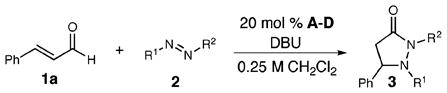
The de novo construction of β-amino acids by the direct construction of the C–N bond continues to be an objective because of the continuing utility of these compounds.1,2 A conventional method to access these molecules is the conjugate addition of a nucleophilic nitrogen source such as azide or an aliphatic amine to an activated ester or ketone (eq 1).3 Alternatively, formal [3+2] reactions with hydroxylamines provide isoxazolidines in which the N–O bond can be easily cleaved.4 A polarity reversal strategy to access β-amino acid derivatives would employ unusual reactivity patterns, such as homoenolates, in the context of a formal cycloaddition. In this Communication, we report the catalytic amination of homoenolates by combining α,β-unsaturated aldehydes with diazenes in the presence of N-heterocyclic carbenes (NHCs) to generate pyrazolidinones (3, eq 2).
 |
(1) (2) |
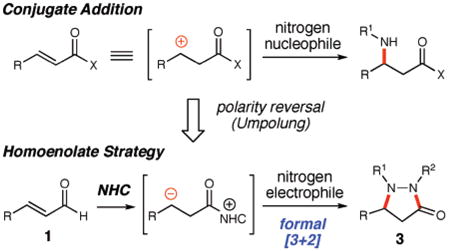
The direct electrophilic amination of a homoenolate generated under catalytic conditions is an unrealized transformation with significant potential. This approach presents the opportunity to obviate the liabilities of standard azide and hydroxylamine nucleophiles and has potential for stereochemical control over the newly formed asymmetric center. A key caveat to the successful realization of electrophilic amination of carbene-generated homoenolates is identifying reactants and conditions that favor carbene addition to the aldehyde over the nonproductive addition to the electrophilic nitrogen reagent.
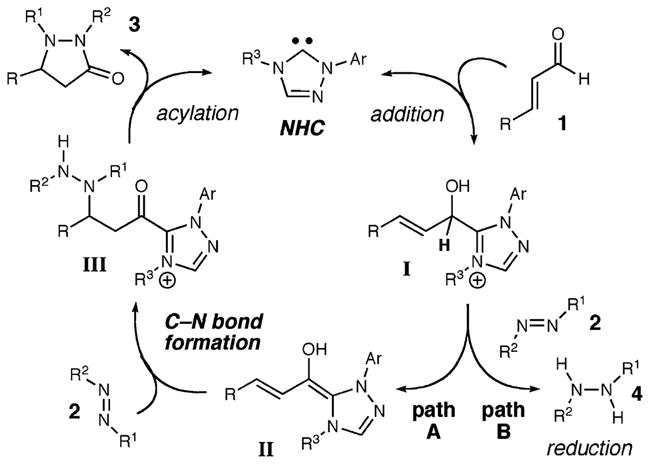
Our previous efforts in the area of N-heterocyclic carbene catalysis5 have generated new ways to form C–H and C–C bonds6 by accessing unique homoenolates from α,β-unsaturated aldehydes.7 In these processes, the aldehyde is converted into an intermediate that possesses nucleophilic character at the β-carbon which upon addition to an electrophile yields an activated carbonyl species. With this general reactivity pattern, the anticipated catalytic pathway for our amination involves the addition of an NHC to the α,β-unsaturated aldehyde 1 to afford the tetrahedral intermediate I. Upon rearrangement, the extended diene homoenolate equivalent II is generated, which undergoes addition to the diazene 2 and subsequently generates ketone III after tautomerization. This activated carbonyl species facilitates catalyst turnover by intramolecular acylation to produce pyrazolidinone 3.
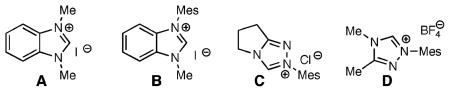
The first goal for the direct amination was to identify a suitable nitrogen-containing electrophile. We began by screening cinnamaldehyde (1a) with heteroazolium salts A–D (Table 1). Diethyl azodicarboxylate provided no desired products (entry 1), but to our delight, diphenyl diazene afforded low yields of pyrazolidinones (entries 2, 3) in the presence of benzimidazolium salts A,B. An intensive investigation of different diazo compounds and azolium salts led to the identification of a new triazolium salt D, which in combination with acylarydiazenes 2d afforded the highest yields of the pyrazolidinone product as a single regioisomer.
Table 1.
Optimization of Amination Conditionsa
| ||||||
|---|---|---|---|---|---|---|
 | ||||||
| entry | catalyst | R1 | R2 | yield (%)b | ||
| 1 | A, B, C, or D | CO2Et | CO2Et (2a) | 0 | ||
| 2 | A | Ph | Ph (2b) | 26 | ||
| 3 | B | Ph | Ph (2b) | 29 | ||
| 4 | C | Ph | Ph (2b) | 18 | ||
| 5 | D | Ph | Ph (2b) | 28 | ||
| 6 | C | Ph | SO2Ph (2c) | 16 | ||
| 7 | D | Ph | SO2Ph (2c) | 16 | ||
| 8 | A or B | Ph | COPh (2d) | 0 | ||
| 9 | C | Ph | COPh (2d) | 42 | ||
| 10 | D | Ph | COPh (2d) | 49 | ||
| 11c | D | Ph | COPh (2d) | 63 | ||
| 12d | D | Ph | COPh (2d) | 54 | ||
20 mol % DBU and 3 equiv of 2, 23 °C.
Isolated yields.
30 mol % DBU, 3 equiv of 2, and 4 Å molecular sieves, 0 °C.
30 mol % DBU, 2 equiv of 2, and 4 Å molecular sieves, 0 °C.
During our optimization studies, we isolated varying amounts of 1-benzoyl-2-phenylhydrazine (4) when employing diazene 2d as the electrophile. On the basis of our carbene-catalyzed hydroacylation studies,5a we postulate that tetrahedral intermediate I (Scheme 1) can form the desired homoenolate intermediate (path A) or collapse to afford an acyl heteroazolium species with concomitant hydride transfer to the diazene (path B). The undesired reaction reduces 2d to the observed hydrazine and sacrifices an equivalent of unsaturated aldehyde. Fortunately, by tuning the structure of the carbene catalyst to D and lowering the reaction temperature, we can maximize the desired homoenolate amination (Table 1, entry 11).8
Scheme 1.
Reaction Pathway
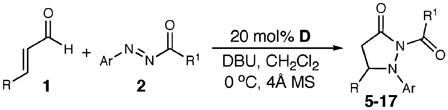
The reaction scope (Table 2) accommodates a variety of substituted α,β-unsaturated aldehydes, including electron-rich and -poor aromatic systems (entries 2–6), and, importantly, β-alkyl-substituted aldehydes provided excellent yields of 11 and 12 (entries 7, 8). An examination of the acylaryldiazene component of the reaction reveals that variously substituted aryl groups are competent substrates. Electron-withdrawing and electron-donating groups on the aryl ring of the benzoyl portion of the diazene afforded good yields of the pyrazolidinone (entries 9–11). However, only electron-rich substituents on the aryl component of the diazene resulted in product formation (entries 12, 13), while electron poor aryl substituents (i.e., Ar = 4-Br-Ph) gave a low yield (25%).
Table 2.
Reaction Scopea
| ||||||
|---|---|---|---|---|---|---|
| entry | R | Ar | R1 | yield (%)b | ||
| 1 | Ph | Ph | Ph | 63 (5) | ||
| 2 | 3-OMe-Ph | Ph | Ph | 60 (6) | ||
| 3 | 2-OMe-Ph | Ph | Ph | 66 (7) | ||
| 4 | 2-Naphthyl | Ph | Ph | 64 (8) | ||
| 5 | 4-Cl-Ph | Ph | Ph | 61 (9) | ||
| 6 | 2-OMe-Ph | Ph | 3-Me-Ph | 64 (10) | ||
| 7 | Me | Ph | 2-Me-Ph | 82 (11) | ||
| 8 | CH2CH2CH3 | Ph | 3-Me-Ph | 84 (12) | ||
| 9 | Ph | Ph | 4-Cl-Ph | 68 (13) | ||
| 10 | Ph | Ph | 4-F-Ph | 61 (14) | ||
| 11 | Ph | Ph | 3-Me-Ph | 73 (15) | ||
| 12 | Ph | 3-Me-Ph | Ph | 71 (16) | ||
| 13 | Ph | 4-Me-Ph | Ph | 63 (17) | ||
3 equiv of 2.
Isolated yields after chromatography.
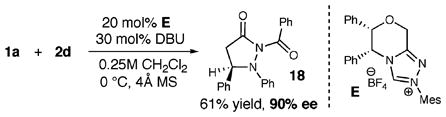
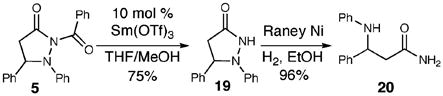
The use of our chiral triazolium salt E5c in this new amination reaction provides pyrazolidinone 18 in good yield (61%) and excellent enantioselectivity (90% ee, eq 5). The pyrazolidinone products can be manipulated to afford β-amino amides by initial exposure to 10 mol % Sm(OTf)3 in THF/MeOH9 to remove the benzoyl functionality (eq 6). A clean reductive cleavage of the N–N bond of pyrazolidinone 19 with Raney nickel produces the β-amino amide 20 in excellent yield (96%).
 |
 |
(6) |
In summary, we have developed a direct electrophilic amination of homoenolates catalyzed by N-heterocyclic carbenes. The addition of a carbene derived from triazolium salt D to an α,β-unsaturated aldehyde generates a homoenolate intermediate which undergoes a formal [3+2] cycloaddition with an 1-acyl-2-aryldiazene to afford pyrazolidinones as a single regioisomer. A chiral triazolium salt can be used to control the newly formed stereocenter of the product, which results in good selectivity. The pyrazolidinone products can be converted into β-amino acid derivatives in excellent yields. Carbene catalysis continues to facilitate new strategies for the creation of valuable molecules from simple precursors.
Supplementary Material
Acknowledgments
Support for this work was generously provided by NIGMS (RO1 GM73072), Abbott Laboratories, Amgen, AstraZeneca, GlaxoSmithKline, 3M, the Sloan Foundation, and Boehringer-Ingelheim. A.C. thanks Dow Chemical Co. for a graduate fellowship.
Footnotes
Supporting Information Available: Experimental procedures and spectral data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Seebach D, Matthews JL. Chem Commun. 1997:2015–2022. [Google Scholar]; (b) Cheng RP, Gellman SH, DeGrado WF. Chem Rev. 2001;101:3219–3232. doi: 10.1021/cr000045i. [DOI] [PubMed] [Google Scholar]; (c) Liu M, Sibi MP. Tetrahedron. 2002;58:7991–8035. [Google Scholar]; (d) Ma JA. Angew Chem, Int Ed. 2003;42:4290–4299. doi: 10.1002/anie.200301600. [DOI] [PubMed] [Google Scholar]; (e) Juaristi E, Soloshonok VA, editors. Enantioselective Synthesis of β-Amino Acids. Wiley-VCH; New York: 2005. [Google Scholar]
- 2.For the use of β-amino acids in peptides, see: Seebach D, Overhand M, Kuehnle FNM, Martinoni B. Helv Chim Acta. 1996;79:913–941.Appella DH, Christianson LA, Karle IL, Powell DR, Gellman SH. J Am Chem Soc. 1996;118:13071–13072.Daniels DS, Petersson EJ, Qiu JX, Schepartz A. J Am Chem Soc. 2007;129:1532–1533. doi: 10.1021/ja068678n.
- 3.For examples, see: Sibi MP, Shay JJ, Liu M, Jasperse CP. J Am Chem Soc. 1998;120:6615–6616.Myers JK, Jacobsen EN. J Am Chem Soc. 1999;121:8959–8960.Horstmann TE, Guerin DJ, Miller SJ. Angew Chem, Int Ed. 2000;39:3635–3638. doi: 10.1002/1521-3773(20001016)39:20<3635::aid-anie3635>3.0.co;2-y.Zhuang W, Hazell RG, Jorgensen KA. Chem Commun. 2001:1240–1241.Xu LW, Li JW, Xia CG, Zhou SL, Hu XX. Synlett. 2003:2425–2427.Doi H, Sakai T, Iguchi M, Yamada K, Tomioka K. J Am Chem Soc. 2003;125:2886–2887. doi: 10.1021/ja029633z.Xu LW, Xia CG, Li JW, Zhou SL. Synlett. 2003:2246–2248.Fadini L, Togni A. Chem Commun. 2003:30–31. doi: 10.1039/b210680a.Palomo C, Oiarbide M, Halder R, Kelso M, Gomez-Bengoa E, Garcia JM. J Am Chem Soc. 2004;126:9188–9189. doi: 10.1021/ja047004e.Shimizu M, Nishi T. Synlett. 2004:889–891.Xu LW, Li L, Xia CG, Zhou SL, Li JW. Tetrahedron Lett. 2004;45:1219–1221.Reboule I, Gil R, Collin J. Tetrahedron–Asymmetry. 2005;16:3881–3886.Sakai T, Doi H, Tomioka K. Tetrahedron. 2006;62:8351–8359.Chen YK, Yoshida M, MacMillan DWC. J Am Chem Soc. 2006;128:9328–9329. doi: 10.1021/ja063267s.
- 4.(a) Niu DQ, Zhao K. J Am Chem Soc. 1999;121:2456–2459. [Google Scholar]; (b) Lee HS, Park JS, Kim BM, Gellman SH. J Org Chem. 2002;68:1575–1578. doi: 10.1021/jo026738b. [DOI] [PubMed] [Google Scholar]; (c) Sibi MP, Prabagaran N, Ghorpade SG, Jasperse CP. J Am Chem Soc. 2003;125:11796–11797. doi: 10.1021/ja0372309. [DOI] [PubMed] [Google Scholar]; (d) Ibrahem I, Rios R, Vesely J, Zhao GL, Cordova A. Chem Commun. 2007:849–851. doi: 10.1039/b613410f. [DOI] [PubMed] [Google Scholar]
- 5.(a) Chan A, Scheidt KA. J Am Chem Soc. 2006;128:4558–4559. doi: 10.1021/ja060833a. [DOI] [PubMed] [Google Scholar]; (b) Phillips EM, Wadamoto M, Chan A, Scheidt KA. Angew Chem, Int Ed. 2007;46:3107–3110. doi: 10.1002/anie.200605235. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wadamoto M, Phillips EM, Reynolds TE, Scheidt KA. J Am Chem Soc. 2007;129:10098–10099. doi: 10.1021/ja073987e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Chan A, Scheidt KA. Org Lett. 2005;7:905–908. doi: 10.1021/ol050100f. [DOI] [PubMed] [Google Scholar]; (b) Chan A, Scheidt KA. J Am Chem Soc. 2007;129:5334–5335. doi: 10.1021/ja0709167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For related studies, see: Burstein C, Glorius F. Angew Chem, Int Ed. 2004;43:6205–6208. doi: 10.1002/anie.200461572.Sohn SS, Rosen EL, Bode JW. J Am Chem Soc. 2004;126:14370–14371. doi: 10.1021/ja044714b.He M, Bode JW. Org Lett. 2005;7:3131–3134. doi: 10.1021/ol051234w.Nair V, Vellalath S, Poonoth M, Mohan R, Suresh E. Org Lett. 2006;8:507–509. doi: 10.1021/ol052926n.Nair V, Vellalath S, Poonoth M, Suresh E. J Am Chem Soc. 2006;128:8736–8737. doi: 10.1021/ja0625677.
- 8.Small amounts (10–15%) of hydrazine byproducts are generated under optimized conditions.
- 9.Evans DA, Scheidt KA, Downey CW. Org Lett. 2001;3:3009–3012. doi: 10.1021/ol016420q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.