fragmented DNA was precipitated with two volume of ethanol at −20 °C for 2 h, collected by centrifugation (15,000 × g, at 4 °C for 15 min), and washed once with 70% ethanol. The DNA pellet was air-dried and dissolved in EB buffer (10 mM Tris-HCl, pH 8.5). The DNA samples were revolved by 1% agarose gel electrophoresis and visualized under a UV illuminator followed by Polaroid photography. The fragmented DNA appeared as multiples of 180 bp.
Immunoblotting
The cells were seeded and harvested as described in the DNA fragmentation assay section and resuspended in 1×cell lysis buffer (Cell Signaling, Beverly, MA) supplemented with cocktails of protease inhibitor, phosphatase inhibitor 1, and phosphatase inhibitor 2 (all inhibitors were used in a 1:100 dilution). The cell suspension was then subjected to sonication for 5 sec at a time for three times. After incubation on ice for 30 min, the supernatant was collected by centrifugation at 15,000 × g, for 10 min. For the analysis of full length HMG-CoA reductase, the cell lysate after incubation on ice was mixed with equal volume of strong denaturing buffer (8M urea, 15% SDS, 20% glycerol, 25 mg/ml DTT, 25 μM ALLN, 0.1 mM PMSF, and 0.25% bromophenol blue). The mixture was then dialyzed in Laemmli’s SDS-sample buffer (50 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol) at room temperature for 2 h. After the protein concentration was quantified by BCA protein assay reagets (Pierce Biotechnology, Rockford, IL), 25-50 μg of protein samples were resolved by SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Proteins of interest were probed using different primary antibodies at the concentrations recommended by manufacturers followed by incubation with suitable secondary antibodies conjugated with infrared-dye. Labeled proteins were visualized using the Odyssey infrared imaging system (LI-COR, NE). The primary antibodies for cleaved caspase-3 (Asp175), cleaved-PARP, and Na+/K+ATPase were from Cell Signaling (Beverly, MA). The primary antibodies for RhoA (26C4), k-Ras (F234), and HMG-CoA reductase were from Santa Cruz Biotechnology (Santa Cruz, CA). The primary antibodies for p21Cip1/Waf1 and β-actin were from Upstate (Lake Placid, NY) and Sigma-Aldrich, respectively.
Cell cytosolic and cytoplasm membrane fraction preparation
Cells were harvested as described in the DNA fragmentation assay section and resuspended in membrane preparation buffer (10 mM Tris-HCl, pH 7.5, 1 mM EDTA, 1 mM EGTA, 0.1 mM dithiothreitol, supplemented with protease and phosphatase inhibitor cocktails at a 1 to 100 dilution). Cells were sonicated on ice 5 sec each time for 4 times, and then centrifuged at 4 °C, 25,000 × g for 1 h. The supernatant was collected as the cytosolic fraction. The pellet was washed once with membrane preparation buffer, solublized with total cell lysis buffer (Cell signaling, Beverly, MA) containing protease and phosphatase inhibitor cocktails and put on ice for 30 min after thorough mixing. The dissolved membrane protein remained in the supernatant were collected after centrifugation at 4 °C, 10,000 × g for 10 min.
Statistical analysis
The analysis of synergistic action was based on Chou and Talalay’s empirical method26 with modifications as described previously.25 It is assumed that the dose response model follows log [E/(1 - E)] = α (log d - log Dm), which is a linear regression model with the response log [E/(1 - E)] and the regressor log (d). This model is used for compound 1, compound 2, and the combination of the two compounds at a fixed ratio of concentrations. E is the fraction of cells that survived, d is the dose applied, Dm is the median effective dose of a compound (IC50), and α is a slope parameter. On the basis of this regression model, median effect plots were constructed using data from MTT assays. If the combination (d1, d2) elicits the same effect x as compound 1 alone at dose level Dx,1, and as compound 2 alone at dose Dx,2, then the interaction index = d1/Dx,1 + d2/Dx,2 (d1, d2, Dx,1, and Dx,2 were calculated from median effect models). The interaction index value of 1, <1, or >1 indicates additive, synergistic, or antagonistic effect of the combination at dose (d1, d2), respectively. The delta method was used to calculate the variance (Var) of the interaction index, which is given by Var (interaction index) = Var(Dx,1)(d12/Dx,14) + Var(Dx,2)(d22/Dx,24). Data were analyzed by R programming language.
Most of the data were presented as mean ± S.D. The difference among more than 2 groups were determined by one-way analyses of variance (ANOVA) followed by Tukey’s B post-hoc test using SPSS statistical analysis software. The significance level used for all the tests was α = 0.05.
RESULTS
Synergistic effect of ATST and TT in the inhibition of HT29 and HCT116 cell growth
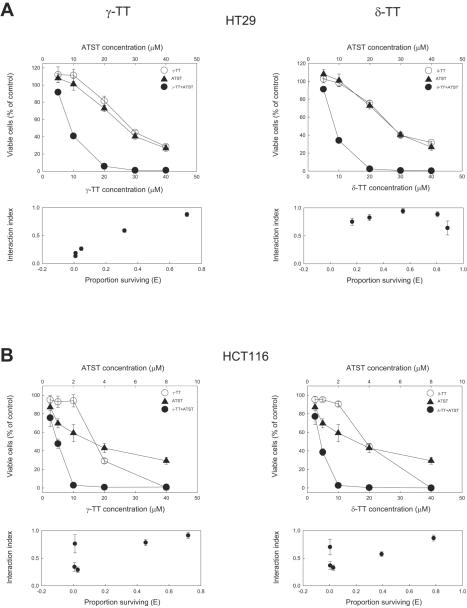
In order to study the possible synergistic action between ATST and vitamin E, we examined the cell growth inhibitory effects of δ- and γ-isomers of tocopherols and tocotrienols in two human colon cancer cell lines HT29 and HCT116. γ-TT and δ-TT were found to have much more inhibitory activity than the corresponding tocopherols (data not shown). Therefore, the possible synergistic action between ATST and γ-TT (or δ-TT) was investigated. Both ATST and γ-TT showed dose-dependent (Fig. 2, left panels) and time-dependent (data not shown) growth inhibition on these two cell lines. HCT116 cells (the IC50 values of ATST and γ-TT are 3.5 μM and 17.5 μM, respectively, Fig. 2B, left panels) were more sensitive than HT29 cells (the IC50 values of both ATST and γ-TT are 30 μM, Fig. 2A, left panels) to these compounds. HCT116 cells were especially more susceptible to the inhibition by ATST. To study the effect of the combination of these two compounds, we used different concentrations of ATST/γ-TT at ratios of 1:1 and 1:5 for HT29 and HCT116 cells, respectively, based on the IC50 values of ATST or γ-TT alone in these two cell lines. The combined treatments produced more pronounced cell growth inhibition on these two cancer cell lines (the IC50 values of both ATST and γ-TT are 9μM in HT29 cells; the IC50 values of ATST and γ-TT are 0.72 μM and 3.6 μM, respectively, in HCT116 cells) To investigate the existence of a possible synergistic effect, an isobologram analysis for the two-drug interaction was performed.26 The interaction index effect plots demonstrated that all combined doses at the fixed ratio generated an interaction index lower than 1.0 in both cell lines, suggesting a synergistic action between ATST and γ-TT (Fig. 2, left panels). δ-TT exhibited a similar pattern of inhibition as γ-TT on colon cancer cell growth when used alone or in combination with ATST (the IC50 values of δ-TT alone are 30 μM and 20 μM for HT29 cells and HCT116 cells, respectively; the IC50 values of δ-TT in combination with ATST are 9 μM and 3.7 μM, respectively, in HCT116 cells). In contrast, the combination of ATST and γ- or δ-tocopherol showed only marginal increases of cell growth inhibition as compared to ATST alone (data not shown).
Fig. 2.
Growth inhibitory effects of ATST, TTs, and their combination. Human colon cancer cells HT29 (A) and HCT116 (B) were treated with different concentrations of ATST, γ-TT (or δ-TT), and their combination for 48 h. Viable cells were measured by MTT assay, and are shown as a percentage of the value of the respective control. The synergistic effect between ATST and TTs was determined by the interaction index plot constructed using the method of Chou and Talalay.16 Synergy was indicated by interaction index lower than 1.0. Error bars in interaction index plots represent 95% confidence intervals for the interaction indices and were calculated using the delta method (n=6).
Mevalonate and geranylgeranyl pyrophosphate counteracted the cell growth inhibitory effect induced by ATST
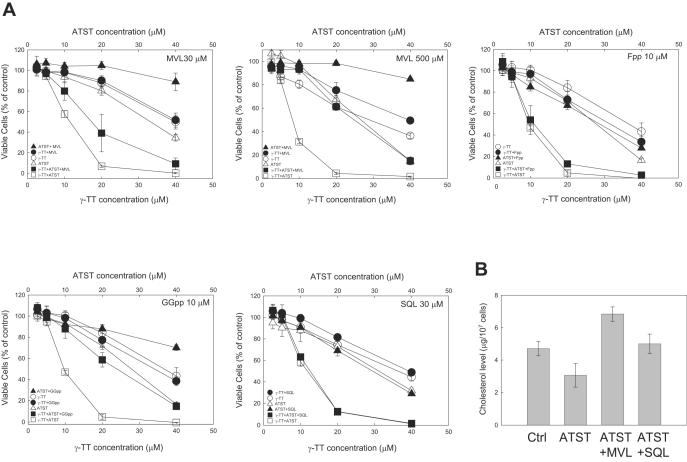
ATST inhibits HMG-CoA reductase and blocks the synthesis of mevalonate and subsequently, the synthesis of several isoprenoids, such as farnesyl pyrophosphate (Fpp) and geranylgeranyl pyrophosphate (GGpp) (Fig. 1). We investigated whether the growth inhibitory effects of ATST, γ-TT, and their combination could be counteracted by adding different intermediates in the cholesterol synthesis pathway, including mevalonate, Fpp, GGpp, and squalene (shown in boxes in Fig. 1), back in the cell culture system. Interestingly, the addition of mevalonate and GGpp eliminated the majority of the growth inhibitory effect of ATST, but had little effect on the inhibitory action of γ-TT (Fig. 3A, the IC50 value of γ-TT remained almost unchanged at about 30 μM; whereas the IC50 value of ATST increased from 30 μM to beyond 40 μM). Fpp and squalene, two major intermediates of cholesterol synthesis, however, did not affect the growth inhibition by ATST or γ-TT (the IC50 values of both ATST and γ-TT remained unchanged at about 30 μM). In the combined treatment of ATST and γ-TT, mevalonate and GGpp appeared only to restore the cell growth that was inhibited by ATST, but not that of γ-TT (the IC50 values of both ATST and γ-TT increased from 9 μM to about 30 μM after the supplement of 500 μM mevalonate or GGpp, but not beyond 40 μM as in ATST treatment alone; whereas the IC50 values remained unchanged at about 30 μM after the supplement of Fpp or squalene). This result suggests that GGpp is important for cell growth; addition of mevalonate could restore GGpp level under ATST treatment. The addition of Fpp could not restore GGpp level because this restoration would require isopentenyl pyrophosphate, which was not available after mevalonate depletion caused by ATST treatment (Fig. 1). Next, we examined whether the depletion of cholesterol caused by ATST was responsible for the cell growth inhibition. Although both mevalonate and squalene restored the cellular cholesterol level that was decreased by ATST, squalene did not counteract the cell growth inhibition induced by ATST, suggesting that the reduced cellular cholesterol level was not responsible for suppressing cell growth in our experimental system (Fig. 3B).
Fig. 3.
Effects of mevalonate (MVL), farnesyl pyrophosphate (Fpp), geranylgeranyl pyrophosphate (GGpp), and squalene (SQL) on cell growth inhibition induced by ATST, γ-TT, and their combination. (A) HT29 cells were treated with different concentrations of ATST, γ-TT, and their combination in the absence or presence of mevalonate (30 and 500 μM), and other intermediates in the cholesterol synthesis pathway including Fpp (10 μM), GGpp (10 μM), and SQL (30 μM) for 48 h. Viable cells were measured by MTT assay. (B) HT29 cells were treated with 20 μM ATST alone, or ATST plus MVL (250 μM) or SQL (250 μM). The total cellular cholesterol level of each treatment was determined by Wako cholesterol E kit.
Both ATST and γ-TT acted on HMG-CoA reductase
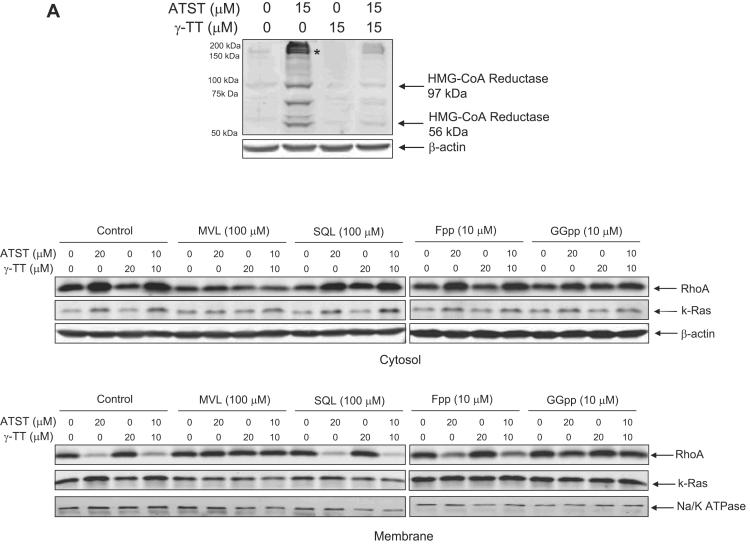
In cells treated with ATST, the inhibition of HMG-CoA reductase activity triggers an increase of HMG-CoA reductase level to compensate for the loss of enzyme activity.27, 28 γ-TT has been suggested to decrease the enhanced level of HMG-CoA reductase.12, 13, 28 The same results were indeed observed in our experimental system (Fig. 4A). ATST increased the level of both the full length HMG-CoA reductase (97 kDa) and a 56-kDa soluble fragment of HMG-CoA reductase.29 The anti-HMG-CoA reductase antibody also detected the same change for a high molecular weight band (indicated with a star in Fig. 4A). This band is likely to be a fraction containing the multimeric form of HMG-CoA reductase.27 Addition of γ-TT attenuated the level of all HMG-CoA reductase bands that were elevated by ATST. These results suggest that ATST inhibited HMG-CoA reductase activity, depleted downstream isoprenoids, and elevated the levels of HMG-CoA reductase, whereas γ-TT neutralized the upregulated level of HMG-CoA reductase, resulting in a synergistic action between the two compounds
Fig. 4.
Effects of ATST, γ-TT, and their combination on the level of HMG-CoA reductase and the cellular distribution of RhoA. (A) HT29 cells were treated with ATST, γ-TT, and their combination for 24 h at the concentrations indicated. The protein level of HMG-CoA reductase was determined by Western-blot with an anti-HMG-CoA reductase antibody. β-actin served as an equal loading control. (B) HT29 cells were treated with ATST, γ-TT, and their combination for 24 h at the concentrations indicated, with the supplement of different intermediate products in the cholesterol biosynthesis pathway including mevalonate (MVL, 100 μM), Fpp (10 μM), GGpp (10 μM) and squalene (SQL, 100 μM). The cytosolic and membrane fractions of HT29 cells were prepared as described previously15 and the levels of RhoA and k-Ras proteins were determined by Western-blots. β-actin and Na+/K+ ATPase served as equal loading controls for the cytosolic and membrane fractions, respectively.
ATST and γ-TT affected the cellular distribution of RhoA protein
The isoprenoids (Fpp, GGpp) are essential for the modification of certain signaling proteins. It is known that small G proteins undergo isoprenylation and their isoprenylation is essential for their translocation from the cytoplasm to the plasma membrane, where they become functionally active.30-33 Since ATST affects the production of GGpp, we examined whether the cellular distribution of RhoA, a small G-protein that is solely under geranylgeranylation, was affected by ATST or γ-TT treatment. Our data demonstrated that ATST treatment reduced the level of membrane-bound RhoA, but increased RhoA level in the cytosol (Fig. 4B). Co-treatment of cells with 10 μM γ-TT in addition to 10 μM ATST led to a similar or stronger effect as treatment with 20 μM ATST, even though γ-TT alone at 20 μM exhibited no effect. Supplement with mevalonate and GGpp, but not Fpp or squalene restored the level of membrane-bound RhoA that was reduced by ATST, consistent with the ability of these compounds to restore cancer cell growth (Fig 4B). Interestingly, k-Ras, another important small G-protein that is mainly farnesylated, did not exhibit a significant change after ATST or γ-TT treatment (Fig. 4B). Similar results were observed in our previous work.25 Moreover, addition of mevalonate, Fpp, GGpp, and squalene did not alter the cellular distribution of k-Ras (Fig 4B). Taken together, these results suggest RhoA, but not k-Ras, is a key protein that is affected by ATST and γ-TT treatment, which contributes to the synergistic action of these two compounds.
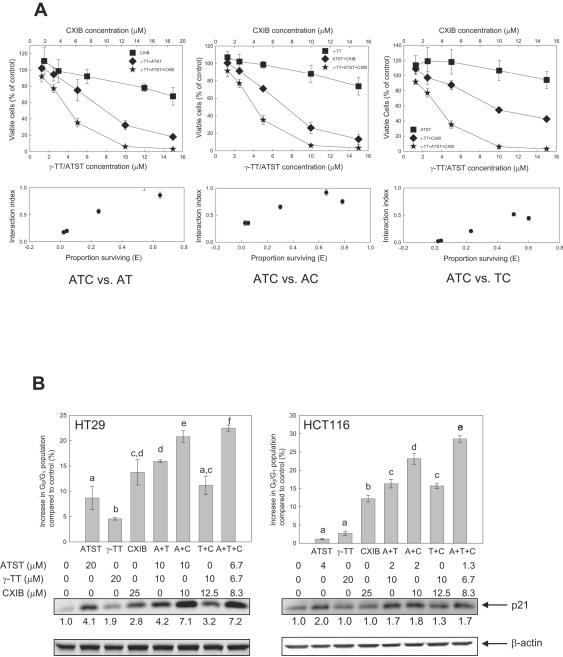
Triple combination of ATST, γ-TT, and CXIB synergistically inhibited cancer cell growth
We previously reported that the combination of ATST and CXIB, a cyclooxyhenase-2 (COX-2) inhibitor, synergistically induced cell cycle arrest and apoptosis in colon cancer cells.25 Here we examined whether the triple combination of ATST, γ-TT, and CXIB could further produce synergy in inhibiting cancer cell growth (Fig. 5A). The synergy of triple combination was studied by considering two compounds at a fixed ratio as one agent and the third compound as the other agent and analyzing the synergy of these two agents. The triple combination of ATST, γ-TT, and CXIB on HT29 cells displayed a synergistic effect between each dual-compound combination (ATST/γ-TT, ATST/CXIB, or γ-TT/CXIB) and the third compound (the IC50 values of the triple combination is about 4 μM as compared to more than 8 μM in double combinations, Fig. 5A, lower panels). δ-TT again showed similar synergistic inhibition as γ-TT in the combination with ATST and CXIB (data not shown).
Fig. 5.
Synergistic cell growth inhibitory effect of the triple combination of ATST, γ-TT, and CXIB. (A) HT29 cells were treated with different concentrations of γ-TT, ATST, CXIB, and with their double and triple combinations for 48 h. Viable cells were measured by MTT assay. The synergy between any double combination and the third compound was determined by the interaction index plot constructed using the method of Chou and Talalay.16 AT, AC, and TC represent the double combinations of ATST plus γ-TT, ATST plus CXIB, and γ-TT plus CXIB, respectively; ATC represents the triple combination of ATST, γ-TT, and CXIB. (B) G0/G1 phase cell cycle arrest effect of ATST, γ-TT, CXIB, and their combinations. HT29 and HCT116 cells were treated with different concentrations of ATST, γ-TT, CXIB, and with their double and triple combinations for 24 h. Cells were stained with propidium iodide and cell cycle was analyzed by flow cytometry. The mean difference among groups was analyzed by one-way ANOVA followed by Tukey’s B test. The level of p21Cip1/Waf1, determined by Western-blot, is shown below the cell cycle analysis data. The numbers below the p21Cip1/Waf1 band are the folds of increase of p21Cip1/Waf1 as compared to control, after normalization by β-actin.
Combined treatment of ATST, γ-TT, and CXIB synergistically induced G0/G1 phase cell cycle arrest
To explore the mechanisms underlying the synergistic action of ATST, γ-TT, and CXIB in cancer cell growth inhibition, we performed cell cycle analyses by flow cytometry on both HT29 and HCT 116 cells 24 h after treatment. In HT29 cells, while ATST (20 μM) or γ-TT (20 μM) alone marginally induced G0/G1 phase cell cycle arrest (for example, the amount of cells in G0/G1 phase among total gated cells is 4.55% more in γ-TT treated group than in the non-treated group), the combination of ATST plus γ-TT and ATST plus CXIB, at a half dose of the corresponding individual compound (10 μM for both ATST and γ-TT, and 12.5 μM for CXIB), led to a significant increase of cells arrested in G0/G1 phase (15.93% more cells in G0/G1 phase). Triple combination of three compounds, at a one third dose of the corresponding individual compound (6.7 μM each for both ATST and γ-TT, and 8.3 μM for CXIB), induced even higher cell cycle arrest (22.47% more cells in G0/G1 phase) (Fig. 5B). A similar pattern was observed when HCT116 cells were treated with ATST (4 μM), γ-TT (20 μM), and CXIB (25 μM), their double (half dose of the corresponding single treatment) and triple (one third dose of the corresponding single treatment) combinations (Fig. 5B).
Cyclin-dependent kinase (CDK) inhibitors, such as p21Cip1/Waf1, are negative regulators of cell cycle progression. We next analyzed whether our treatments with ATST, γ-TT, CXIB, and with their double and triple combinations could affect the level of p21Cip1/Waf1. After 24 h of treatment when cell cycle arrest was observed, ATST alone induced an increase of p21Cip1/Waf1 level in HT29 cells. The double combination of ATST and γ-TT, at a half dose of the corresponding individual compound, led to a higher level of p21Cip1/Waf1. The double combination of ATST and CXIB, at a half dose of the corresponding individual compound; the triple combination of three compounds, at a one-third dose of the corresponding single compound, caused an even higher increase of p21Cip1/Waf1 level (Fig. 5B). The change in p21Cip1/Waf1 levels after triple combination treatment in HCT116 cells did not change significantly as compared to the double combinations, even though the increase of cell cycle arrest at G0/G1 phase was still observed.
Combined treatment of ATST, γ-TT, and CXIB synergistically induced cell apoptosis
Next, we examined whether the combined treatment of ATST, γ-TT, and CXIB synergistically induced cell apoptosis. HCT116 cells were treated with ATST, γ-TT, CXIB, and with their double and triple combinations for 48 h. The cells were then subjected to Annexin V and propidium iodide (PI) co-staining followed by flow cytometry analysis. Annexin V positive and PI negative cells are regarded as early apoptotic cells, whereas Annexin V and PI double stained cells are considered as late apoptotic or necrotic cells. Dot intensity of the Annexin V positive and PI negative region (B4) was not increased after any single compound treatment, but the combined treatment with ATST and γ-TT caused a significantly higher level of early apoptosis (45.17% of total gated cells). Moreover, triple combination of ATST, γ-TT, and CXIB induced higher apoptosis (53.46% of total gated cells) than any double combination (Fig. 6A).
Fig. 6.
Effect of ATST, γ-TT, CXIB, and their combinations on cancer cell apoptosis. (A) HCT116 cells were treated with ATST, γ-TT, CXIB, and their double and triple combinations with the concentrations indicated for 48 h. The cells were then co-stained with Annexin-V and propidium iodide (PI) and analyzed by flow cytometry. Dots located in the B4 region of the intensity dot plot are considered as early apoptosis. The percentage of early apoptotic cells among total gated cells is shown in the figure. (B). HT29 and HCT116 cells were treated with different concentrations of ATST, γ-TT, CXIB, and their double and triple combinations as indicated in the absence or presence of mevalonate (MVL, 250 μM) for 48 h. Cell apoptosis was determined by Western blot with antibodies against cleaved caspase-3 and cleaved PARP, and by DNA fragmentation assay.
To further investigate the apoptosis induced by ATST and γ-TT, we analyzed the protein level of cleaved caspase-3, a hallmark of cell apoptosis, by Western blot analysis. As shown in Fig. 6B, while the level of cleaved caspase-3 was not changed or only marginally increased after treatment with ATST alone, combined treatment with ATST and γ-TT, at a half dose of the corresponding individual compound, significantly increased the cleavage of caspase-3 (more prominent in HCT116 cells). Also, the triple combination, at a one-third dose of the corresponding individual compound, caused an even higher increase of cleaved caspase-3 in both cells, consistent with the Annexin V/PI co-staining results (Fig. 6A). We also analyzed the activation of poly ADP-ribose polymerase (PARP), a marker of DNA damage in apoptosis and a downstream effector of caspase-3 (Fig. 6B). ATST alone induced the cleavage of PARP. The combined treatment with ATST and CXIB, at a half dose of the corresponding individual compound, induced significantly more PARP cleavage in HT29 cells, but the change was not very significant in HCT116 cells when compared to the change induced by the treatment of ATST alone. The combination of ATST and γ-TT, at a half-dose of the corresponding individual compound, and the triple combination of ATST, γ-TT, and CXIB, at a one-third dose of the corresponding individual compound, caused a significantly higher level of cleaved PARP as compared to the levels associated with ATST treatment alone in both cell lines. DNA fragmentation, another indicator of cell apoptosis, was significantly increased in the double combination of ATST and γ-TT and in the triple combination of ATST, γ-TT, and CXIB as compared to the marginal DNA fragmentation in the treatment with ATST alone (Fig. 6B). These results suggest that the combined action of ATST, γ-TT, and CXIB was due to apoptosis induced by these compounds. The cleavage of caspase-3 and PARP, as well as DNA fragmentation was not observed after the addition of mevalonate, suggesting that the presence of mevalonate prevented cell apoptosis induced by ATST, γ-TT, CXIB, and their combinations (Fig. 6B).
DISCUSSION
In this study, we demonstrated that the combination of ATST and γ-TT synergistically inhibited colon cancer cell growth based on the results from MTT assays followed by isobologram analysis (Fig. 2). A normal human intestinal epithelial cell line INT407 also showed a dose-dependent inhibitory response with the treatment of ATST or γ-TT alone, but the combination of these two compounds did not produce any synergistic inhibition of cell growth (data not shown).
Our study demonstrated that ATST treatment resulted in an up-regulation of the full length HMG-CoA reductase (97 kDa) band and related bands with lower molecular weight (ranging from 56kDa to about 70 kDa) (Fig. 4A). The full length HMG-CoA reductase is an integral membrane protein of the endoplasmic reticulum, which could only be completely dissolved under strong denaturing conditions (15% SDS and 8 M urea). The 56-kDa-band is a soluble, enzymatically active HMG-CoA reductase fragment.29 The bands between the full length HMG-CoA reductase and the 56 kDa fragment are likely to be some intermediate products of HMG-CoA reductase degradation. The same antibody also detected a high molecular weight band (around 200 kDa); this band is likely to be the multimeric form of HMG-CoA reductase resulted from disulfide bond formation.27 The effects of ATST and γ-TT on the activity and the protein level of HMG-CoA reductase, respectively, could partly explain the synergistic action of these two compounds. The cell growth inhibition by γ-TT, when used alone, appears to be due to mechanisms other than the regulation of HMG-CoA reductase, because addition of mevalonate did not eliminate the cell growth inhibition induced by γ-TT (Fig. 3A, the IC50 value of γ-TT remained unchanged with or without the supplement of mevalonate).
We analyzed the effect of the treatment with ATST and γ-TT on the distribution of RhoA, a small G-protein that undergoes geranylgeranylation, but not farnesylation.1 The membrane-bound RhoA was significantly reduced as more RhoA was sequestered in cytosolic fraction after ATST treatment (Fig. 4B). Addition of γ-TT further reduced the level of membrane-bound RhoA, which is consistent with our result that γ-TT counteracted the induction of HMG-CoA reductase by ATST (Fig. 4A). Since ATST inhibited HMG-CoA reductase activity and the subsequent production of GGpp and Fpp (Fig. 1), and addition of GGpp, but not Fpp restored the membrane-bound RhoA, it is likely that ATST affected RhoA distribution and activity by regulating its geranylgeranylation. Noguchi et al. reported that RhoA protein undergoes a recycling process that is essential for the G1/S transition in the cell cycle. RhoA is eliminated from the membrane in mid-G1 phase and then re-associated with the membrane in late G1 phase; whereas Ras protein level in the membrane is unchanged during G1/S progression.34 Therefore, it is very likely that the depletion of GGpp by ATST inhibits the reappearance of RhoA on the membrane, and subsequently blocks G1/S transition and causes cell cycle arrest at the G1 phase (Fig. 5B). Since Ras protein is not actively translocated through cell progression from the G1 to S phase, farnesylation of Ras is not required, at least for G1/S transition.34 In the time frame of our study (24 h), more Ras protein was localized in the membrane fraction, and the level of Ras protein in the cytosol was relatively low. Treatment with ATST and γ-TT might slightly increase Ras protein level in the cytosol by sequestering Ras protein due to the depletion of Fpp. However, the membrane-bound Ras protein level did not significantly decrease (Fig. 4B). Under this condition, Ras protein does not contribute to the cancer cell growth inhibition induced by ATST and γ-TT. The relative stable membrane-distribution of Ras protein also abrogated the requirement for Fpp.
ATST and γ-TT exhibited their actions on colon cancer cell growth by synergistically inducing cell cycle arrest at the G0/G1 phase and apoptosis (Fig. 5B and Fig. 6). It has been reported that the protein level of p21Cip1/Waf1, a negative regulator of cell proliferation, can be repressed by RhoA protein.35 ATST inhibited the activation of RhoA and therefore reduced the level of p21Cip1/Waf1 (Fig. 5B). This is one of the molecular mechanisms that ATST induces cell growth inhibition through RhoA. The treatment with ATST and γ-TT also induced cell apoptosis activation of caspase-3, a hallmark of cell apoptosis, and subsequently induced the cleavage and activation of PARP and DNA fragmentation (Fig. 6B). The mechanisms of how ATST mediates cell apoptosis are not clear, but are very likely to be related to the depletion of isoprenoids because addition of mevalonate significantly reduced the activation of caspase-3 and PARP, and eliminated DNA fragmentation (Fig. 6B). Previously, we reported that the combination of ATST and CXIB also exhibited synergistic action to inhibit colon cancer cell growth.25 However, when we compared these two types of combinations side by side, we found that the combination of ATST and γ-TT tended to induce more cell apoptosis, whereas the combination of ATST and CXIB induced more cell cycle arrest at G0/G1 phase (Fig. 5B and Fig. 6). Therefore, it is likely that the triple combination of ATST, γ-TT, and CXIB would display more synergistic anti-cancer action than the combinations of any two compounds. Indeed, the triple combination showed synergistic inhibitory action between any double combination (which is regarded as a single agent) and the third compound in MTT assays (Fig. 5A). The synergy among these three compound can also be directly analyzed using CompuSyn program developed by Chou et al.36 The interaction index calculated by this program was less than 1 for every concentration of the triple-compound combination in Fig. 5A, indicating that there was a synergy among ATST, γ-TT, and CXIB.
We noticed that ATST is the key agent in the combination studies; γ-TT or CXIB alone, or their combination did not exhibit a significant inhibition of cancer cell growth. However, γ-TT and CXIB synergistically potentiated the inhibitory effect of ATST. The mechanisms behind this phenomenon still need to be further elucidated.
In summary, we demonstrated that ATST and γ-TT exhibited a strong synergy against human colon cancer cells in causing cell cycle arrest and apoptosis. We proposed that the modulation of HMG-CoA reductase and small G-protein geranylgeranylation are the key mechanisms for the observed synergistic action. Triple combination of ATST, γ-TT, and CXIB further inhibited cancer cell growth. Our combination strategy produced a significant inhibition of cancer cell growth at a relatively low concentration of each individual compound, thus reducing the potential toxicity of these compounds. We anticipate that these results will encourage further studies on the use of the combinations of these compounds in cancer prevention and therapy.
ACKNOWLEDGMENTS
The authors thank Dr. Sonia Picinich, Dr. Yang Zhao, and Ms. Shea Loy for their suggestions and assistance in the preparation of this manuscript. This work was supported by NIH grants CA120915 and CA133021.
Abbreviation
- ATST
atorvastatin
- Cox-2
cyclooxyhenase-2
- CXIB
celecoxib
- FPP
farnesyl pyrophosphate
- GGpp
geranylgeranyl pyrophosphate
- HMG-CoA
3-hydroxy-3-methylglutaryl coenzyme A
- MTT
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
- PARP
poly ADP-ribose polymerase
- TT
tocotrienol
REFERENCES
- 1.Holstein SA, Wohlford-Lenane CL, Wiemer DF, Hohl RJ. Isoprenoid pyrophosphate analogues regulate expression of Ras-related proteins. Biochemistry. 2003;42:4384–91. doi: 10.1021/bi027227m. [DOI] [PubMed] [Google Scholar]
- 2.Aznar S, Lacal JC. Rho signals to cell growth and apoptosis. Cancer Lett. 2001;165:1–10. doi: 10.1016/s0304-3835(01)00412-8. [DOI] [PubMed] [Google Scholar]
- 3.Fromigué O, Haÿ E, Modrowski D, Bouvet S, Jacquel A, Auberger P, Marie PJ. RhoA GTPase inactivation by statins induces osteosarcoma cell apoptosis by inhibiting p42/p44-MAPKs-Bcl-2 signaling independently of BMP-2 and cell differentiation. Cell Death Differ. 2006;13:1845–56. doi: 10.1038/sj.cdd.4401873. [DOI] [PubMed] [Google Scholar]
- 4.Fromigué O, Hamidouche Z, Marie PJ. Blockade of the RhoA-JNK-c-Jun-MMP2 cascade by atorvastatin reduces osteosarcoma cell invasion. J Biol Chem. 2008;283:30549–56. doi: 10.1074/jbc.M801436200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn KS, Sethi G, Krishnan K, Aggarwal BB. Gamma-tocotrienol inhibits nuclear factor-kappaB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J Biol Chem. 2007;282:809–20. doi: 10.1074/jbc.M610028200. [DOI] [PubMed] [Google Scholar]
- 6.Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, Cottens S, Takada Y, Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7:687–92. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 7.Rao S, Porter DC, Chen X, Herliczek T, Lowe M, Keyomarsi K. Lovastatin-mediated G1 arrest is through inhibition of the proteasome, independent of hydroxymethyl glutaryl-CoA reductase. Proc Natl Acad Sci USA. 1999;96:7797–802. doi: 10.1073/pnas.96.14.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–42. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- 9.Constantinou C, Papas A, Constantinou AI. Vitamin E and cancer: An insight into the anticancer activities of vitamin E isomers and analogs. Int J Cancer. 2008;123:739–52. doi: 10.1002/ijc.23689. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal MK, Agarwal ML, Athar M, Gupta S. Tocotrienol-rich fraction of palm oil activates p53, modulates Bax/Bcl2 ratio and induces apoptosis independent of cell cycle association. Cell Cycle. 2004;3:205–11. doi: 10.4161/cc.3.2.654. [DOI] [PubMed] [Google Scholar]
- 11.Theriault A, Chao JT, Gapor A. Tocotrienol is the most effective vitamin E for reducing endothelial expression of adhesion molecules and adhesion to monocytes. Atherosclerosis. 2002;160:21–30. doi: 10.1016/s0021-9150(01)00540-8. [DOI] [PubMed] [Google Scholar]
- 12.Parker RA, Pearce BC, Clark RW, Gordon DA, Wright JJ. Tocotrienols regulate cholesterol production in mammalian cells by post- transcriptional suppression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem. 1993;268:11230–38. [PubMed] [Google Scholar]
- 13.Song BL, DeBose-Boyd RA. Insig-dependent ubiquitination and degradation of 3-hydroxy-3-methylglutaryl coenzyme a reductase stimulated by delta- and gamma-tocotrienols. J Biol Chem. 2006;281:25054–61. doi: 10.1074/jbc.M605575200. [DOI] [PubMed] [Google Scholar]
- 14.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci U S A. 1997;94:3336–40. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–6. [PubMed] [Google Scholar]
- 16.Liu XH, Kirschenbaum A, Yao S, Stearns ME, Holland JF, Claffey K, Levine AC. Upregulation of vascular endothelial growth factor by cobalt chloride-simulated hypoxia is mediated by persistent induction of cyclooxygenase-2 in a metastatic human prostate cancer cell line. Clin Exp Metastasis. 1999;17:687–94. doi: 10.1023/a:1006728119549. [DOI] [PubMed] [Google Scholar]
- 17.Grösch S, Maier TJ, Schiffmann S, Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst. 2006;98:736–47. doi: 10.1093/jnci/djj206. [DOI] [PubMed] [Google Scholar]
- 18.Kardosh A, Blumenthal M, Wang WJ, Chen TC, Schönthal AH. Differential effects of selective COX-2 inhibitors on cell cycle regulation and proliferation of glioblastoma cell lines. Cancer Biol Ther. 2004;3:55–62. doi: 10.4161/cbt.3.1.571. [DOI] [PubMed] [Google Scholar]
- 19.Grösch S, Tegeder I, Niederberger E, Bräutigam L, Geisslinger G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 2001;15:2742–4. doi: 10.1096/fj.01-0299fje. [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, Song SH, Kim SG, Chun KS, Lim SY, Na HK, Kim JW, Surh YJ, Bang YJ, Song YS. Celecoxib induces apoptosis in cervical cancer cells independent of cyclooxygenase using NF-kappaB as a possible target. J Cancer Res Clin Oncol. 2004;130:551–60. doi: 10.1007/s00432-004-0567-6. [DOI] [PubMed] [Google Scholar]
- 21.Maier TJ, Janssen A, Schmidt R, Geisslinger G, Grösch S. Targeting the beta-catenin/APC pathway: a novel mechanism to explain the cyclooxygenase-2-independent anticarcinogenic effects of celecoxib in human colon carcinoma cells. FASEB J. 2005;19:1353–5. doi: 10.1096/fj.04-3274fje. [DOI] [PubMed] [Google Scholar]
- 22.Wallace JL. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997;112:1000–16. doi: 10.1053/gast.1997.v112.pm9041264. [DOI] [PubMed] [Google Scholar]
- 23.Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M, Adenoma Prevention with Celecoxib (APC) Study Investigators Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–80. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 24.Psaty BM, Potter JD. Risks and benefits of celecoxib to prevent recurrent adenomas. N Engl J Med. 2006;355:950–2. doi: 10.1056/NEJMe068158. [DOI] [PubMed] [Google Scholar]
- 25.Xiao H, Zhang Q, Lin Y, Reddy BS, Yang CS. Combination of atorvastatin and celecoxib synergistically induces cell cycle arrest and apoptosis in colon cancer cells. Int J Cancer. 2008;122:2115–24. doi: 10.1002/ijc.23315. [DOI] [PubMed] [Google Scholar]
- 26.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 27.Parker RA, Miller SJ, Gibson DM. Phosphorylation of native 97-kDa 3-hydroxy-3-methylglutaryl-coenzyme A reductase from rat liver. Impact on activity and degradation of the enzyme. J Biol Chem. 1989;264:4877–87. [PubMed] [Google Scholar]
- 28.Ravid T, Doolman R, Avner R, Harats D, Roitelman J. The Ubiquitin-Proteasome Pathway Mediates the Regulated Degradation of Mammalian 3-Hydroxy-3-methylglutaryl-coenzyme A Reductase. J Biol Chem. 2000;275:35840–47. doi: 10.1074/jbc.M004793200. [DOI] [PubMed] [Google Scholar]
- 29.Ness GC, Way SC, Wickham PS. Proteinase involvement in the solubilization of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Biochem Biophys Res Commun. 1981;102:81–5. doi: 10.1016/0006-291x(81)91491-1. [DOI] [PubMed] [Google Scholar]
- 30.Willumsen BM, Christensen A, Hubbert NL, Papageorge AG, Lowy DR. The p21 ras C-terminus is required for transformation and membrane association. Nature. 1984;310:583–6. doi: 10.1038/310583a0. [DOI] [PubMed] [Google Scholar]
- 31.Casey PJ, Thissen JA, Moomaw JF. Enzymatic modification of proteins with a geranylgeranyl isoprenoid. Proc Natl Acad Sci U S A. 1991;88:8631–5. doi: 10.1073/pnas.88.19.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bokoch GM, Bohl BP, Chuang TH. Guanine nucleotide exchange regulates membrane translocation of Rac/Rho GTP-binding proteins. J Biol Chem. 1994;269:31674–9. [PubMed] [Google Scholar]
- 33.Ellenbroek SI, Collard JG. Rho GTPases: functions and association with cancer. Clin Exp Metastasis. 2007;24:657–72. doi: 10.1007/s10585-007-9119-1. [DOI] [PubMed] [Google Scholar]
- 34.Noguchi Y, Nakamura S, Yasuda T, Kitagawa M, Kohn LD, Saito Y, Hirai A. Newly synthesized Rho A, not Ras, is isoprenylated and translocated to membranes coincident with progression of the G1 to S phase of growth-stimulated rat FRTL-5 cells. J Biol Chem. 1998;273:3649–53. doi: 10.1074/jbc.273.6.3649. [DOI] [PubMed] [Google Scholar]
- 35.Adnane J, Bizouarn FA, Qian Y, Hamilton AD, Sebti SM. p21(WAF1/CIP1) is upregulated by the geranylgeranyltransferase I inhibitor GGTI-298 through a transforming growth factor beta- and Sp1-responsive element: involvement of the small GTPase rhoA. Mol Cell Biol. 1998;18:6962–70. doi: 10.1128/mcb.18.12.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chou TC. Preclinical versus clinical drug combination studies. Leuk Lymphoma. 2008;49:2059–80. doi: 10.1080/10428190802353591. [DOI] [PubMed] [Google Scholar]