Abstract
The proximate mechanisms that regulate transitions in mammalian female reproductive effort have not been widely studied. However, variation in circulating levels of the androgenic steroid hormone testosterone (T) appears to mediate a trade-off between investment in current and future offspring in males. The purpose of this study was to investigate the possibility that T is also associated with transitions in the reproductive effort of females, by examining the relationship between urinary T excretion, maternal caregiving behavior, and the timing of the postpartum conception in female Wied's black tufted-ear marmosets (Callithrix kuhlii). We examined the maternal carrying effort and peripartum T profiles of six females across two conditions: (1) when they conceived during the period of infant dependence (DPID), such that gestation was coupled with lactation; and (2) when the same females conceived after the period of infant dependence (APID). We also assessed the relationship between postpartum T levels and caregiving effort. When female marmosets conceived DPID, they dramatically reduced their caregiving effort, and had higher levels of urinary T, relative to when they conceived APID. Further, the litter-to-litter changes in maternal caregiving effort that we observed were related to variation in urinary T excretion; as weekly levels of urinary T excretion increased, concurrent caregiving effort declined. Our results suggest that variation in T secretion may regulate transitions in female reproductive behavior, and that the regulation of male and female parental behavior may be mediated by homologous neuroendocrine mechanisms.
Keywords: Reproduction, Maternal behavior, Infant care, Trade-offs, Conception, Postpartum, Testosterone, Callitrichid, Marmoset, Callithrix kuhlii
Introduction
Decades of research on the behavioral ecology of mammalian maternal care have demonstrated that females are anything but indiscriminate about the care they provide to their young. When they have difficulty meeting their own subsistence needs, and when their physical condition is poor, females may invest less in their current offspring's fitness in lieu of maintaining or improving maternal condition and the possibility of producing future offspring (e.g., Clutton-Brock, 1991; Hrdy, 1999; Lee et al., 1991). It appears, therefore, that the quality and quantity of care that mothers provide to any given offspring is the result of complex trade-offs between their ability to invest in current offspring and the probability of producing offspring in the future (Trivers, 1974).
The proximate mechanisms that regulate transitions in mammalian female reproductive effort—toward preparation for and production of future offspring, and away from the rearing of current offspring—have not been widely studied. However, variation in circulating levels of the androgenic steroid hormone testosterone (T) appears to mediate a tradeoff between investment in current and future offspring in males (Ketterson and Nolan, 1992, 1994, 1999). For example, male dark-eyed juncos (Junco hyemalis) with experimentally elevated T spent less time provisioning and defending nestlings but spent more time singing, maintaining their territories, and obtaining extra-pair fertilizations, than controls (e.g., Cawthorn et al., 1998; Chandler et al., 1994; Hegner and Wingfield, 1987; Ketterson and Nolan, 1992; Raouf et al., 1997). Male house finches (Carpodacus mexicanus) treated with exogenous T sang at a higher rate, but fed their nestlings at a lower rate, than controls (Stoehr and Hill, 2000). Clark and her colleagues reported that male Mongolian gerbils (Meriones unguiculatus) positioned between two males in utero (2M males) not only had higher circulating levels of T in adulthood (Clark et al., 1992b), but that they also spent less time in contact with pups (Clark et al., 1997) and were more successful at impregnating females (Clark et al., 1992a), than males positioned between two females (2F males). In contrast, 2F males engaged in less sexual behavior than 2M males, but spent more time in contact with pups, even when their mates were away from the nest (Clark et al., 1997). Variation in circulating levels of T is also associated with variation in the reproductive effort of individual males. For instance, decreases in T are known to accompany the onset of paternal care in fish (e.g., bluegill, Lepomis macrochirus: Kindler et al., 1991; plainfin midshipman, Porichthys notatus: Knapp et al., 1999; black-chinned tilapia, Sarotherodon melanotheron: Specker and Kishida, 2000), birds (e.g., red-cockaded woodpecker, Picoides borealis: Khan et al., 2001; white-crowned sparrow, Zonotrichia leucophrys pugetensis: Wingfield and Farner, 1978; song sparrow, Melospiza melodia: Wingfield and Goldsmith, 1990), rodents (e.g., Mongolian gerbil: Brown et al., 1995; Djungarian hamster, Phodopus campbelli: Reburn and Wynne-Edwards, 1999; but also see Trainor and Marler, 2001, 2002), and primates (e.g., Wied's black tufted-ear marmoset, Callithrix kuhlii: Nunes et al., 2000; human: Berg and Wynne-Edwards, 2001; Fleming, 2002; Storey et al., 2000). To the degree that male and female parental behaviors appear to be regulated by common neuroendocrinological mechanisms (e.g., Kelley, 1988; Rosenblatt and Ceus, 1998; Wynne-Edwards, 2001; Wynne-Edwards and Reburn, 2000), it seems likely that T might also facilitate trade-offs between investment in current versus future offspring in females.
In fact, there are reports that maternal caregiving behavior is, at least to some extent, androgen-dependent. Pre- and perinatal exposure to androgens, for instance, has been shown to profoundly influence parental effort in adulthood. Female rats (Rattus norvegicus) treated with testosterone propionate in utero, for example, showed suppressed responsiveness to pups in adulthood (Ichikawa and Fujii, 1982; Juárez et al., 1998; Quadagno and Rockwell, 1972; but also see Lonstein et al., 2002; Stern and Strait, 1983). Additionally, there are numerous reports that T levels decrease with the onset of maternal care and/or exposure to offspring (e.g., plainfin midshipman: Knapp et al., 1999; domestic chicken, Gallus domesticus: Richard-Yris et al., 1987; white-crowned sparrow: Wingfield and Farner, 1978; rat: Bridges et al., 1982; rabbit, Oryctolagus cuniculus: González-Mariscal, 2001; sheep, Ovis aries: Strott et al., 1974; human: Fleming et al., 1997). Taken together, these studies provide some evidence, albeit preliminary, to suggest that T inhibits parental effort in females, as it does in males.
Primates belonging to the New World family Callitrichidae, the marmosets and tamarins, are characterized by distinctive reproductive traits that make them ideally suited for investigations into the proximate mechanisms that regulate maternal trade-offs between future and current offspring. A postpartum ovulation occurs 2–4 weeks following birth (French et al., 1996; Ziegler et al., 1990) so that females can conceive while nursing, carrying, and otherwise caring for their current litter of (typically) twin infants, which at birth can weigh as much as 15–25% of the female's own body weight (Kleiman, 1977). In every species studied to date, females share the responsibility of infant care with members of their family or social group (e.g., Cleveland and Snowdon, 1984; Goldizen, 1987; Snowdon, 1996). However, we recently reported that the degree to which individual female marmosets relinquished the responsibility of infant care to others varied from litter to litter and depended, at least in part, on the timing of the postpartum conception (Fite et al., submitted for publication). When females conceived after the period of infant dependence (APID), at which time their current litters had begun independent locomotion and feeding, they only gradually reduced the amount of time they spent carrying their current litters over subsequent weeks. Yet, when the same females conceived during the period of infant dependence (DPID), during which time females face the energetic challenge of nursing infants every few hours and, along with other group members, carrying infants almost constantly, they exhibited an abrupt and significant decrease in the amount of time that they spent carrying their current litters—a decrease that occurred when infants were only 2 weeks of age. It seems likely then that callitrichid females are equipped with the ability to make trade-offs between current and future offspring when energetic demands are increased—when gestation was coupled with lactation, the female marmosets in our study appeared to have exhibited a shift of investment away from their current litters and toward developing fetuses. In light of reports that female primates exhibit increases in maternal serum androgens early in gestation (see review in Castracane et al., 1998), it also seems likely that T could have mediated the redirection of female reproductive effort.
The purpose of this study was to investigate the possibility that T is associated with transitions in the reproductive effort of individual females, by examining the relationship between urinary T excretion, maternal caregiving behavior, and the timing of the postpartum conception in female Wied's black tufted-ear marmosets (C. kuhlii). Previous studies have associated transitory variation in urinary levels of T with transitory shifts in the reproductive effort of C. kuhlii males. In fact, decreased urinary T excretion was found to correspond with the postpartum shift from mating effort to parental effort (Nunes et al., 2000, 2001). Therefore, we hypothesized that if T mediates a trade-off between current and future offspring in females, and if female marmosets shift investment away from their current litters and toward developing fetuses when conception occurs DPID (Fite et al., submitted for publication), then females should have higher peripartum levels of T and exhibit less maternal caregiving effort when conception occurs DPID versus APID. Additionally, we examined the relationship between postpartum urinary T levels and infant-carrying effort, to determine whether observed changes in maternal investment were associated with variation in T excretion.
Methods
Subjects and housing
The subjects of this study were adult female Wied's black tufted-ear marmosets, and their families, housed at the University of Nebraska at Omaha's Callitrichid Research Center. Marmosets were housed in wire mesh cages (1.6 × 0.9 × 2.4 m), which were furnished with natural branches, a feeding platform, a nest box, and an assortment of enrichment devices. A 12 h/12 h light/dark cycle was controlled by automatic timers, with light onset occurring at 0800 h. Neighboring family groups were always at least 1 m apart and were denied visual, but not auditory or olfactory, contact. Our routine husbandry practices were designed to minimize disturbance to the normal day-to-day activities of the animals. We limited marmosets’ exposure to unfamiliar humans as much as possible, and we handled the animals only when it was necessary to administer veterinary care. For further details of animal housing and husbandry, see Schaffner et al. (1995).
The selection criterion for individuals to include in this study was the timing of females’ postpartum conception. We identified adult females (N = 6) who gave birth to full-term, surviving litters followed by conception DPID and, at a separate reproductive attempt, APID. When more than one DPID or APID conception occurred for a female, we randomly selected one conception per female. To distinguish between DPID and APID conditions, we referred to Tardif et al.'s (1998) chronology of early marmoset development, which emphasized weekly changes in the degree to which infants rely on caregivers for nutrition and transport. We operationally defined conception DPID as conception during the first 3 weeks postpartum, because infants exhibit little, if any, independent feeding or locomotion during this time (common marmoset, C. jacchus: Tardif et al., 1998). During this phase of development, females nurse their infants every few hours (common marmoset: Missler et al., 1992) and, along with other group members, carry infants more than 90% of the time (common marmoset; cotton-top tamarin; golden lion tamarin, Leontopithecus rosalia; saddle-back tamarin, S. fuscicollis; silvery marmoset, C. argentata; see review in Tardif et al., 1998). This care is energetically costly for females; lactation is the most energetically expensive component of reproduction for female mammals (see review in Gittleman and Thompson, 1988), and infant carrying comes at a 21% increase in the caloric cost of traveling (Tardif, 1996). We operationally defined conception APID as conception occurring 4 weeks following birth, and later. Weeks 4–6 postpartum are a transitional period for marmoset infants. During this time period, males replace females as the primary caregiver (Wied's black tufted-ear marmoset: Fite et al., submitted for publication; Nunes et al., 2000), and infant locomotion and feeding becomes increasingly independent (common marmoset: Tardif et al., 1998). By weeks 7–10, infants exhibit locomotion that is completely independent, as well as independent feeding, although sporadic nursing bouts can still occur.
Table 1 presents demographic and reproductive data for each female. Individual females did not differ significantly in age between DPID and APID conditions (t5 = 0.57, NS), nor did their male partners (t5 = 0.56, NS). Neither litter sizes (t5 = 0.00, NS) nor the number of alloparents present to assist each female in the rearing of offspring (t5 = –0.42, NS) differed significantly between DPID and APID conditions. Each of the females in this study had experience caring for infants, prior to the commencement of this study; all six females had extensive sibling-rearing experience as alloparents, and three females (Bas, Jin, Pix) had experience rearing their own offspring.
Table 1.
Demographic data for mothers when they conceived DPID and APID
| Female ID | Timing of conception (postpartum week)a |
Female age (year) |
Male age (year) |
Litter size |
Number of alloparents |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conception DPID | Conception APID | Conception DPID | Conception APID | Conception DPID | Conception APID | Conception DPID | Conception APID | Conception DPID | Conception APID | |
| Bas | 2 | 9 | 3.00 | 3.42 | 2.39 | 2.82 | 2 | 2 | 2 | 3 |
| Bon | 2 | 4 | 7.33 | 6.91 | 12.97 | 12.55 | 2 | 1 | 2 | 1 |
| Jin | 2 | 7 | 5.05 | 4.63 | 8.43 | 8.01 | 1 | 2 | 1 | 1 |
| Luc | 2 | 6 | 4.48 | 3.56 | 4.58 | 3.66 | 1 | 2 | 1 | 2 |
| Pix | 2 | 7 | 5.78 | 5.36 | 6.42 | 6.01 | 2 | 2 | 2 | 1 |
| Xux | 2 | 6 | 2.11 | 2.96 | 5.74 | 6.58 | 2 | 1 | 2 | 3 |
| Mean ± SE | 2.00 ± 0.00 | 6.50 ± 0.67 | 4.63 ± 0.77 | 4.47 ± 0.61 | 6.76 ± 1.49 | 6.61 ± 1.42 | 1.67 ± 0.21 | 1.67 ± 0.21 | 1.67 ± 0.21 | 1.83 ± 0.40 |
Postpartum week during which conception occurred (i.e., conception during week 1 postpartum occurred 1-7 days postpartum, conception during week 2 postpartum occurred 8-14 days postpartum, etc.).
Behavioral measures
Observations of maternal carrying effort were conducted between August 1996 and August 2001. Our observational protocol employed the “all-occurrences” recording technique (Martin and Bateson, 1993), using the Observer 3.0® (Noldus Information Technology, Leesburg, VA, USA) computerized behavioral recording program. Family groups were observed for 20 min, five times per week, for the first 9 weeks of infant life (total observation time per litter, across 9 weeks = 15 h; total observation time for study = 180 h). Observations were conducted at randomly selected times between 0700 and 1800 h, but never occurred less than 1 h preceding, or subsequent to, the a.m. feeding. All behavioral observations were conducted by one individual (J.E.F.), with whom the animals were very familiar. The observer sat approximately 2 m from each cage, and began conducting a behavioral observation only after a 10-min habituation period. Mothers were determined to be carrying infants when one or more infants were observed clinging to the body or pelage of a parent or alloparent. We did not distinguish between the carrying of one versus two infants, because infants born into large social groups are not generally carried by the same individual (cotton-top tamarin: Price, 1990, 1992a,b), and there is evidence that there are few additional costs associated with carrying more than one infant at a time (e.g., saddle-back tamarin: Goldizen, 1987; cotton-top tamarin: Price, 1992b).
Endocrine measures
Urine collection
Urine samples were collected two to five times per week from the six females in this study, as well as from other animals in our colony, as part of routine colony husbandry. A noninvasive, stress-free collection procedure previously described (French et al., 1996) was utilized. Urine samples were collected between 0600 and 0800 h, centrifuged at 7000 rpm for 2 min to remove detritus, and the supernatant was then transferred to a clean mini-vial for storage. All samples collected were catalogued and stored at –20°C until assayed.
Hormone assays
For each female, two urine samples per week were assayed for T and pregnanediol glucuronide (PdG) and their values averaged, providing a weekly estimate of urinary T and PdG excretion. Concentrations of T and PdG in peripartum urine samples were determined using enzyme immunoassays previously described and validated for use with C. kuhlii (T: Nunes et al., 2000;PdG: French et al., 1996). T was extracted from samples with diethyl ether before assays were performed. We measured hormone concentrations in 12 T assays and 12 PdG assays. Intra-assay coefficients of variation, determined from duplicate evaluations of pooled marmoset urine run within assays, were 5.37% and 6.91% for high concentration pools and 3.10% and 11.15% for low concentration pools in T and PdG assays, respectively. Inter-assay coefficients of variation, determined from evaluations of pooled marmoset urine run between assays, were 11.36% and 6.48% for high concentration pools and 13.74% and 17.87% for low concentration pools in T and PdG, respectively. All hormone concentrations were corrected for the creatinine concentration of each sample. Creatinine concentrations were measured by a modified Jaffé end-point assay (Tietz, 1976), which was previously described and validated for C. kuhlii (French et al., 1996).
Identification of postpartum conception
To determine the timing of females’ postpartum conception, we monitored females’ postpartum urinary PdG levels across the first 9 weeks of infant life. French et al. (1996) previously described patterns of urinary PdG excretion across the reproductive cycle in C. kuhlii, and we used these parameters to identify the postpartum week in which conception occurred. While there were individual differences in absolute urinary PdG concentrations, qualitative changes in PdG profiles across the postpartum period were similar among females in each condition. The first postpartum ovulation occurred approximately 14 days postpartum (mean postpartum to ovulation interval: 13.6 ± 1.2 days; French et al., 1996). For nonconceptive cycles, PdG levels returned to preovulatory follicular levels within 25 days (mean duration in days from successive luteinizing hormone (LH) peaks: 24.9 ± 0.60 days; French et al., 1996). For conceptive cycles, there was a rapid elevation in urinary PdG excretion during the first 30 days of pregnancy, and PdG levels remained high for the first two trimesters of pregnancy (mean first trimester urinary PdG levels: 33.7 ± 8.4 μg/mg Cr; mean second trimester urinary PdG levels: 39.0 ± 10.9 μg/mg Cr; mean gestation length: 143.1 ± 1.6 days; French et al., 1996). Therefore, the postpartum week during which females’ PdG levels initially rose beyond preovulatory follicular levels was identified as the week of conception. The timing of each female's conception in DPID and APID conditions is presented in Table 1.
Statistical analyses
To compare levels of maternal investment in offspring when females conceived DPID versus APID, a 2-way completely within-subjects ANOVA (2 × 9; conception condition × postpartum week) was conducted on infant carrying effort across the first 9 postpartum weeks. To compare patterns of androgen excretion when females conceived DPID versus APID, a 2-way completely within-subjects ANOVA (2 × 13; conception condition × peripartum week) was conducted on urinary T concentrations across the peripartum period-from week –4 prepartum though week 9 postpartum. Post hoc analyses were conducted using the Tukey test (Keppel, 1991). Additionally, we used Pearson correlation coefficients to examine the relationship between T and maternal effort. An a level of 0.05 was adopted for all statistical tests, and all data are presented as X̄ ± SEM.
Results
Maternal caregiving behavior and timing of conception
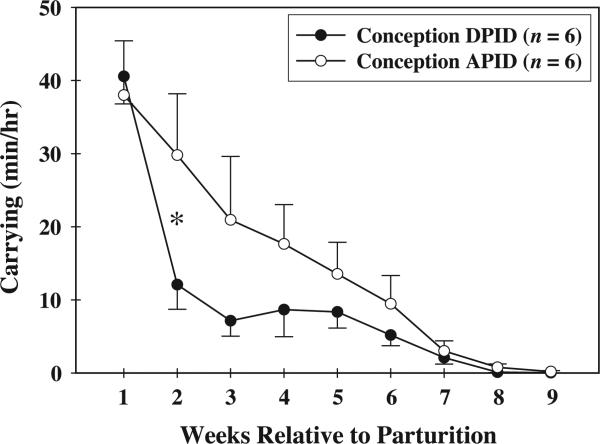
Maternal carrying effort was influenced by the timing of the postpartum conception. A significant interaction between conception condition and postpartum week [F(8, 40) = 2.43, P = 0.03] indicated that the effect of conception condition on infant carrying effort varied by postpartum week. As illustrated in Fig. 1, when females conceived DPID, their carrying effort at week 2 postpartum (12.09 ± 3.38 min/hr) was significantly less than when they conceived APID (29.80 ± 8.39 min/h; P < 0.05). Individual carrying data, by female, are presented in Table 2. With the exception of one female (Bon: carrying in DPID condition = 7.51 ± 4.29 min/h; carrying in APID condition = 7.31 ± 2.15 min/h), females spent less time carrying their infants when they conceived DPID than when they conceived APID.
Fig. 1.
Mean (± SEM) maternal carrying effort when females conceived DPID and APID, *P < 0.05.
Table 2.
Mean (±SEM) maternal carrying effort and urinary T excretion by timing of conception
| Female ID | Carrying efforta (min/h) |
Peripartum urinary Tb (ng/mg Cr) |
||||
|---|---|---|---|---|---|---|
| Conception DPID | Conception APID | Conception DPID | Conception APID | |||
| Bas | 8.90 ± 5.48 | < | 12.52 ± 4.62 | 267.62 ± 36.68 | > | 265.19 ± 45.51 |
| Bon | 7.51 ± 4.29 | > | 7.31 ± 2.15 | 524.39 ± 60.80 | > | 467.25 ± 75.72 |
| Jin | 13.27 ± 4.80 | < | 14.35 ± 4.15 | 696.70 ± 115.56 | > | 344.20 ± 29.93 |
| Luc | 6.74 ± 2.79 | < | 7.62 ± 4.76 | 367.60 ± 31.97 | > | 243.34 ± 57.85 |
| Pix | 12.25 ± 5.15 | < | 29.94 ± 8.27 | 845.82 ± 130.48 | > | 666.41 ± 63.57 |
| Xux | 7.51 ± 3.68 | < | 17.18 ± 6.97 | 439.98 ± 45.92 | > | 343.61 ± 53.33 |
| Mean ± SE | 9.36 ± 1.77 | 14.82 ± 2.39 | 523.69 ± 38.74 | 388.33 ± 27.56 | ||
| Conceptc | NS | 0.042 | ||||
| Wksd | 0.001 | 0.001 | ||||
| Concept × Wkse | 0.03 | NS | ||||
Mean (±SEM) carrying effort during postpartum weeks 1–9.
Mean (±SEM) urinary T excretion during the peripartum period, week –4 prepartum though week 9 postpartum.
Concept, main effect for conception condition; numerical value indicates significance (P) level; NS, not significant.
Wks, main effect for post- and peri-partum weeks.
Concept × Wks, interaction between conception condition and weeks relative to parturition.
Urinary testosterone excretion and timing of conception
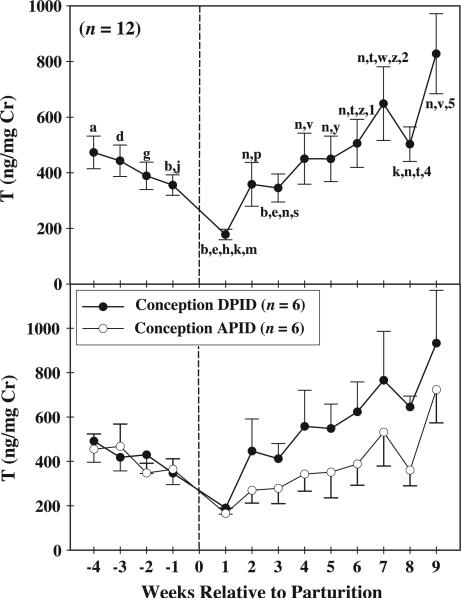
Females’ urinary T profiles (Fig. 2, top panel) were characterized by decreasing levels between week –4 prepartum (473.05 ± 56.19 ng/mg Cr) and week 1 postpartum (177.96 ± 18.11 ng/mg Cr), with the greatest week-to-week decrease (177.78 ± 31.48 ng/mg Cr) occurring between week –1 prepartum (355.74 ± 33.58 ng/mg Cr) and week 1 postpartum (177.96 ± 18.11 ng/mg Cr). T levels were lowest at week 1 postpartum (177.96 ± 18.11 ng/mg Cr), and rose across the following 8 weeks. The greatest week-to-week increase (325.11 ± 115.94 ng/mg Cr) in T levels occurred between weeks 8 (502.82 ± 59.40 ng/mg Cr) and 9 (827.93 ± 137.81 ng/mg Cr) postpartum. T levels were higher at week 9 postpartum than at any other peripartum time point. Peripartum urinary T excretion varied significantly among weeks [F(12, 60) = 7.19, P < 0.01]. Significant (P < 0.05) between-weeks differences in urinary T levels are illustrated in Fig. 2 (top panel).
Fig. 2.
Data are presented relative to the week of parturition (week 0), which is indicated by the dashed vertical line. [top panel] Mean (±SEM) peripartum concentrations of excreted urinary T for all females. Weeks demarked by consecutive letters (e.g., a–b, d–e) or numbers (e.g., 1–2, 4–5) differ significantly from one another, P < 0.05. [bottom panel] Mean (±SEM) peripartum concentrations of excreted urinary T when females conceived DPID and APID, *P < 0.05.
Urinary T excretion was related to the timing of females’ postpartum conception. When females conceived DPID, their overall urinary T concentrations were significantly higher (523.68 ± 38.74 ng/mg Cr) than at breeding attempts in which the same females conceived APID [388.33 ± 27.56 ng/mg Cr; F(1, 5) = 7.37, P = 0.04]. Although the main effects of peripartum week and conception condition were significant and the interaction was not, different levels of T excretion for females in each condition can be accounted for by postpartum changes in T excretion that occurred after week 1 postpartum (Fig. 2, bottom panel). Individual data for each female are presented in Table 2. All six females had higher urinary T levels when they conceived DPID than when they conceived APID.
Urinary testosterone excretion and maternal caregiving behavior
Urinary T excretion during the postpartum period was related to concurrent maternal behavior. Females’ weekly postpartum urinary T concentrations were negatively and significantly correlated with their carrying effort during the same postpartum week [r(108) = –0.29, P = 0.002]. Individual Pearson correlation coefficients, by female, are presented in Table 3, along with associated P values. For all six females, maternal caregiving effort decreased as urinary T levels increased. For three females (Bon, Pix, and Xux), the relationship between urinary T levels and carrying effort was highly significant (P ≤ 0.01). For two females, this relationship approached statistical significance (P ≤ 0.07).
Table 3.
Correlations between weekly postpartum urinary T excretion and concurrent maternal carrying effort for each female
| Female ID | n | r | P |
|---|---|---|---|
| Bas | 18 | –0.44 | 0.07 |
| Bon | 18 | –0.57 | 0.01 |
| Jin | 18 | –0.45 | 0.06 |
| Luc | 18 | –0.24 | 0.34 |
| Pix | 18 | –0.70 | 0.001 |
| Xux | 18 | –0.67 | 0.003 |
| Mean r | –0.51 |
Discussion
A growing body of literature (e.g., Cawthorn et al., 1998; Chandler et al., 1994; Clark et al., 1992a,b, 1997; Hegner and Wingfield, 1987, Raouf et al., 1997; Stoehr and Hill, 2000) has provided empirical support for Ketterson and Nolan's (1992, 1994, 1999) proposal that male birds and mammals maximize their reproductive success by making T-mediated trade-offs between investment in current and future offspring. The results of our study suggest that females might do the same. When female marmosets conceived APID, during which time their infants were not being nursed or carried as intensively, maternal carrying effort gradually declined across the postpartum period. Yet, when the same females conceived DPID and gestation was coupled with lactation, they abruptly and significantly reduced the amount of time spent carrying their current litters, leaving the care of their 2-week-old litters to other family-group members. Although our data are correlational in nature, two analyses provide converging evidence consistent with the hypothesis that T regulates maternal behavior. First, the decrease in maternal responsiveness was accompanied by significantly higher levels of urinary T excretion, relative to breeding attempts in which females conceived APID. Second, mean weekly urinary T levels were found to be negatively and significantly correlated with concurrent carrying effort. Therefore, it appears that the litter-to-litter variation in maternal caregiving effort we observed was reflective of a T-mediated shift of investment away from females’ current litters and toward their developing fetuses, in the face of elevated costs associated with producing and caring for offspring.
Although the results of our study seem to suggest that the timing of females’ postpartum conception affected maternal caregiving behavior, perhaps via modulation of T excretion, Ziegler et al. (1990) reported that in cotton-top tamarins variation in the timing of the postpartum ovulation was a consequence of variation in nursing effort (but also see French, 1983; Lunn and McNeilly, 1982; McNeilly, 1979; Sousa et al., 1999). In our study, we did not record the amount of time spent nursing, due to the difficulty of distinguishing between “time on the nipple” and suckling. Therefore, we were unable to address the possibility that variation in maternal care was the cause, rather than the consequence, of variation in the timing of the postpartum conception. We are quite confident that variation in litter size did not affect our results, however, because singleton and twin litters were equally distributed across both conditions.
To the best of our knowledge, the possibility that T might mediate trade-offs between current and future offspring in individual female mammals has not been investigated prior to this study. In our study, individual female marmosets had significantly higher urinary T levels when they conceived DPID than when they conceived APID. Moreover, differences in T levels for the females in each condition could be accounted for by changes in T excretion that occurred 2 weeks postpartum and later—the time of postpartum conception for females in the DPID condition. Urinary T levels were not only significantly and negatively correlated with concurrent maternal carrying effort, but they were also lowest at week 1 postpartum, when mothers provided the most care for their current litters. These results are particularly exciting in light of reports by Nunes et al. (2000, 2001) that variation in the urinary T levels of male marmosets corresponded to changes in their reproductive effort. For instance, male T levels were at high, prepartum levels, during the first 2 weeks postpartum when males mate most frequently with their female partners. And, as one might expect, T levels decreased significantly, and were at their lowest levels, during the period of maximum paternal caregiving effort (weeks 3–4 postpartum). Moreover, as the weaning period progressed, urinary T levels returned to prepartum levels. It seems, then, that the transition from predominantly maternal care to predominantly paternal care, which occurs approximately 2 weeks postpartum in C. kuhlii families (Fite et al., submitted for publication; Nunes et al., 2000), is regulated by changes in the T levels of males and females. Our results are also in line with reports that, in fish (e.g., Knapp et al., 1999), birds (e.g., Richard-Yris et al., 1987; Wingfield and Farner, 1978), and mammals (e.g., Bridges et al., 1982; Fleming et al., 1997; González-Mariscal, 2001; Strott et al., 1974), T levels decrease with the onset of maternal care and/or exposure to offspring. The results of our study, therefore, indicate a high degree of similarity in the relationship between T and parental effort in male and female marmosets, and shed light on one proximate mechanism by which transitions in female reproductive behavior might be regulated.
The results of our study indicated that conception DPID coincided, to the week, with both behavioral and endocrinological changes in marmoset females. When females conceived DPID (i.e., during the second post-partum week), they exhibited a significant reduction in carrying effort and elevated urinary T levels during week 2 postpartum, relative to when they conceived APID. Unfortunately, we were unable to address the precise timing, to the day, of conception, or the precise timing of changes in maternal behavior and T excretion. While it is possible that changes in maternal behavior may have preceded early conception, and that elevated T levels may have preceded early conception, we do know that female primates exhibit increases in serum androgens early in gestation (see review in Castracane et al., 1998), and that there is reason to believe that T inhibits maternal care (e.g., Bridges et al., 1982; Fleming et al., 1997; González-Mariscal, 2001; Ichikawa and Fujii, 1982; Juárez et al., 1998; Knapp et al., 1999; Quadagno and Rockwell, 1972; Richard-Yris et al., 1987; Strott et al., 1974; Wingfield and Farner, 1978), as it does paternal care in some males. Further research on the psychobiological precursors and consequences of variation in the timing of conception will shed light on the exact nature of the within-female interactions between maternal physiology and behavior, and encourage investigations into potential fitness consequences of these processes.
Although our study did not specifically address the issue of the origin of elevated urinary T in females postpartum, it is a central question that bears on the interpretation of the results. If elevated T simply represents peripheral metabolism of steroid precursors prior to excretion, then our measure of urinary T has little relevance to the possibility that circulating T acts on neural circuits critical for maternal motivation and care. However, we have demonstrated that experimental modification of circulating concentrations of plasma T in female marmosets produces the expected changes in levels of excreted T (Armstrong et al., 2003). Further, there is compelling comparative evidence that the differences we noted in androgen production in female marmosets on the basis on postpartum conception status represents a common process in a number of mammalian species. Androgen production (both androstenedione (A4) and T) is higher in the luteal phase of conceptive ovulatory cycles than in the luteal phase if nonconceptive cycles in dogs, baboons, and human females (Castracane and Goldzieher, 1983; Castracane et al., 1998; Concannon and Castracane, 1985). In the case of human females, dehydroepiandrosterone sulfate (an androgen of primarily adrenal origin; Burger, 2002) levels did not differ between conceptive and nonconceptive studies, suggesting that the differences in luteal T and A4 as a function of conception status reflects differences in ovarian and/or luteal androgen production (Castracane et al., 1998).
Even though male and female parental behaviors are thought to be mediated by common neuroendocrinological underpinnings (e.g., Kelley, 1988; Rosenblatt and Ceus, 1998; Wynne-Edwards, 2001; Wynne-Edwards and Reburn, 2000), investigations into the role of T in the expression of female reproductive behavior have largely focused on T's influence on sexual behavior. Thus, the possibility that T might also play an important role in the expression of maternal behavior, as it affects the expression of paternal behavior (e.g., Ketterson and Nolan, 1992, 1994, 1999), has remained largely unexplored. In the study presented here, we examined changes in maternal care-giving behavior, and concurrent T excretion, in female marmosets faced with the costs of gestation coupled with lactation. Not surprisingly, the results of this study suggest that patterns of maternal caregiving behavior were sensitive to the costs of producing and caring for offspring. Furthermore, our results suggest that females, like males, may make trade-offs between investment in current and future offspring, and that elevated urinary T excretion accompanies shifts of marmoset maternal investment away from current offspring and toward developing fetuses. It may be, then, that the expression and regulation of male and female parental behavior is even more analogous than previously recognized.
Acknowledgments
This study was a portion of the dissertation research of J.E. Fite in the Department of Psychology at the University of Nebraska at Omaha. The research presented was described in Animal Research Protocol No. 95-103-07, and was approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center/University of Nebraska at Omaha. Financial support was provided by grants from the National Institutes of Health (HD-42882), the National Science Foundation (IBN 97-23842 and 00-91030), and from the UNOmaha Animal Care Fund.
References
- Armstrong D, French JA, Puffer AM. Testosterone administration alters urinary excretion of T and E2 in female marmosets. Am. J. Primatol. 2003;60(Supplement 1):110. [Google Scholar]
- Berg SJ, Wynne-Edwards KE. Changes in testosterone, cortisol, and estradiol levels in men becoming fathers. Mayo Clin. Proc. 2001;76(6):582–592. doi: 10.4065/76.6.582. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Todd RB, Logue CM. Serum concentrations of testosterone throughout pregnancy in rats. J. Endocrinol. 1982;94:21–27. doi: 10.1677/joe.0.0940021. [DOI] [PubMed] [Google Scholar]
- Brown RE, Murdoch T, Murphy PR, Moger WH. Hormonal responses of male gerbils to stimuli from the mate and pups. Horm. Behav. 1995;29:474–491. doi: 10.1006/hbeh.1995.1275. [DOI] [PubMed] [Google Scholar]
- Burger HG. Androgen production in women. Fertil. Steril. 2002;77(4):S3–S5. doi: 10.1016/s0015-0282(02)02985-0. [DOI] [PubMed] [Google Scholar]
- Castracane VD, Goldzieher JW. Plasma androgens during early pregnancy in the baboon (Papio cynocephalus). Fertil. Steril. 1983;39:553–559. doi: 10.1016/s0015-0282(16)46950-5. [DOI] [PubMed] [Google Scholar]
- Castracane VD, Stewart DR, Gimpel T, Overstreet JW, Lasley BL. Maternal serum androgens in human pregnancy: early increases within the cycle of conception. Hum. Reprod. 1998;13(2):460–464. doi: 10.1093/humrep/13.2.460. [DOI] [PubMed] [Google Scholar]
- Cawthorn JM, Morris DL, Ketterson ED, Nolan V., Jr. Influence of experimentally elevated testosterone on nest defence in dark-eyed juncos. Anim. Behav. 1998;56:617–621. doi: 10.1006/anbe.1998.0849. [DOI] [PubMed] [Google Scholar]
- Chandler CR, Ketterson ED, Nolan V, Jr., Ziegenfus C. Effects of testosterone on spatial activity in free-ranging male dark-eyed juncos, Junco hyemalis. Anim. Behav. 1994;47:1445–1455. [Google Scholar]
- Clark MM, Tucker L, Galef BG., Jr. Stud males and dud males: intra-uterine position effects on the reproductive success of male gerbils. Anim. Behav. 1992a;43:215–221. [Google Scholar]
- Clark MM, vom Saal FS, Galef BG., Jr. Intrauterine positions and testosterone levels of adult male gerbils are correlated. Physiol. Behav. 1992b;51:957–960. doi: 10.1016/0031-9384(92)90077-f. [DOI] [PubMed] [Google Scholar]
- Clark MM, Desousa D, Vonk J, Galef BG., Jr. Parenting and potency: alternative routes to reproductive success in male Mongolian gerbils. Anim. Behav. 1997;54:635–642. doi: 10.1006/anbe.1997.0468. [DOI] [PubMed] [Google Scholar]
- Cleveland J, Snowdon CT. Social development during the first twenty weeks in the cotton-top tamarin (Saguinus oedipus). Anim. Behav. 1984;32:432–444. [Google Scholar]
- Clutton-Brock TH. The Evolution of Parental Care. Princeton Univ. Press; Princeton, NJ: 1991. [Google Scholar]
- Concannon PW, Castracane VD. Serum androstenedione and testosterone concentrations during pregnancy and nonpregnant cycles in dogs. Biol. Reprod. 1985;33:1078–1083. doi: 10.1095/biolreprod33.5.1078. [DOI] [PubMed] [Google Scholar]
- Fite JE, French JA, Patera KJ, Rukstalis MB, Hopkins E. Opportunistic mothers: marmoset females with elevated and diminished energetic constraints reduce their investment in offspring. 2004. Manuscript submitted for publication.
- Fleming AS. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Horm. Behav. 2002;42:399–413. doi: 10.1006/hbeh.2002.1840. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Ruble DN, Krieger H, Wong PY. Hormonal and experiential correlates of maternal responsiveness during pregnancy and the puerperium in human mothers. Horm. Behav. 1997;31:145–158. doi: 10.1006/hbeh.1997.1376. [DOI] [PubMed] [Google Scholar]
- French JA. Lactation and fertility: an examination of nursing and interbirth intervals in cotton-top tamarins (Saguinus o. oedipus). Folia Primatol. (Basel) 1983;40:276–282. doi: 10.1159/000156110. [DOI] [PubMed] [Google Scholar]
- French JA, Brewer KJ, Schaffner CM, Schalley J, Hightower-Merritt D, Smith TE, Bell SM. Urinary steroid and gonadotropin excretion across the reproductive cycle in female Wied's black tufted-ear marmosets (Callithrix kuhli). Am. J. Primatol. 1996;40:231–246. doi: 10.1002/(SICI)1098-2345(1996)40:3<231::AID-AJP2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Gittleman JL, Thompson SD. Energy allocation in mammalian reproduction. Am. Zool. 1988;28:863–875. [Google Scholar]
- Goldizen AW. Facultative polyandry and the role of infant-carrying in wild saddle-back tamarins (Saguinus fuscicollis). Behav. Ecol. Sociobiol. 1987;20:99–109. [Google Scholar]
- González-Mariscal G. Neuroendocrinology of maternal behavior in the rabbit. Horm. Behav. 2001;40:125–132. doi: 10.1006/hbeh.2001.1692. [DOI] [PubMed] [Google Scholar]
- Hegner RE, Wingfield JC. Effects of experimental manipulation of testosterone levels on parental investment and breeding success in male house sparrows. Auk. 1987;104:462–469. [Google Scholar]
- Hrdy SB. Mother Nature: A History of Mothers, Infants, and Natural Selection. Pantheon Books; New York: 1999. [DOI] [PubMed] [Google Scholar]
- Ichikawa S, Fujii Y. Effect of prenatal androgen treatment on maternal behavior in the female rat. Horm. Behav. 1982;16:224–233. doi: 10.1016/0018-506x(82)90021-6. [DOI] [PubMed] [Google Scholar]
- Juárez J, del Roi-Portilla I, Corsi-Cabrera M. Effects of prenatal testosterone on sex and age differences in behavior elicited by stimulus pups in the rat. Dev. Psychobiol. 1998;32:121–129. doi: 10.1002/(sici)1098-2302(199803)32:2<121::aid-dev5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Kelley DB. Sexually dimorphic behaviors. Annu. Rev. Neurosci. 1988;11:225–251. doi: 10.1146/annurev.ne.11.030188.001301. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and Analysis: A Researcher's Handbook. 3rd ed. Prentice Hall; Englewood Cliffs, NJ: 1991. [Google Scholar]
- Ketterson ED, Nolan V., Jr. Hormones and life histories: an integrative approach. Am. Nat. 1992;140:S33–S62. doi: 10.1086/285396. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V., Jr. Hormones and life histories: an integrative approach. In: Real LA, editor. Behavioral Mechanisms in Evolutionary Ecology. University of Chicago Press; Chicago: 1994. pp. 327–353. [Google Scholar]
- Ketterson ED, Nolan V., Jr. Adaptation, exaptation, and constraint: a hormonal perspective. Am. Nat. 1999;154S:S4–S25. doi: 10.1086/303280. [DOI] [PubMed] [Google Scholar]
- Khan MZ, McNabb FMA, Walters JR, Sharp PJ. Patterns of testosterone and prolactin concentrations and reproductive behavior of helpers and breeders in the cooperatively breeding red-cockaded woodpecker (Picoides borealis). Horm. Behav. 2001;40:1–13. doi: 10.1006/hbeh.2001.1658. [DOI] [PubMed] [Google Scholar]
- Kindler PM, Bahr JM, Phillips DP. The effects of exogenous 11-ketotestosterone, testosterone, and cyproterone acetate on prespawning and parental care behaviors of male bluegill. Horm. Behav. 1991;25:410–423. doi: 10.1016/0018-506x(91)90011-6. [DOI] [PubMed] [Google Scholar]
- Kleiman DG. Monogamy in mammals. Q. Rev. Biol. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Knapp R, Wingfield JC, Bass AH. Steroid hormones and paternal care in plainfin midshipman fish (Porichthys notatus). Horm. Behav. 1999;35:81–89. doi: 10.1006/hbeh.1998.1499. [DOI] [PubMed] [Google Scholar]
- Lee PC, Majluf P, Gordon IJ. Growth, weaning and maternal investment from a comparative perspective. J. Zool. 1991;225:99–114. [Google Scholar]
- Lonstein JS, Rood BD, De Vries GJ. Parental responsiveness is feminized after neonatal castration in virgin male prairie voles, but is not masculinized by perinatal testosterone in virgin females. Horm. Behav. 2002;41:80–87. doi: 10.1006/hbeh.2001.1740. [DOI] [PubMed] [Google Scholar]
- Lunn SF, McNeilly AS. Failure of lactation to have a consistent effect on interbirth interval in the common marmoset, Callithrix jacchus jacchus. Folia Primatol. (Basel) 1982;37:99–105. doi: 10.1159/000156023. [DOI] [PubMed] [Google Scholar]
- Martin P, Bateson P. Measuring Behaviour: An Introductory Guide. Cambridge Univ. Press; Cambridge: 1993. [Google Scholar]
- McNeilly AS. Effects of lactation on fertility. Br. Med. J. 1979;35:151–154. doi: 10.1093/oxfordjournals.bmb.a071562. [DOI] [PubMed] [Google Scholar]
- Missler M, Wolff JR, Rothe H, Heger W, Merker H-J, Treiber A, Scheid R, Crook GA. Developmental biology of the common marmoset: proposal for a bpostnatal stagingQ. J. Med. Primatol. 1992;21:285–298. [PubMed] [Google Scholar]
- Nunes S, Fite JE, French JA. Variation in steroid hormones associated with infant-care behaviour and experience in male marmosets (Callithrix kuhlii). Anim. Behav. 2000;60(6):857–865. doi: 10.1006/anbe.2000.1524. [DOI] [PubMed] [Google Scholar]
- Nunes S, Fite JE, Patera KJ, French JA. Interactions among paternal behavior, steroid hormones, and parental experience in male marmosets. Horm. Behav. 2001;39(1):70–82. doi: 10.1006/hbeh.2000.1631. [DOI] [PubMed] [Google Scholar]
- Price EC. Reproductive strategies of cotton-top tamarins (Doctoral dissertation, University of Sterling, 1990). DAI. 1990;52:0700. [Google Scholar]
- Price EC. The benefits of helpers: effects of group and litter size on infant care in tamarins (Saguinus oedipus). Am. J. Primatol. 1992a;26:179–190. doi: 10.1002/ajp.1350260304. [DOI] [PubMed] [Google Scholar]
- Price EC. The costs of infant carrying in captive cotton-top tamarins. Am. J. Primatol. 1992b;26:23–33. doi: 10.1002/ajp.1350260106. [DOI] [PubMed] [Google Scholar]
- Quadagno DM, Rockwell J. The effects of gonadal hormones in infancy on maternal behavior in the adult rat. Horm. Behav. 1972;3:55–62. doi: 10.1016/0018-506x(72)90007-4. [DOI] [PubMed] [Google Scholar]
- Raouf SA, Parker PG, Ketterson ED, Nolan V, Jr., Ziegenfus C. Testosterone affects reproductive success by influencing extra-pair fertilizations in male dark-eyed juncos (Aves: Junco hyemalis). Proc. R. Soc. Lond., B Biol. Sci. 1997;264:1599–1603. [Google Scholar]
- Reburn CJ, Wynne-Edwards KE. Hormonal changes in males of a naturally biparental and a uniparental mammal. Horm. Behav. 1999;35(2):163–176. doi: 10.1006/hbeh.1998.1509. [DOI] [PubMed] [Google Scholar]
- Richard-Yris M-A, Leboucher G, Chadwick A, Garnier DH. Induction of maternal behavior in incubating and non-incubating hens: influence of hormones. Physiol. Behav. 1987;40:193–199. doi: 10.1016/0031-9384(87)90207-1. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, Ceus K. Estrogen implants in the medial preoptic area stimulate maternal behavior in male rats. Horm. Behav. 1998;33:23–30. doi: 10.1006/hbeh.1997.1430. [DOI] [PubMed] [Google Scholar]
- Schaffner CM, Shepherd RE, Santos CV, French JA. Development of heterosexual relationships in Wied's black tufted-ear marmosets (Callithrix kuhli). Am. J. Primatol. 1995;36:185–200. doi: 10.1002/ajp.1350360303. [DOI] [PubMed] [Google Scholar]
- Snowdon CT. Infant care in cooperatively breeding species. Adv. Study Behav. 1996;25:643–689. [Google Scholar]
- Sousa MBC, Silva HPA, Vidal JF. Litter size does not interfere with fertility in common marmosets, Callithrix jacchus. Folia Primatol. (Basel) 1999;70:41–46. doi: 10.1159/000021674. [DOI] [PubMed] [Google Scholar]
- Specker JL, Kishida M. Mouthbrooding in the black-chinned tilapia, Sarotherodon melanotheron (Pisces: Cichlidae): the presence of eggs reduces androgen and estradiol levels during paternal and maternal parental behavior. Horm. Behav. 2000;38:44–51. doi: 10.1006/hbeh.2000.1601. [DOI] [PubMed] [Google Scholar]
- Stern JM, Strait T. Reproductive success, postpartum maternal behavior, and masculine sexual behavior of neonatally androgenized female hamsters. Horm. Behav. 1983;17:208–224. doi: 10.1016/0018-506x(83)90008-9. [DOI] [PubMed] [Google Scholar]
- Stoehr AM, Hill GE. Testosterone and the allocation of reproductive effort in male house finches (Carpodacus mexicanus). Behav. Ecol. Sociobiol. 2000;48:407–411. [Google Scholar]
- Storey AE, Walsh CJ, Quinton RL, Wynne-Edwards KE. Hormonal correlates of paternal responsiveness in new and expectant fathers. Evol. Hum. Behav. 2000;21:79–95. doi: 10.1016/s1090-5138(99)00042-2. [DOI] [PubMed] [Google Scholar]
- Strott CA, Sundel H, Stahlman MT. Maternal and fetal plasma progesterone, cortisol, testosterone and 17β -estradiol in preparturient sheep: response to fetal ACTH infusion. Endocrinology. 1974;95:1327–1339. doi: 10.1210/endo-95-5-1327. [DOI] [PubMed] [Google Scholar]
- Tardif SD. The bioenergetics of parental behavior and the evolution of alloparental care in marmosets and tamarins. In: Solomon NG, French JA, editors. Cooperative Breeding in Mammals. Cambridge Univ. Press; Cambridge: 1996. pp. 11–33. [Google Scholar]
- Tardif S, Jaquish C, Layne D, Bales K, Power M, Power R, Oftedal O. Growth variation in common marmosets monkeys (Callithrix jacchus) fed a purified diet: relation to care-giving and weaning behaviors. Lab. Anim. Sci. 1998;48:264–269. [PubMed] [Google Scholar]
- Tietz N. Fundamentals of Clinical Chemistry. W. Saunders; Philadelphia: 1976. [Google Scholar]
- Trainor BC, Marler CA. Testosterone, paternal behavior, and aggression in the monogamous California mouse (Peromyscus californicus). Horm. Behav. 2001;40:32–42. doi: 10.1006/hbeh.2001.1652. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Marler CA. Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc. R. Soc. Lond., B Biol. Sci. 2002;269:823–829. doi: 10.1098/rspb.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers RL. Parent–offspring conflict. Am. Zool. 1974;14:249–264. [Google Scholar]
- Wingfield JC, Farner DS. The endocrinology of a natural breeding population of the white-crowned sparrow (Zonotrichia leucophrys pugetensis). Physiol. Zool. 1978;51:188–205. [Google Scholar]
- Wingfield JC, Goldsmith AR. Plasma levels of prolactin and gonadal steroids in relation to multiple-brooding and renesting in free-living populations of the song sparrow, Melospiza melodia. Horm. Behav. 1990;24:89–103. doi: 10.1016/0018-506x(90)90029-w. [DOI] [PubMed] [Google Scholar]
- Wynne-Edwards KE. Hormonal changes in mammalian fathers. Horm. Behav. 2001;40:139–145. doi: 10.1006/hbeh.2001.1699. [DOI] [PubMed] [Google Scholar]
- Wynne-Edwards KE, Reburn CJ. Behavioral endocrinology of mammalian fatherhood. Trends Ecol. Evol. 2000;15(11):464–468. doi: 10.1016/s0169-5347(00)01972-8. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Widowski TM, Larson ML, Snowdon CT. Nursing does affect the duration of the post-partum to ovulation interval in cotton-top tamarins (Saguinus oedipus). J. Reprod. Fertil. 1990;90:563–570. doi: 10.1530/jrf.0.0900563. [DOI] [PubMed] [Google Scholar]