Abstract
Objective and Methods
Russia has one of the fastest growing HIV epidemics in the world, and screening has been widespread. Whether such screening is an effective use of resources is unclear. We used epidemiologic and economic data from Russia to develop a Markov model to estimate costs, quality of life, and survival associated with a voluntary HIV screening program compared to no screening in Russia. We measured discounted lifetime health care costs and quality-adjusted life years (QALYs) gained. We varied our inputs in sensitivity analysis.
Results
Early identification of HIV through screening provided a substantial benefit to persons with HIV, increasing life expectancy by 2.1 years and 1.7 QALYs. At a base-case prevalence of 1.2%, once-per-lifetime screening cost $13,396 per QALY gained, exclusive of benefit from reduced transmission. Cost-effectiveness of screening remained favorable until prevalence dropped below 0.04%. When HIV transmission-related costs and benefits were included, once-per-lifetime screening cost $6,910 per QALY gained, and screening every two years cost $27,696 per QALY gained. An important determinant of the cost-effectiveness of screening was effectiveness of counseling about risk reduction.
Conclusions
Early identification of HIV infection through screening in Russia is effective and cost-effective in all but the lowest prevalence groups.
Keywords: Russia, HIV, AIDS, screening, prevention, cost effectiveness
INTRODUCTION
Russia has one of the world’s fastest growing HIV epidemics. Driven by an increase in injection drug use and commercial sex after the dissolution of the Soviet Union, HIV prevalence doubled annually between 1995 and 2001 (1–3). Recent data suggests the epidemic is becoming generalized, with many cases now associated with heterosexual contact or vertical transmission (4, 5). While there are more than 380,000 officially registered HIV/AIDS cases in Russia (1), experts estimate that more than one million people are infected, which corresponds to a prevalence of 1.2% in the 15- to 49-year-old age group (2, 5–8). Since 80% of cases are in individuals under 30 years of age, in stark contrast to Western epidemics (5, 8), HIV could be especially detrimental to Russia’s economy, compounding effects of its already declining population size.
Early detection of HIV through voluntary screening is important for treatment and for reducing HIV transmission. Early identification can provide the opportunity for timely treatment of infected individuals, thus reducing morbidity and mortality (9). Additionally, the decrease in risky behavior resulting from HIV counseling and the reduction in infectivity due to use of antiretroviral therapy (ART) (9–14) can translate into a significant benefit from reduced HIV transmission (9).
Since the late 1980’s, screening for HIV in Russia has been widespread (4, 8). Whether such screening is an effective and efficient use of resources is unclear, particularly since many individuals are screened multiple times each year, and high-risk individuals may not undergo screening at all (2, 15, 16). Consequently, many infections are likely undiagnosed, leading experts to report that the prevalence of HIV is at least three times the officially registered number (5, 8). Furthermore, cases are often detected late in the course of illness: most people with HIV/AIDS in Russia survive only 3–5 years after initial diagnosis (8, 17). While pre- and post-test counseling are mandated by the Russian Federal AIDS Act of 1995, the extent and nature of counseling is unclear (18), potentially reducing the program’s effectiveness in decreasing the spread of HIV.
In the United States, a number of recent studies have demonstrated the cost-effectiveness of expanded screening for HIV (9, 19–21). However, the generalizability of this finding to other countries is unclear, and there have been extremely limited evaluations of costs and benefits of screening in middle- and low-income countries (22). Because of the importance of the epidemic in Russia, we sought to evaluate the cost-effectiveness of a voluntary HIV screening and counseling program in Russia.
METHODS
Model overview
We developed a Markov model using Decision Maker software to estimate the health-related costs and benefits of a voluntary HIV screening program in Russia. We followed a cohort of 15- to 49-year-old individuals over their lifetimes. Our model included HIV natural history, voluntary testing and counseling, HIV transmission, and treatment with ART. Our model included natural history data to estimate disease progression without ART, and estimated relative hazard of death based on changing viral loads and CD4 counts (9). We measured health-related costs and life expectancy in quality-adjusted life years (QALYs), both discounted at 3% annually. A detailed description of the model structure, assumptions, and parameters has been published previously (9).
We evaluated three voluntary screening strategies: no HIV screening, once-per-lifetime HIV screening, and repeat HIV screening. Each month, patients could undergo HIV testing through symptom-based case finding and/or a screening program. We evaluated screening intervals of every one to five years in the repeat HIV screening strategies; such screening intervals are commonly reported in the literature (9, 21).
For each screening program, we calculated lifetime per person costs and QALYs, as well as incremental cost-effectiveness ratios. Cost-effectiveness ratios were interpreted using criteria developed by the World Health Organization (23). WHO guidelines propose that interventions that cost less than three times the per capita gross domestic product (GDP) are cost-effective, and interventions costing less than the per capita GDP are very cost-effective. Russia’s per capita GDP in 2006 was approximately $12,000.
Treatment regimens
Patients identified with HIV initiated ART once their CD4 counts dropped below 350 cells per mm3. In our base-case analyses, we estimated health outcomes and costs for the case in which 50% of patients had access to treatment with ART; we examined lower and higher rates in sensitivity analyses. We modeled treatment with three suppressive ART regimens, followed by lifelong nonsuppressive therapy. Such comprehensive treatment is not always available currently, but reflects the recent emphasis on expanding ART in Russia (6, 24)..
HIV epidemic data
Our analysis focused on individuals aged 15 to 49 in health care settings whose HIV status was unknown (Table 1). The cohort had an average age of 32.5 years, which is the average age of 15- to 49-year-olds in Russia (25). Based on data from the Joint United Nations Programme on HIV/AIDS (UNAIDS) and the World Health Organization (WHO), we calculated an HIV prevalence of 1.2% among 15- to 49-year-olds, with two-thirds of cases undiagnosed (5, 6, 8).
Table 1.
Model Parameters and Sources*
| Parameter | Base-Case Value | Range | Source |
|---|---|---|---|
| Demographic variables | |||
| Age of screened population (yrs) | 32.5 | 20 – 40 | (25) |
| Prevalence of HIV (%) | 1.2 | 0.01–30 | (1, 5, 6, 25, 44) |
| Prevalence of unidentified HIV (%) | 0.83 | 0.01 – 40 | (1, 5, 6, 25, 44) |
| Annual HIV incidence, 15 to 49 year olds (%) | 0.075 | 0.0375 – 0.3 | (1) |
| Proportion of uninfected population that is female (%) | 50.4 | 40 – 60 | (5, 6, 44, 45) |
| Proportion of infected population that is male (%) | 65 | 50 – 75 | (5, 6, 45) |
| Proportion of infected males who are MSM (%) | 19 | 0.2 – 20 | (46) |
| Age of index case’s sexual partners (yrs) | 32.5 | 20 – 40 | (25) |
| HIV testing and treatment variables | |||
| Probability that patients return for test results (%) | 80 | 50 – 100 | (9, 47–53) |
| Probability that eligible patients receive ART (%) | 50 | 5 – 100 | Estimated |
| Effectiveness of testing and counseling in reducing sexual transmission (%) | 20 | 0 – 25 | (9, 12–14, 27–30) |
| Sensitivity of screening test (%) | |||
| First 3 months after infection | 60 | 11 – 83 | (9, 54–56) |
| Established disease | 99.5 | 98.0 – 99.9 | (9, 54, 55, 57) |
| Specificity of entire sequence of screening tests (%) | 99.9994 | 99 – 100 | (9, 54, 55, 58) |
| Cost variables | |||
| Cost of negative HIV test ($) | 1 | 1 – 10 | Bulletins of Laboratory Services, interviews with HIV experts in Russia |
| Cost of positive HIV test ($) | 70 | 50 – 100 | Bulletins of Laboratory Services, interviews with HIV experts in Russia |
| Cost of HIV counseling ($) | 3 | 1 – 10 | Interviews with HIV experts in Russia |
| Cost of CD4 count test ($) | 7 | 1 – 10 | Interviews with HIV experts in Russia |
| Cost of viral load test ($) | 80 | 50 – 120 | Interviews with HIV experts in Russia |
| Annual healthcare costs (non-HIV related) ($) | 115 | 80 – 250 | (59) |
| Annual cost of HIV infection ($) | 570 | 400 – 1000 | (60) |
| Annual cost of three-drug therapy ($) | 1700 | 500 – 2000 | (33, 34) |
| Annual cost of fourth drug ($) | 600 | 100 – 1000 | Interviews with HIV experts in Russia |
| Annual cost of salvage therapy ($) | 2300 | 2000 – 3000 | Interviews with HIV experts in Russia |
| Annual cost of additional support services while on therapy ($) | 600 | 100 – 1000 | Interviews with HIV experts in Russia |
| Cost of ART side effect per episode ($) | 5 | 1 – 20 | Interviews with HIV experts in Russia |
| Quality-of-life variables | |||
| Unknown asymptomatic HIV infection | 0.91 | 0.85 – 1.00 | (9, 37) |
| Diagnosed asymptomatic HIV infection | 0.84 | 0.68 – 1.00 | (9, 37) |
| Symptomatic (untreated) HIV infection | 0.79 | 0.45 – 1.00 | (9, 37, 39–42) |
| HIV infection during HAART | 0.83 | 0.45 – 1.00 | (9, 37, 39–42) |
| AIDS | 0.73 | 0.30 – 0.80 | (9, 37, 39–42) |
| Decrease in quality of life due to side effects of HAART (multiplier) | 0.53 | 0.44 – 0.62 | (35, 36, 38) |
Parameter values and ranges were estimated based on sources listed. MSM = men who have sex with men. ART = antiretroviral therapy.
Considerable uncertainty exists regarding HIV incidence in Russia. Russia’s Federal AIDS Center has recorded all newly diagnosed cases since 1987 (1). However, given likely under-detection and late detection, these recorded cases underestimate true incidence. One small population-based study that evaluated incidence in a cohort of injection drug users demonstrated seroconversion at a rate as high as 4.5% per year (26). Based on Federal AIDS Center data (1), annual HIV incidence is approximately 0.05% in the 15- to 49-year-old age group. Because of likely under-detection, we estimated a higher incidence of 0.075% for our base-case analyses. Since the age distribution of HIV in Russia is markedly skewed toward young people (8), we estimated a ninety percent reduction in incidence per decade of life after age 50. We varied these assumptions widely in sensitivity analysis.
HIV transmission
We considered sexual and needle sharing transmission of HIV. The probability of sexual transmission of HIV depended on the patient’s gender, partner’s gender, number of partners, knowledge of HIV status, and viral load. The effectiveness of counseling in reducing risk behavior is an important determinant of the reduction in transmission that can occur through screening (9). Based on evaluations of the effectiveness of counseling and testing in the U.S., we estimated that counseling reduced risk behaviors by 20%, which is likely conservative for counseling as practiced in the U.S. (9, 12–14, 27–30). However, because little direct evidence is available about the effectiveness of counseling in Russia, we evaluated scenarios in which counseling has minimal effectiveness. Because needle exchange programs and substitution therapy for substance abuse are rarely available in Russia (2, 31, 32), we assumed that counseling did not alter the risk of transmission via injection drug use.
Costs
We obtained Russia-specific costs through a number of different sources, including Russian Bulletins of Laboratory Services, Russian Regional AIDS Center directors, and non-government organizations (Table 1). As costs of medical visits and laboratory tests were often not standardized across clinics, we obtained prices from several regions in Russia, and varied cost parameters in sensitivity analysis. Our analysis included costs of testing and counseling, follow up, treatment, and support services while on treatment
The cost of ART in Russia is evolving. The price of three drug ART recently dropped from approximately $8000 to $1700 per year in 2006, and is expected to decrease three-fold over the next several years (6, 33, 34). Our base-case assumed an annual cost of $1700 for ART. We explored lower costs of treatment in sensitivity analysis.
Quality of life with HIV
We based our estimates of quality of life with HIV on published assessments (9, 35–42). Because utility-based estimates of quality of life are not available from Russia, we evaluated whether changes in quality of life influenced our results in sensitivity analyses.
RESULTS
Benefit to infected individuals
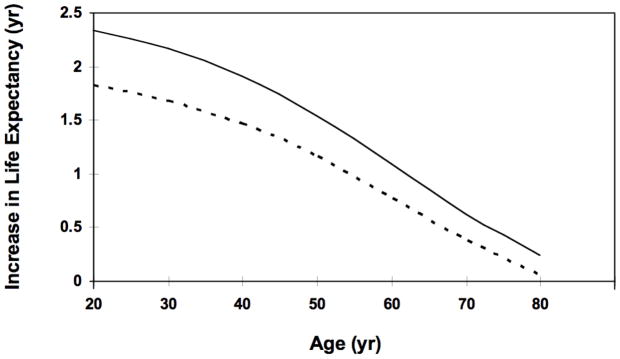
We calculated the benefit of early identification and treatment of HIV compared to symptom-based case finding. HIV-infected individuals identified early through screening who initiated ART did so at a CD4 count of 350 cells per mm3, compared to an average CD4 count of 175 cells per mm3 for those identified through symptom-based case finding. In our base-case analysis, screening and early treatment resulted in an additional 2.1 years of life expectancy for HIV-infected individuals and an additional 1.7 years of quality-adjusted life expectancy. This benefit varied by age, but persisted into the eighth decade of life (Figure 1).
Figure 1. Effect of Early Identification and Treatment of HIV Infection on Life Expectancy.
Solid line depicts the increase in life expectancy associated with identification of asymptomatic HIV, compared to symptom-based case finding. Dotted line shows increase in quality-adjusted life expectancy.
Base case health and economic outcomes
When we included costs and benefits for index cases only, once-per-lifetime screening cost $56 more per person screened than symptom-based case finding alone, and increased life expectancy by 1.5 quality-adjusted days per person screened, yielding an incremental cost-effectiveness ratio of $13,396 per QALY gained (Table 2). Repeat screening every five years cost $25,388 per QALY gained compared to once-per-lifetime screening.
Table 2.
Health and Economic Outcomes*
| Strategy | Cost per person screened, $ | Incremental Cost, $ | Quality- Adjusted Life Expectancy, in Years | Incremental Quality-Adjusted Life Expectancy, in Days | Incremental Cost- Effectiveness, $/QALY |
|---|---|---|---|---|---|
| Index cases only | |||||
| No screening | 3,121 | 23.813 | |||
| Once-per-lifetime screening | 3,177 | 56 | 23.817 | 1.5 | 13,396 |
| Screening every 5 years | 3,282 | 105 | 23.822 | 1.5 | 25,388 |
| Index cases and sexual partners | |||||
| No screening | 3,289 | 23.697 | |||
| Once-per-lifetime screening | 3,345 | 56 | 23.705 | 3.0 | 6,910 |
| Screening every 5 years | 3,450 | 105 | 23.719 | 5.2 | 7,402 |
Analysis was based on HIV prevalence of 1.2% in 15- to 49-year-olds, undiagnosed HIV prevalence of 0.83%, and 50% of identified individuals receiving antiretroviral therapy. QALY = quality-adjusted life year.
When we included costs and benefits for index cases and their sexual partners, the cost-effectiveness of once-per-lifetime screening improved to $6,910 per QALY gained, and screening every five years cost $7,402 per QALY.
Prevalence
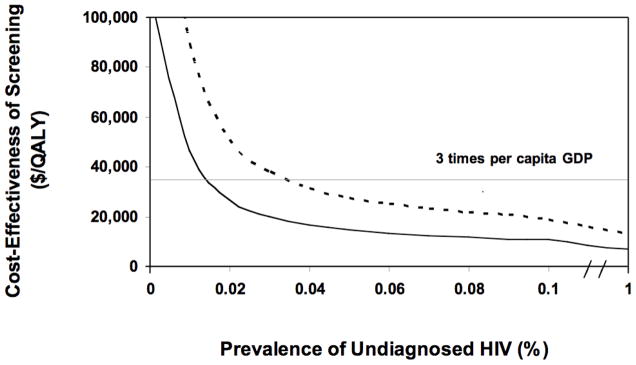
We evaluated the effect of HIV prevalence on the cost-effectiveness of screening. The cost effectiveness of screening for HIV remained favorable until prevalence dropped substantially below one-tenth of our base case estimate of 1.2% (Figure 2). When including benefits and costs for index cases only, once-per-lifetime screening cost less than $36,000 per QALY gained (three times the per capita GDP) if prevalence was at least 0.04% (Figure 2). With inclusion of costs and benefits to sexual partners, screening cost less than $36,000 per QALY gained if HIV prevalence was at least 0.02%, and cost less than $12,000 per QALY gained (the per capita GDP) if prevalence was at least 0.08%.
Figure 2. Effect of Prevalence of Unidentified HIV on the Incremental Cost-Effectiveness of Once-per-lifetime HIV Screening.
Solid line depicts incremental cost-effectiveness ratio when costs and benefits to sexual partners are included. Dotted line includes costs and benefits to index cases only.
Repeat HIV screening
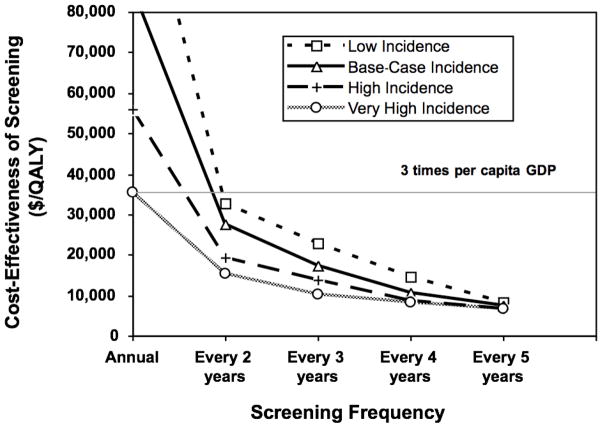
The cost-effectiveness of repeat HIV screening was determined primarily by the incidence of HIV in the screened population. In our base-case analysis, including costs and benefits to both index cases and their sexual partners, screening every five years cost $7,402 per QALY gained compared with once-per-lifetime screening (Figure 3). At half of the base-case incidence, screening every five years cost $8,421 per QALY gained compared with once-per-lifetime screening. At double the base-case incidence, screening every five years cost $6,847 per QALY gained.
Figure 3. Incremental Cost-Effectiveness of Recurrent HIV Screening in Very High, High, Middle, and Low Incidence Settings.
Base-case incidence corresponds to annual incidence of 0.075% in 15 to 49 year olds, with 90% decrease per subsequent decade of life. Low, high, and very high incidence corresponds to half, double, and four times the base-case incidence, respectively.
The impact of incidence on cost-effectiveness was more apparent at more frequent screening intervals. The cost-effectiveness of screening every two years compared to screening every three years was $27,696 per QALY gained in the base case, $32,841 per QALY gained when incidence was half that of the base case, and $19,301 per QALY gained at double the base-case incidence.
Annual screening was expensive, costing $85,972 per QALY gained in the base case, $123,626 per QALY gained when incidence was half that of the base case, and $55,812 per QALY gained at double the base-case incidence. Incidence would need to be approximately four times that of our base case, or 0.3% per year in the 15 to 49 age group, for annual screening to cost less than $36,000 per QALY gained.
Impact of counseling
The degree to which screening and counseling reduced risky behavior was an influential determinant of the cost-effectiveness of screening. Our base-case analysis of the cost-effectiveness of screening ($6,910 per QALY gained) included a 20% reduction in risky sexual behavior. If counseling was half as effective, decreasing risky behavior by only 10%, screening cost $9,100 per QALY gained.
The influence of counseling on the cost-effectiveness of periodic screening was substantial. Screening every five years cost $7,402 per QALY gained in our base case, and $10,711 per QALY gained if counseling was half as effective. Screening every two years cost $27,696 per QALY gained in our base case, and $77,008 per QALY gained if counseling was half as effective.
Additional sensitivity analysis
The cost-effectiveness of screening was moderately influenced by quality of life associated with diagnosed or symptomatic HIV infection. When the quality of life decrement associated with diagnosed or symptomatic infection was twice that of the base case, cost-effectiveness worsened from $6,910 to $11,942 per QALY gained for once-per-lifetime screening, and from $7,401 to $12,530 per QALY gained for screening every five years.
Reduction in the cost of ART has been a worldwide public health priority. When the annual price of a three-drug ART regimen was reduced from $1,700 in our base case to $500, once-per-lifetime screening cost $4,913 per QALY gained after including costs and benefits to sexual partners. Screening every five years cost $5,495 per QALY gained, but annual screening remained expensive at $85,309 per QALY gained.
If effective counseling was included with screening, our results were minimally influenced by changes in the proportion of individuals who received ART; however, if counseling was ineffective, the cost-effectiveness of screening became less favorable if access to ART was reduced.
DISCUSSION
We evaluated the health outcomes, costs, and cost-effectiveness of voluntary HIV screening and counseling among 15- to 49-year-olds in Russia. The costs and benefits of HIV screening have not been evaluated rigorously in middle income countries, and therefore, the value of screening has been uncertain. The effectiveness and efficiency of HIV screening in Russia is particularly important because it is prototypic of the epidemics in Eastern Europe.
Our analysis has three main findings. First, early diagnosis through screening resulted in a substantial gain in life expectancy and quality-adjusted life expectancy for HIV-infected individuals. The life expectancy increase of approximately 2 years is a large increment and is similar to the gain we estimated for individuals identified through screening in the U.S. (9). Second, one-time screening is cost-effective, even when prevalence is extremely low, if it is accompanied by modestly effective counseling to reduce risk behavior and at least partial access to ART. Because HIV testing is inexpensive in Russia, counseling of modest efficacy and less-than-universal access to ART are sufficient to justify the costs of screening. Third, as expected, periodic screening is most cost-effective in high-incidence risk groups.
Based on our estimate of 1.2% HIV prevalence, once-per-lifetime screening cost $6,910 per QALY gained, which is just over half of Russia’s per capita GDP. WHO guidelines consider interventions that cost less than the per capita GDP very cost-effective, and interventions that cost less than three times the per capita GDP cost-effective (23). Screening is very cost-effective because the cost of a negative HIV test and counseling in Russia are low; counseling and treatment reduce HIV transmission; and the survival benefit due to ART is substantial.
Using WHO guidelines, screening was cost-effective even with very low prevalence of undiagnosed HIV cases. Disregarding transmission, screening was cost-effective if prevalence was at least 0.04%. When transmission-related costs and benefits were included, once-per-lifetime HIV screening was cost-effective if prevalence was at least 0.02%. The considerable survival benefit associated with early identification and treatment resulted in favorable cost-effectiveness ratios for HIV screening at low prevalence, even when transmission-related benefits were not taken into account.
The ideal repeat screening interval varied depending on HIV incidence, but our findings were robust across a wide range of incidence. When annual incidence ranged from 0.0375% to 0.15% per year among 15- to 49-year-old individuals, screening as frequently as every two years remained cost-effective. Incidence would need to be at least 0.3% per year for annual screening to be cost-effective, suggesting that annual screening could be appropriate for high-risk groups, but would not be an efficient use of resources for the general population.
Our analysis highlights the critical importance of including risk-reduction counseling in HIV screening programs in Russia. In our base-case analysis, we assumed counseling reduced risky sexual behavior by 20% and had no effect on injection drug use behavior. The degree to which counseling reduces risky behavior has a large impact on the cost-effectiveness of once-per-lifetime and repeat screening because effective counseling can lead to substantial reductions in HIV transmission.
It is important to note that we analyzed voluntary screening. If serious adverse outcomes relating to HIV diagnosis, such as discrimination or stigmatization, were to occur, our results would not be applicable. In addition to ethical considerations, such consequences could substantially reduce quality of life, which would make screening less cost-effective as shown in our sensitivity analysis.
Our analysis has limitations. We included only the benefit from reduced sexual transmission of HIV. Given the limited availability of needle exchange and the lack of substitution therapy in Russia (2, 31, 32), we assumed no change in transmission via injection drug use, as a conservative approach. Should such programs expand in Russia, HIV screening would likely become even more cost-effective than we estimated due to additional reduction in transmission (43).
In addition, in our analysis, HIV-infected individuals who did have access to ART could receive up to three ART regimens aimed at suppressing viral load, followed by lifelong nonsuppressive therapy. Given limited access to ART in Russia, it is possible that some individuals on treatment will have access to fewer drug regimens, which could lead to lower lifetime costs as well as fewer benefits. Nonetheless, our findings were robust to the proportion of patients receiving ART because screening in Russia is relatively inexpensive and counseling alone can offer substantial transmission benefit. Therefore, even in the setting of limited ART access, screening can be an efficient use of resources.
In conclusion, early detection and treatment of HIV in Russia result in substantial improvements in life expectancy among infected individuals. Voluntary HIV screening of 15- to 49-year-olds every two years is cost-effective by WHO guidelines. Such screening identifies HIV-infected individuals earlier, providing health benefits to infected individuals and to the rest of the population due to reduced transmission. Effective counseling is a key component of both the effectiveness and cost-effectiveness of these programs. The health benefit that we projected for screening will be fully realized only if HIV-infected individuals do not suffer adverse outcomes from stigmatization and discrimination.
Acknowledgments
We thank our colleagues in Russia who provided data for our analyses. This work was supported by the National Institute on Drug Abuse (grant 2-R01- DA15612-016), the Agency for Healthcare Research and Quality (grant HS000028), and the Department of Veterans Affairs.
References
- 1.AIDS Foundation East-West (AFEW) Officially Registered Cases in the Russian Federation; 1 January 1987 through 30 September 2007. Moscow: AFEW; 2007. [Google Scholar]
- 2.Csete J. Lessons not learned: human rights abuses and HIV/AIDS in the Russian Federation. Human Rights Watch. 2004;16(5):1–64. [Google Scholar]
- 3.United Nations Development Programme (UNDP) HIV/AIDS in Eastern Europe and the Commonwealth of Independent States: Reversing the Epidemic, Facts and Policy Options. Bratislava: UNDP; 2004. [Google Scholar]
- 4.EuroHIV. HIV/AIDS Surveillance in Europe: End-year report 2005. Saint-Maurice: Institut de Veille Sanitaire; 2006. 2006. Report No.: 72. [Google Scholar]
- 5.Joint United Nations Programme on HIV/AIDS (UNAIDS) 2006 AIDS epidemic update: Eastern Europe and Central Asia. Geneva: UNAIDS; 2006. [Google Scholar]
- 6.World Health Organization (WHO) Summary of Country Profile for HIV/AIDS Treatment Scale-up: Russian Federation, December 2005. Geneva: WHO; 2006. 11-14-07. [Google Scholar]
- 7.National Intelligence Council. The Next Wave of HIV/AIDS: Nigeria, Ethiopia, Russia, India, and China. Washington DC: National Intelligence Council; 2002. [Google Scholar]
- 8.Feshbach M, Galvin CM. HIV/AIDS in Russia - An Analysis of Statistics. Washington DC: Woodrow Wilson International Center for Scholars; 2005. [Google Scholar]
- 9.Sanders GD, Bayoumi AM, Sundaram V, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005 Feb 10;352(6):570–85. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- 10.Chou R, Huffman LH, Fu R, Smits AK, Korthuis PT. Screening for HIV: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2005 Jul 5;143(1):55–73. doi: 10.7326/0003-4819-143-1-200507050-00010. [DOI] [PubMed] [Google Scholar]
- 11.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000 Mar 30;342(13):921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 12.The National Institute of Mental Health (NIMH) Multisite HIV Prevention Trial Group. The NIMH Multisite HIV Prevention Trial: reducing HIV sexual risk behavior. Science. 1998 Jun 19;280(5371):1889–94. doi: 10.1126/science.280.5371.1889. [DOI] [PubMed] [Google Scholar]
- 13.DiClemente RJ, Wingood GM. A randomized controlled trial of an HIV sexual risk-reduction intervention for young African-American women. JAMA. 1995 Oct 25;274(16):1271–6. [PubMed] [Google Scholar]
- 14.Kamb ML, Fishbein M, Douglas JM, Jr, et al. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: a randomized controlled trial. Project RESPECT Study Group. JAMA. 1998 Oct 7;280(13):1161–7. doi: 10.1001/jama.280.13.1161. [DOI] [PubMed] [Google Scholar]
- 15.Cartwright K. Problems with Russia's Compulsory Screening for HIV/AIDS. Washington DC: Urban Institute and World Bank; 2001. [Google Scholar]
- 16.Twigg JL, Skolnik R. Evaluation of the World Bank's Assistance in Responding to the AIDS Epidemic: Russia Case Study. Washington DC: World Bank; 2005. [Google Scholar]
- 17.Moskovsky Komsomolets. 1999;35(Sect 4) [Google Scholar]
- 18.Irwin K, Heimer R, Smoyer A. Drug Policy and HIV Prevention in Russia: The Case of HIV/AIDS Prevention. New Haven: Center for Interdisciplinary Research on AIDS, Yale University; 2004. [Google Scholar]
- 19.Owens DK, Sundaram V, Lazzeroni LC, et al. Prevalence of HIV Infection Among Inpatients and Outpatients in Department of Veterans Affairs Health Care Systems: Implications for Screening Programs for HIV. Am J Public Health. 2007 Oct 30; doi: 10.2105/AJPH.2007.110700. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paltiel AD, Walensky RP, Schackman BR, et al. Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med. 2006 Dec 5;145(11):797–806. doi: 10.7326/0003-4819-145-11-200612050-00004. [DOI] [PubMed] [Google Scholar]
- 21.Paltiel AD, Weinstein MC, Kimmel AD, et al. Expanded screening for HIV in the United States--an analysis of cost-effectiveness. N Engl J Med. 2005 Feb 10;352(6):586–95. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 22.Walker D. Cost and cost-effectiveness of HIV/AIDS prevention strategies in developing countries: is there an evidence base? Health Policy Plan. 2003 Mar;18(1):4–17. doi: 10.1093/heapol/18.1.4. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization (WHO) The World Health Report 2002 - Reducing Risks, Promoting Healthy Life. Geneva: WHO; 2002. [Google Scholar]
- 24.World Health Organization (WHO) Progress on global access to HIV antiretroviral therapy: a report on "3 by 5" and beyond. Geneva: WHO; 2006. [Google Scholar]
- 25.Federal State Statistics Service. Population by age groups. Goskomstat. 2004 [Google Scholar]
- 26.Kozlov AP, Shaboltas AV, Toussova OV, et al. HIV incidence and factors associated with HIV acquisition among injection drug users in St Petersburg, Russia. AIDS. 2006 Apr 4;20(6):901–6. doi: 10.1097/01.aids.0000218555.36661.9c. [DOI] [PubMed] [Google Scholar]
- 27.Hogan D, Salomon J. Prevention and treatment of human immunodeficiency virus/acquired immunodeficiency syndrome in resource-limited settings. Bulletin of the World Health Organization. 2005;83(2):135–43. [PMC free article] [PubMed] [Google Scholar]
- 28.Kalichman SC, Cain D, Weinhardt L, et al. Experimental components analysis of brief theory-based HIV/AIDS risk-reduction counseling for sexually transmitted infection patients. Health Psychol. 2005 Mar;24(2):198–208. doi: 10.1037/0278-6133.24.2.198. [DOI] [PubMed] [Google Scholar]
- 29.Valdiserri RO, Ogden LL, McCray E. Accomplishments in HIV prevention science: implications for stemming the epidemic. Nat Med. 2003 Jul;9(7):881–6. doi: 10.1038/nm0703-881. [DOI] [PubMed] [Google Scholar]
- 30.Wiktor SZ, Abouya L, Angoran H, et al. Effect of an HIV counseling and testing program on AIDS-related knowledge and practices in tuberculosis clinics in Abidjan, Cote d'Ivoire. Int J Tuberc Lung Dis. 2004 Apr;8(4):445–50. [PubMed] [Google Scholar]
- 31.Finnerty E. Opiate substitution treatment in the former Soviet Union. Lancet. 2006 Sep 23;368(9541):1066. doi: 10.1016/S0140-6736(06)69439-7. [DOI] [PubMed] [Google Scholar]
- 32.Zandonella C. International AIDS Vaccine Initiative (IAVI) Report. New York: IAVI; 2006. pp. 9–2006. [PubMed] [Google Scholar]
- 33.Bobrik A. Expected price of the cheapest ARV Regimens. Moscow: Open Health Institute; 2005. [Google Scholar]
- 34.Boykewich S. Moscow Times 2005. Aug 16, 2005. Supply of Anti-HIV Drugs Falls Short. [Google Scholar]
- 35.Bayoumi AM, Redelmeier DA. Preventing Mycobacterium avium complex in patients who are using protease inhibitors: a cost-effectiveness analysis. AIDS. 1998 Aug 20;12(12):1503–12. doi: 10.1097/00002030-199812000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Gable CB, Tierce JC, Simison D, Ward D, Motte K. Costs of HIV+/AIDS at CD4+ counts disease stages based on treatment protocols. J Acquir Immune Defic Syndr Hum Retrovirol. 1996 Aug 1;12(4):413–20. doi: 10.1097/00042560-199608010-00013. [DOI] [PubMed] [Google Scholar]
- 37.Honiden S, Sundaram V, Nease RF, et al. The effect of diagnosis with HIV infection on health-related quality of Life. Qual Life Res. 2006 Feb;15(1):69–82. doi: 10.1007/s11136-005-8485-x. [DOI] [PubMed] [Google Scholar]
- 38.Keiser P, Kvanli MB, Turner D, et al. Protease inhibitor-based therapy is associated with decreased HIV-related health care costs in men treated at a Veterans Administration hospital. J Acquir Immune Defic Syndr Hum Retrovirol. 1999 Jan 1;20(1):28–33. doi: 10.1097/00042560-199901010-00004. [DOI] [PubMed] [Google Scholar]
- 39.Revicki DA, Wu AW, Murray MI. Change in clinical status, health status, and health utility outcomes in HIV-infected patients. Med Care. 1995 Apr;33(4 Suppl):AS173–82. [PubMed] [Google Scholar]
- 40.Tengs TO, Lin TH. A meta-analysis of utility estimates for HIV/AIDS. Med Decis Making. 2002 Nov–Dec;22(6):475–81. doi: 10.1177/0272989X02238300. [DOI] [PubMed] [Google Scholar]
- 41.Tsevat J, Sherman SN, McElwee JA, et al. The will to live among HIV-infected patients. Ann Intern Med. 1999 Aug 3;131(3):194–8. doi: 10.7326/0003-4819-131-3-199908030-00006. [DOI] [PubMed] [Google Scholar]
- 42.Tsevat J, Solzan JG, Kuntz KM, et al. Health values of patients infected with human immunodeficiency virus. Relationship to mental health and physical functioning. Med Care. 1996 Jan;34(1):44–57. doi: 10.1097/00005650-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Zaric GS, Barnett PG, Brandeau ML. HIV transmission and the cost-effectiveness of methadone maintenance. Am J Public Health. 2000 Jul;90(7):1100–11. doi: 10.2105/ajph.90.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health Organization (WHO) Life Tables for WHO Member States: Russian Federation - 2004. Geneva: WHO; 2004. [Google Scholar]
- 45.Rakhmanova A. VICH Infektsiya v Rossii [HIV Infection in Russia]. Epidemic Situation and Priorities In Halting the Spread of HIV Infection in the Russian Federation. AIDS Journal: AIDS, Sex, Health. 2005 [Google Scholar]
- 46.Amirkhanian YKJA, McAuliffe T. Psychosocial needs, mental health, and HIV transmission risk behavior among people living with HIV/AIDS in St. Petersburg, Russia. AIDS. 2003;17(16):2367–74. doi: 10.1097/00002030-200311070-00012. [DOI] [PubMed] [Google Scholar]
- 47.Erickson B, Wasserheit J, Rompalo A, Brathwaite W, Glasser D, Hook Er. Routine voluntary HIV screening in STD clinic clients: characterization of infected clients. Sex Transm Dis. 1990 Oct-Dec;17(4):194–9. doi: 10.1097/00007435-199010000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Hightow L, Miller W, Leone P, Wohl D, Smurzynski M, Kaplan A. Failure to return for HIV posttest counseling in an STD clinic population. AIDS Educ Prev. 2003 Jun;15(3):282–90. doi: 10.1521/aeap.15.4.282.23826. [DOI] [PubMed] [Google Scholar]
- 49.Holman S, Sorin M, Crossette J, LaChance-McCullough M. A state program for postpartum HIV counseling and testing. Public Health Rep. 1994 Jul–Aug;109(4):521–9. [PMC free article] [PubMed] [Google Scholar]
- 50.Irwin K, Olivo N, Schable C, Weber J, Janssen R, Ernst J. Performance characteristics of a rapid HIV antibody assay in a hospital with a high prevalence of HIV infection. CDC-Bronx-Lebanon HIV Serosurvey Team. Annals of Internal Medicine. 1996 Sep 15;125(6):471–5. doi: 10.7326/0003-4819-125-6-199609150-00007. [DOI] [PubMed] [Google Scholar]
- 51.Kassler W, Dillon B, Haley C, Jones W, Goldman A. On-site, rapid HIV testing with same-day results and counseling. AIDS. 1997 Jul;11(8):1045–51. doi: 10.1097/00002030-199708000-00014. [DOI] [PubMed] [Google Scholar]
- 52.Kelen G, Hexter D, Hansen K, et al. Feasibility of an emergency department-based, risk-targeted voluntary HIV screening program. Annals of Emergency Medicine. 1996 Jun;27(7):687–92. doi: 10.1016/s0196-0644(96)70184-1. [DOI] [PubMed] [Google Scholar]
- 53.Sullivan P, Lansky A, Drake A. Failure to return for HIV test results among persons at high risk for HIV infection: results from a multistate interview project. J Acquir Immune Defic Syndr. 2004 Apr 15;35(5):511–8. doi: 10.1097/00126334-200404150-00009. [DOI] [PubMed] [Google Scholar]
- 54.Mylonakis E, Paliou M, Lally M, Flanigan TP, Rich JD. Laboratory testing for infection with the human immunodeficiency virus: established and novel approaches. Am J Med. 2000 Nov;109(7):568–76. doi: 10.1016/s0002-9343(00)00583-0. [DOI] [PubMed] [Google Scholar]
- 55.Owens DK, Nease RF, Jr, Harris RA. Cost-effectiveness of HIV screening in acute care settings. Arch Intern Med. 1996 Feb 26;156(4):394–404. [PubMed] [Google Scholar]
- 56.Schwartz JS, Kinosian BP, Pierskalla WP, Lee H. Strategies for screening blood for human immunodeficiency virus antibody. Use of a decision support system. JAMA. 1990 Oct 3;264(13):1704–10. [PubMed] [Google Scholar]
- 57.Update: serologic testing for antibody to human immunodeficiency virus. MMWR Morb Mortal Wkly Rep. 1988 Jan 8;36(52):833–40. 45. [PubMed] [Google Scholar]
- 58.MacDonald KL, Jackson JB, Bowman RJ, et al. Performance characteristics of serologic tests for human immunodeficiency virus type 1 (HIV-1) antibody among Minnesota blood donors. Public health and clinical implications. Ann Intern Med. 1989 Apr 15;110(8):617–21. doi: 10.7326/0003-4819-110-8-617. [DOI] [PubMed] [Google Scholar]
- 59.World Health Organization (WHO) Per Capita Total Expenditure on Health at Average Exchange Rate (US$) Geneva: WHO; 2001. [Google Scholar]
- 60.Adeyi O, Baris E, Chakraborty S, Novotny T, Pavis R. Averting AIDS crises in Eastern Europe and Central Asia. Washington DC: World Bank; 2003. [Google Scholar]