Abstract
Background
Ischemic mitral regurgitation (MR) relates to displacement of the papillary muscles from ischemic ventricular distortion. We tested the hypothesis that repositioning of the papillary muscles can be achieved by injection of polyvinyl-alcohol (PVA) polymer, a biologically inert biomaterial that has been specially formulated to produce an encapsulated, stable, resilient gel once injected into the myocardium. The purpose is to materially support the infarcted myocardium while at the same time repositioning the papillary muscles that become apically tethered in MR.
Methods and Results
Nine sheep underwent ligation of circumflex branches to produce acute ischemic MR. PVA polymer was then injected by echo guidance into the myocardium underlying the infarcted papillary muscle. Hemodynamic data, left ventricular ejection fraction, elastance, tau (relaxation constant), left ventricular stiffness coefficient, and 2-dimensional and 3-dimensional echocardiograms were obtained post-MR and post-PVA injection. One animal died after coronary ligation and 2 did not develop MR. In the remaining 6, moderate MR developed. With PVA injection, the MR decreased significantly from moderate to trace-mild (vena contracta: 5±0.4 mm versus 2±0.7 mm, post-MR versus post-PVA injection; P<0.0001). This was associated with a decrease in infarcted papillary muscle-to-mitral annulus tethering distance (27±4 to 24±4 mm, post-MR versus post-PVA, P<0.001). Importantly, PVA injection was not associated with significant decreases in left ventricular ejection fraction (43±6% versus 37±4%, post-MR versus post-PVA, P=nonsignificant), elastance (3.5±1.4 versus 2.9±1.3; post-MR versus post-PVA injection, P=nonsignificant). Measures of left ventricular diastolic function, tau (100±51 ms to 84±37 ms, post-MR versus post-PVA; P=nonsignificant), and left ventricular stiffness coefficient (0.18±0.12 versus 0.14±0.08, post-MR versus post-PVA; P=nonsignificant) did not increase post-PVA.
Conclusions
PVA polymer injection resulted in acute reverse remodeling of the ventricle with papillary muscle repositioning to decrease MR. This was not associated with an adverse effect on left ventricular systolic and diastolic function. This new approach to alter pathological anatomy after infarction may offer an alternative strategy for relieving ischemic MR by correcting the position of the affected papillary muscle, thus relieving apical tethering.
Keywords: coronary artery disease, left ventricular remodeling, mitral regurgitation
Ischemic mitral regurgitation (MR) is a common complication of coronary artery disease that doubles late mortality.1,2 Extensive evidence has shown that ischemic MR results from left ventricular (LV) distortion, which displaces the papillary muscles (PMs) and tethers the mitral leaflets apically, restricting their closure.3–5 A key element in the pathogenesis of MR, therefore, relates to ventricular thinning with displacement of the PMs.
Therapy for ischemic MR remains problematic. Mitral ring annuloplasty, often applied at the time of bypass surgery, reduces annular size but does not directly address the broader problem of ischemic LV distortion with tethering; its benefits are therefore incomplete,6–8 particularly when LV remodeling continues to progress postoperatively.9,10
Previous studies have demonstrated that therapies that reverse the remodeling of the distorted LV wall can reposition the PMs to restore mitral leaflet coaptation and have included either surgical plication of the infarcted LV or placing an external patch over the infarct.11,12 An alternative approach, which potentially involves less manipulation and disruption to the LV wall and hence more widely applicable, uses biomaterials injected directly into the myocardium underlying the PMs, acting as a tissue-bulking agent to reposition the PMs without placating or suturing a patch over the epicardium. Biomaterials have been successfully used as bulking and stiffening agents in a variety of urologic, plastic, orthopedic, and reconstructive surgical applications.13–16 Biomaterial injections could have the effect of displacing the PM toward the mitral annulus, thereby relieving tethering (Figure 1) and potentially stiffening the weakened muscle wall. Ideally, this biomaterial would have the following characteristics: (1) injectable with gelling at body temperature; (2) causes gelation in a rapid, controlled, and contained manner without detrimental effects on myocardial function; and (3) has sufficient tissue-bulking properties to displace myocardium and thus support the existing diseased tissue.
Figure 1.
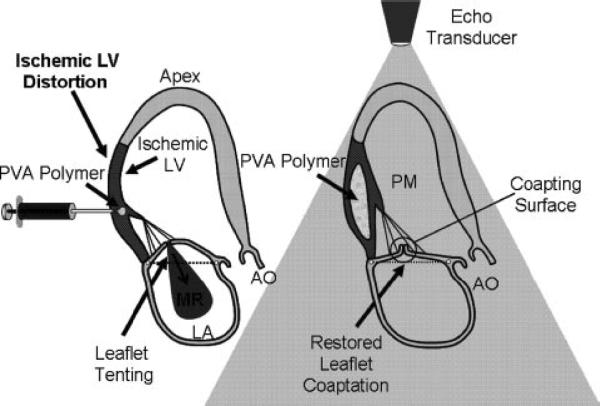
Schematic of PVA polymer injection into infarcted myocardium to reposition PMs and reducing MR.
One type of biomaterial that has physical and mechanical properties that can be modulated for use as a tissue-bulking agent in the beating heart is polyvinyl alcohol (PVA). This polymer is an ideal candidate for this kind of application because it forms a stable, resilient, yet compressible polymer gel complex that is biocompatible and biologically inert with proven effectiveness in a number of clinical applications in humans.17,18
We aimed to test the hypothesis that displaced PMs can be repositioned by injecting PVA polymer, which has been specially formulated to produce an encapsulated, stable, resilient gel into the myocardium. Application of PVA polymer would produce tissue bulking and alter the compliance of infarcted myocardium to realign the displaced PMs.
Methods
As detailed by Llaneras et al,19 anesthesia was induced in Dorsett hybrid sheep with sodium thiopental (12.5 mg/kg intravenously) and the trachea intubated and ventilated at 15 mL/kg with a mixture of 2% isoflurane and oxygen. All animals received glycopyrrolate (0.4 mg intravenously) and vancomycin (0.5 g intravenously) 1 hour before incision. The heart was exposed through a left thoracotomy. A micromanometer-tipped Millar catheter was placed in the LV to measure LV pressure. Sonomicrometry crystals were placed as described subsequently for pressure–volume loop studies.
Acute ischemic MR was produced by ligating the second and third circumflex obtuse marginal branches and the posterior descending artery through a left thoracotomy.19 When MR developed approximately 30 to 60 minutes after ligation, hemodynamic measurements and echocardiographic imaging were repeated. Data acquisition with echocardiographic imaging and hemodynamics, including sonomicrometry, was performed at 3 stages: baseline, acute MR, and PVA polymer injection (Figure 2). This study was reviewed and approved by our institutional Animal Care Committee.
Figure 2.
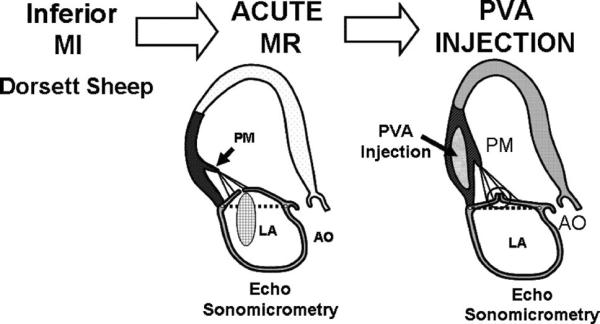
Experimental protocol using an ovine model of acute ischemic MR. Echocardiography and hemodynamic assessment were performed at baseline, post-MR, and post-PVA polymer injection.
Polymer Injection
A 15% PVA polymer aqueous solution with 28% polyethylene glycol (used as a gellant; Cambridge Polymer, Inc, Boston, Mass) was preformulated and stored in 10-mL syringes at room temperature (20°C to 30°C) as a solid gel. This PVA formulation was designed to begin to gel at or near body temperature (below 45°C). The PVA polymer syringes were heated to over 80°C in a water bath to achieve liquid state and then allowed to cool. The cooled PVA formulation remains injectable for a reasonable period (7 to 8 minutes). The temperature of the polymer solution is monitored as it exits the needle with a thermometer attached to the needle exit point to ensure that injection occurs at 39°C to 40°C. Once injected into the myocardium, the PVA substantially solidifies over a 5- to 10-minute period. The PVA polymer was injected in 1- to 2-mL aliquots into the myocardium underlying the papillary muscles using a 18- to 20-gauge short-bevel needle. The location of the injections was guided by direct visualization of anatomic landmarks as well as by real-time echocardiography.
Data Collection and Analysis
LV pressure and electrocardiograms were recorded on a multichannel physiological recorder (Sonometrics Inc, London, Ontario, Canada). Two-dimensional, Doppler, and 3-dimensional echo data were collected using an X3 matrix array transducer and iE33 Ultrasound machine (Philips Medical Systems, Andover, Mass). Epicardial imaging was performed through an agarose standoff to minimize near field effects. For 3-dimensional reconstruction, the probe was positioned along the apex to align the LV apex through the center of the mitral valve. By adjusting the mitral valve to the center of the screen, sector setting were optimized for image and color resolution. Three-dimensional data sets were acquired using the full-volume mode over 4 to 7 sequential heart beats. During acquisition, respiration was suspended to minimize motion artifact.
All images were stored on a DVD disk and transferred for offline analysis using QLAB Advanced Quantification Software, version 5.0 (Philips Medical Systems). Three-dimensional data sets were exported from QLAB in a Cartesian volumetric format and analyzed using customized software.20
Left Ventricular Measures
LV end diastolic and end systolic volumes were measured by 3-dimensional echo using endocardial borders from 6 planes at 30° apart and using a surfacing algorithm.20 LV volumes and contractile performance were assessed using 4 sonomicrometer crystals (Sonometrics) placed over the LV epicardium at the base and apex (long axis) and the anterior and posterior walls (short axis). Pressure–volume loops were constructed from continuous tracings of LV volume, calculated using a standard algorithm, and Millar micromanometer pressure. Sonomicrometry and pressure data were measured using CardioSOFT Pro 3.4 (Sonometrics). The slope of the end systolic pressure–volume relationship or elastance as a relatively load-independent measure of LV contractility was obtained by transiently occluding the inferior vena cava with umbilical tape, thereby rapidly producing beats with varying systolic pressures and LV volumes. End systole was defined as the maximum ratio of LV pressure to LV volume and the end systolic points fitted to a linear equation; its slope (elastance) was taken as a measure of contractile state.21 End diastole was defined by the trough in the LV pressure tracing after atrial contraction. The end diastolic pressure–volume relationship data from caval occlusion were fitted to an exponential equation: LVEDP=D*exp(Kp*LV[EDV]) where D is a curve fitting variable and Kp is the stiffness coefficient, which is a measure of the compliance of the ventricle.22 The relaxation time constant (tau) was calculated as the time for LV pressure to fall from peak negative dP/dt to half its value.
Mitral Regurgitation and Mitral Valve Geometry
MR was quantitated by measuring the vena contracta (narrowest jet origin) in a long-axis view perpendicular to the coaptation line averaged in 3 cardiac cycles. Vena contracta width ≥5 mm was considered moderate in degree.23,24,24a Polymer application was adjusted to reduce MR based on visual assessment of the proximal jet width.
Mitral geometry was reconstructed from rotated images at midsystole, when the leaflets most closely approach the annulus. The PMs traced to identify their tips by reviewing several adjacent images. The tethering length over which the mitral leaflets and chordae are stretched between the PMs and the relatively fixed fibrous portion of the annulus was measured from each PM tip to the medial trigone of the aortic valve (medial junction of aortic and mitral annuli).3 These 3-dimensional measurements have correlated and agreed well with distances measured by sonomicrometer crystal array (Sonometrics) in vitro and in vivo.3,12
Histological Studies
In a subset of animals, after euthanasia, hearts were excised for histological examination to examine the tissue architecture after injection. Injected myocardium was fixed in 10% formalin and histopathologic examination performed on 5-μm sections stained with hematoxylin– eosin and Masson's trichrome stain for microscopic examination.
MRI Diffusion Imaging to Assess Fiber Architecture
To assess effect of PVA polymer injection on surrounding myocardial fiber architecture, 2 explanted lamb hearts (Hatfield Meats, Hatfield, Pa) were injected with PVA polymer in the inferior myocardium adjacent to the papillary muscles. The excised ventricular specimens containing the injected region were placed in a perfluorocarbon solution (Fomblin Oil) and diffusion spectrum imaging was performed using a 4.7-T magnet using an EPI-3DFT hybrid SE 1000/50 hybrid acquisition, 515 diffusion encodings with bmax 10000 s/mm2 and 700-μm spatial resolution. Fiber architecture mapping was obtained using these MRI protocols as described previously.25,26
Statistical Analysis
The efficacy of PVA polymer injection was tested by nonparametric repeated measures analysis of variance (baseline, post-MR, post-PVA polymer) using Friedman's test. Significant differences were examined by paired t test using Fisher F-test criterion for multiple comparisons. A 2-tailed probability value of 0.05 was considered significant. Statistical analysis was performed using SPSS 16.0 (SPSS Inc, Chicago, Ill).
Results
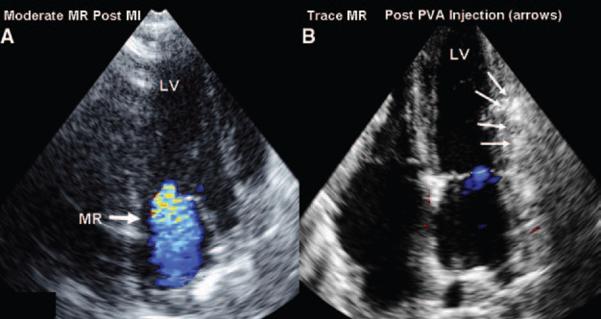
A total of 9 sheep sustained an infarction by ligation of left circumflex branches. Two animals did not develop MR and one animal died after ligation but before PVA polymer injection. The remaining 6 animals developed moderate MR. PVA injection resulted in a decrease in MR from moderate to trace-mild (vena contracta from 5±0.4 mm to 2±0.7 mm (P<0.0001; Figure 3; Supplemental movie clips). The tethering length from infarcted PM to mitral annulus decreased after PVA injection (27±4 to 24±4 mm, P<0.001) indicating reduced leaflet tethering after PVA injection. Figure 4 demonstrates visualization by 3-dimensional echo of the PVA polymer within the myocardium underlying the infarcted papillary muscle along the inferior wall.
Figure 3.
A, Moderate ischemic MR that has developed after ligation of left circumflex branches. B, After injection of PVA polymer into infarcted myocardium over the PMs (arrows), the MR has decreased to trace.
Figure 4.
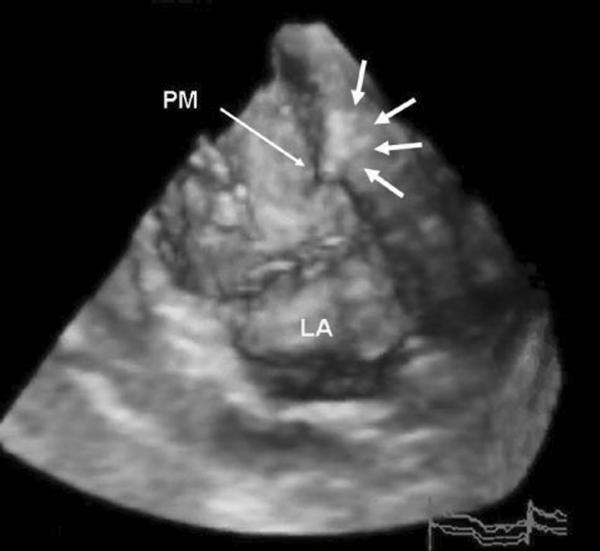
Three-dimensional echo image of LV after PVA polymer injection into myocardium underlying infarcted posterior medial PM. Arrows outline the PVA gel within the myocardium.
LV function parameters were evaluated using sonomicrometry and echocardiography. The Table summarizes these data. There was no change in heart rate, peak LV pressure, or LV end diastolic pressure across baseline, post-MR, and post-PVA stages. As expected, there was a significant decrease in LV ejection fraction after infarction (post-MR stage) compared with baseline. However, there was no change in LV ejection fraction (43±6% versus 37±4%, post-MR versus post-PVA, P=nonsignificant) and there were no new wall motion abnormalities post-PVA injection compared with the post-MR stage. Elastance, a load-independent measure of contractility, decreased after infarction but was not significantly decreased post-PVA injection. In addition, PVA injection was not associated with a worsening of diastolic function. There was no significant increase in the LV stiffness coefficient as derived from end diastolic pressure volume relationship (Table). Furthermore, the relaxation constant, tau, was not significantly increased after PVA injection. These data suggest no detrimental effect on both LV systolic and diastolic function with localized PVA injection into the myocardium.
Table.
Hemodynamic and LV Function Data
| Baseline | Post-MR | Post-PVA | P* | P† | |
|---|---|---|---|---|---|
| Heart rate, beats/min | 105±4 | 100±9 | 99±13 | 0.51 | NS |
| LV pressure, mm Hg | 81±16 | 70±8 | 68±9 | 0.31 | NS |
| EDV, mL | 52±12 | 57±12 | 49±5 | 0.11 | NS |
| ESV, mL | 25±5 | 32±4 | 31±4 | 0.03 | NS |
| LVEF, % | 52±8 | 43±6 | 37±4 | 0.006 | NS |
| Elastance | 5.6±1.4 | 3.5±1.4 | 2.9±1.3 | 0.009 | NS |
| LVEDP, mm Hg | 4.6±2.9 | 6.7±3.8 | 5.1±2.7 | 0.31 | NS |
| Tau, ms | 89±28 | 100±51 | 84±37 | 0.54 | NS |
| Stiffness coefficient | 0.16±0.14 | 0.18±0.12 | 0.14±0.08 | 0.29 | NS |
P<0.05 for baseline versus post-MR and post-PVA; p = Friedman's test analysis of variance.
For post-MR versus post-PVA.
EDV indicates end diastolic volume; ESV, end systolic volume; LVEF, LV ejection fraction; LVEDP, LV end diastolic pressure; NS, nonsignificant.
Histological analysis was available on 4 of the 6 animals and demonstrated that PVA polymer formed a localized gel contained within the myocardium and without disruption of the surrounding myocardial fibers (Figure 5).
Figure 5.
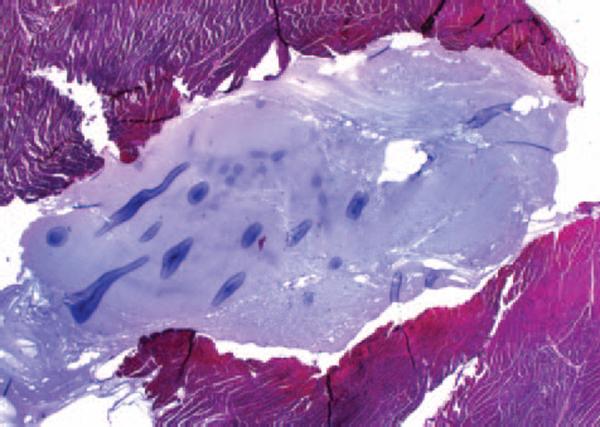
Histological examination of explanted infarcted myocardium that underwent PVA injection demonstrates that the PVA polymer formed a localized gel (blue) contained within the myocardium and without disruption of the surrounding myocardial fibers (red).
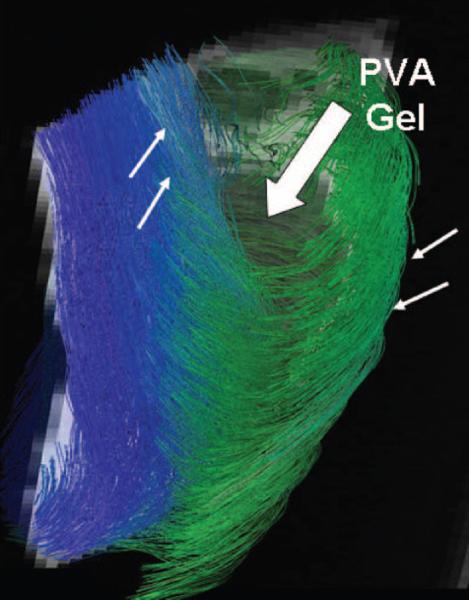
Effects of PVA gel injection on the fiber architecture of the heart were assessed using MRI diffusion spectrum imaging. Figure 6 shows a diffusion scan of a lamb heart performed on a 4.7-T magnet focusing on the gel injection site region. The PVA gel is the circular void shown in gray (big arrow). There are no fibers noted interweaving or within the volume of the PVA gel consistent with gelling occurring in an encapsulated manner (as suggested by the histology data) without “stranding” of gel into the myocardial layers. The surrounding myocardium is evenly displaced around the gel without disruption of basic architecture as assessed in terms of fiber and sheet continuity, including continuity of shear planes. The fiber architecture surrounding the PVA gel shown in green (subepicardial fibers) and blue (subendocardial fibers; arrows) appears in opposite helical orientations as in the normal heart, continuous, orderly, and intact throughout.
Figure 6.
MRI diffusion spectrum imaging of an injected lamb heart to display fiber architecture. Fiber map showing course of fibers (green and blue fibers, thin arrows) in the region of PVA gel (gray void, big arrow). The fibers in the myocardium remain continuous at gel site, retaining helical architecture.
Discussion
The results of this study demonstrate that injection of a polymer into infarcted myocardium underlying the papillary muscles results in reverse remodeling of the ventricle with reduction of ischemic MR.
Advances in polymer chemistry have led to a tremendous variety of polymers that can be selected and modified based on their physical properties and suitability for use in biological systems. Biomaterials such as PVA have been increasingly used in a spectrum of clinical disciplines, including urologic, plastic and reconstructive, orthopedic, and interventional radiological specialties to augment and reconstruct soft tissue.13–17 More recently, biocompatible matrices formed from natural compounds such as collagen or synthetic components have been used in conjunction with cells to provide the scaffolding for a variety of tissue-engineering applications.27–31 In addition, studies have demonstrated improved myocardial function after injection of embryonic stem cells into infarcted rat hearts32 and reduction in ischemic MR after injection of skeletal myoblasts in an ovine infarct model of ischemic MR.33
The use of biomaterials has previously been applied as a tissue-bulking agent in a rat infarct model. Dai et al injected collagen into infarcted myocardium, thereby thickening the myocardium and showed favorable remodeling changes with a decrease in LV volumes and an improvement LV ejection fraction.34 Although important in demonstrating efficacy in tissue bulking with a biomaterial, collagen is not an ideal long-term biomaterial for application in the beating heart because it has a low modulus (soft) and also is biodegradable.35
PVA polymer is better suited for use as a tissue-bulking agent in the heart. It has a chemical formula of (C2H4O)n and is manufactured by the hydrolysis of polyvinyl acetate. PVA is highly water-soluble and elicits little or no host biological response17,36 when implanted in animals or humans. For these reasons, PVA is also used in a variety of biomedical applications, including drug delivery, cell encapsulation, artificial tears, contact lenses, and more recently for longer-term implantation as nerve cuffs and in cartilage repair.37,38 PVA polymer has long-term durability and elasticity that are adjustable and capable of withstanding the pulsatile loading conditions in the beating heart. Furthermore, PVA polymers can be designed to “gel” at controlled rates at, or near, body temperature, making delivery of this novel biomaterial suitable for injection systems.
PVA injection did not result in a decrease in measures of global LV systolic or diastolic function. Because the PVA injections are focused on a relatively small area of the heart, the reduction in MR likely result from reverse localized remodeling. PVA polymer injection may reposition the papillary muscles both by causing tissue displacement and in principle altering the myocardial mechanics so that the underlying myocardium bulges less. The localized nature and relatively small volume of PVA injections would not be expected to have a significant impact, if at all, on LV function or volumes. This was reflected in the lack of change in our measures of LV function after PVA injection compared with post-MR stage. Finite element modeling of material injections into the myocardium has demonstrated favorable effects on cardiac mechanics with no effect on diastolic function and corroborates these results.39
PVA injections did not result in disruption of the surrounding myocardial fibers. Histological examination showed that PVA injections produced a well-defined crosslinked gel without disruption of the surrounding myocardium or dispersion of the polymer into the myocardial fibers. MRI diffusion spectrum imaging of the myocardial fibers surrounding PVA gel injection also did not show disruption or loss of the normal helical fiber architecture in in vitro specimens. Work on other PVA gels indicates a similar effect on tissue architecture in the nucleus pulposus of baboons.40 Biological examination after injection of other polymers such as alginate or collagen-based polymer injections into the myocardium (used to provide a temporary tissue scaffold during cellular transplantation) has demonstrated fibrous cap formation surrounding an intact polymer gel without disruption of the tissue architecture.41
Clinical Implications
Polymer injection has several potential advantages over current approaches to treatment of ischemic MR. Current methods based on solely annular approaches are limited in that they do not directly address ischemic ventricular distortion and papillary muscle displacement. Approaches that directly address ventricular distortion such as the Coapsys or Patch Balloon device by reverse remodeling at the level of the papillary muscles remain relatively invasive11,42,43 Polymer injection can affect papillary muscle repositioning in a less invasive manner and has the potential for minimally invasive application. Another effect of polymer injections may be to act to internally constrain the myocardium from expanding, limiting LV remodeling. Furthermore, polymer injections can be extended toward the mitral annulus to combine papillary muscle repositioning with annular remodeling if necessary to reduce MR. In addition, directly controlled injections of polymer at the annular level may circumvent problems associated with percutaneous methods of annular reduction through a coronary sinus approach such as variable coronary sinus and left circumflex anatomy.44
Limitations
This study examined only an acute ischemic model of MR and in a relatively small number of sheep. Further study will be necessary to ensure consistency and effect of polymer therapy on chronic ischemic MR models.
Conclusion
PVA polymer injection can acutely reverse LV remodeling to reposition displaced PMs and decrease ischemic MR without detectable adverse effects on LV systolic or diastolic function. This new approach offers a potential alternative for relieving ischemic MR by correcting papillary muscle position, thus relieving tethering that causes ischemic MR.
Supplementary Material
Acknowledgments
Sources of Funding This work was supported in part by National Institutes of Health/National Institute for Biomedical Imaging and Bioengineering R21 EB005294 (J.H.), National Institutes of Health/National Heart Lung Blood Institute R01 038176 and K24 HL67434 (R.A.L.), and an American Society of Echocardiography Career Development Award (J.S.).
Footnotes
Presented at the American Heart Association Scientific Sessions, November 4–7, 2007, Orlando, Fla.
The online Data Supplement can be found with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.107.756502/DCI.
Disclosures G.J.C.B. is an employee of Cambridge Polymer Inc.
Statement of Responsibility The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written.
References
- 1.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–1764. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 2.Lamas GA, Mitchell GF, Flaker GC, Smith SC, Jr, Gersh BJ, Basta L, Moye L, Braunwald E, Pfeffer MA. Clinical significance of mitral regurgitation after acute myocardial infarction. Circulation. 1997;96:827–833. doi: 10.1161/01.cir.96.3.827. [DOI] [PubMed] [Google Scholar]
- 3.Otsuji Y, Handschumacher M, Schwammenthal E, Jiang L, Song JK, Guerrero JL, Vlahakes GJ, Levine RA. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation. 1997;96:1999–2008. doi: 10.1161/01.cir.96.6.1999. [DOI] [PubMed] [Google Scholar]
- 4.He S, Fontaine AA, Schwammenthal E, Yoganathan AP, Levine RA. Integrated mechanism for functional mitral regurgitation: leaflet restriction versus coapting force: in vitro studies. Circulation. 1997;96:1826–1834. doi: 10.1161/01.cir.96.6.1826. [DOI] [PubMed] [Google Scholar]
- 5.Komeda M, Glasson JR, Bolger AF, Daughters GT, II, Ingels NB, Jr, Miller DC. Papillary muscle–left ventricular wall `complex'. J Thorac Cardiovasc Surg. 1997;113:292–301. doi: 10.1016/s0022-5223(97)70326-x. [DOI] [PubMed] [Google Scholar]
- 6.Calafiore AM, Gallina S, Di Mauro M, Gaeta F, Iaco AL, D'Alessandro S, Mazzei V, Di Giammarco G. Mitral valve procedure in dilated cardiomyopathy: repair or replacement? Ann Thorac Surg. 2001;71:1146–1152. doi: 10.1016/s0003-4975(00)02650-3. [DOI] [PubMed] [Google Scholar]
- 7.McGee EC, Gillinov MA, Blackstone EH, Rajeswaran J, Cohen G, Najam F, Shiota T, Sabik JF, Lytle BW, McCarthy PM, Cosgrove DM. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2004;128:916–924. doi: 10.1016/j.jtcvs.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 8.Tahta SA, Oury JH, Maxwell JM, Hiro SP, Duran CM. Outcome after mitral valve repair for functional ischemic mitral regurgitation. J Heart Valve Disease. 2001;11:11–18. [PubMed] [Google Scholar]
- 9.Hung J, Papakostas L, Tahta SA, Hardy BG, Bollen BA, Duran CM, Levine RA. Mechanism of recurrent ischemic mitral regurgitation after annuloplasty: continued LV remodeling as a moving target. Circulation. 2004;100:II85–II90. doi: 10.1161/01.CIR.0000138192.65015.45. [DOI] [PubMed] [Google Scholar]
- 10.Guy TS, IV, Moainie SL, Gorman JH, III, Jackson BM, Plappert T, Enomoto Y, St John-Sutton MG, Edmunds LH, Jr, Gorman RC. Prevention of ischemic mitral regurgitation does not influence the outcome of remodeling after posterolateral myocardial infarction. J Am Coll Cardiol. 2004;43:377–383. doi: 10.1016/j.jacc.2003.07.045. [DOI] [PubMed] [Google Scholar]
- 11.Hung J, Guerrero JL, Handschumacher MD, Supple G, Sullivan S, Levine RA. Reverse ventricular remodeling reduces ischemic mitral regurgitation: echo-guided device application in the beating heart. Circulation. 2002;106:2594–2600. doi: 10.1161/01.cir.0000038363.83133.6d. [DOI] [PubMed] [Google Scholar]
- 12.Liel-Cohen N, Guerrero JL, Otsuji Y, Handschumacher MD, Rudski LG, Hunziker PR, Tanabe H, Scherrer-Crosbie M, Sullivan S, Levine RA. Design of a new surgical approach for ventricular remodeling to relieve ischemic mitral regurgitation: insights from three-dimensional echocardiography. Circulation. 2000;101:2756–2763. doi: 10.1161/01.cir.101.23.2756. [DOI] [PubMed] [Google Scholar]
- 13.Radovan C. Tissue expansion in soft-tissue reconstruction. Plast Reconstr Surg. 1984;74:482–492. doi: 10.1097/00006534-198410000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Orr TE, Patel AM, Wong B, Hatzigiannis GP, Minas T, Spector M. Attachment of periosteal grafts to articular cartilage with fibrin sealant. J Biomed Mater Res. 1999;44:308–313. doi: 10.1002/(sici)1097-4636(19990305)44:3<308::aid-jbm9>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 15.Kershen RT, Atala A. New advances in injectable therapies for the treatment of incontinence and vesicoureteral reflux. Urol Clin North Am. 1999;26:81–94. viii. doi: 10.1016/s0094-0143(99)80008-1. [DOI] [PubMed] [Google Scholar]
- 16.Niimi Y, Berenstein A, Setton A, Neophytides A. Embolization of spinal dural arteriovenous fistulae: results and follow-up. Neurosurgery. 1997;40:675–682. doi: 10.1097/00006123-199704000-00004. discussion: 682–683. [DOI] [PubMed] [Google Scholar]
- 17.Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J. Physico-chemical foundations and structural design of hydrogels in medicine and biology. Annu Rev Biomed Eng. 2000;2:9–29. doi: 10.1146/annurev.bioeng.2.1.9. [DOI] [PubMed] [Google Scholar]
- 18.Ku DB, Wootton DM. Reinforced Uncrosslinked Poly (vinyl alcohol) Cryogel. Georgia Tech Research Corporation; Atlanta, Ga: 1999. [Google Scholar]
- 19.Llaneras MR, Nance ML, Streicher JT, Lima JA, Savino JS, Bogen DK, Deac RF, Ratcliffe MB, Edmunds LH., Jr Large animal model of ischemic mitral regurgitation. Ann Thorac Surg. 1994;57:432–439. doi: 10.1016/0003-4975(94)91012-x. [DOI] [PubMed] [Google Scholar]
- 20.Handschumacher M, Lethor JP, Siu SC, Mele D, Rivera JM, Picard MH, Weyman AE, Levine RA. A new integrated system for three-dimensional echocardiographic reconstruction: development and validation for ventricular volume with application in human subjects. J Am Coll Cardiol. 1993;21:743–753. doi: 10.1016/0735-1097(93)90108-d. [DOI] [PubMed] [Google Scholar]
- 21.Suga H, Sagawa K, Shoukas AA. Load independence for the instantaneous pressure–volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circ Res. 1973;32:314–322. doi: 10.1161/01.res.32.3.314. [DOI] [PubMed] [Google Scholar]
- 22.Pagel PS, Kampine JP, Schmeling WT, Warltier DC. Alteration of left ventricular diastolic function by desflurane, isoflurane, and halothane in the chronically instrumented dog with autonomic nervous system blockade. Anesthesiology. 1991;74:1103–1114. doi: 10.1097/00000542-199106000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Grayburn PA, Peshock RM. Noninvasive quantification of valvular regurgitation: getting to the core of the matter. Circulation. 1996;94:119–121. doi: 10.1161/01.cir.94.2.119. [DOI] [PubMed] [Google Scholar]
- 24.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 24a.Mele D, Vandervoort P, Palacios I, Rivera JM, Dinsmore RE, Schwammenthal E, Marshall JE, Weyman AE, Levine RA. Proximal jet size by Doppler color flow mapping predicts severity of mitral regurgitation. Clinical Studies. Circulation. 1995;91:746–754. doi: 10.1161/01.cir.91.3.746. [DOI] [PubMed] [Google Scholar]
- 25.Tseng WY, Reese TG, Weisskoff RM, Wedeen VJ. Cardiac diffusion tensor MRI in vivo without strain correction. Magn Reson Med. 1999;42:393–403. doi: 10.1002/(sici)1522-2594(199908)42:2<393::aid-mrm22>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 26.Wedeen VJ, Hagmann P, Tseng WY, Reese TG, Weisskoff RM. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med. 2005;54:1377–1386. doi: 10.1002/mrm.20642. [DOI] [PubMed] [Google Scholar]
- 27.Stock UA, Vacanti JP. Tissue engineering: current state and prospects. Annu Rev Med. 2001;52:443–451. doi: 10.1146/annurev.med.52.1.443. [DOI] [PubMed] [Google Scholar]
- 28.Hadlock TA, Vacanti JP, Cheney ML. Tissue engineering in facial plastic and reconstructive surgery. Facial Plastic Surgery. 1998;14:197–203. doi: 10.1055/s-2008-1064345. [DOI] [PubMed] [Google Scholar]
- 29.Nasseri BK, Ogawa K, Vacanti JP. Tissue engineering: an evolving 21st-century science to provide biologic replacement for reconstruction and transplantation. Surgery. 2001;130:781–784. doi: 10.1067/msy.2001.112960. [DOI] [PubMed] [Google Scholar]
- 30.Sodian R, Hoerstrup SP, Sperling JS, Martin DP, Daebritz S, Mayer JE, Jr, Vacanti JP. Evaluation of biodegradable, three-dimensional matrices for tissue engineering of heart valves. Asaio Journal. 2000;46:107–110. doi: 10.1097/00002480-200001000-00025. [DOI] [PubMed] [Google Scholar]
- 31.Leor J, Aboulafia-Etzion S, Dar A, Shapiro L, Barbash IM, Battler A, Granot Y, Cohen S. Bioengineered cardiac grafts: a new approach to repair the infarcted myocardium? Circulation. 2000;102:III56–III61. doi: 10.1161/01.cir.102.suppl_3.iii-56. [DOI] [PubMed] [Google Scholar]
- 32.Leor J, Gerecht S, Cohen S, Miller L, Holbova R, Ziskind A, Shachar M, Feinberg MS, Guetta E, Itskovitz-Eldor J. Human embryonic stem cell transplantation to repair the infarcted myocardium. Heart. 2007;93:1278–1284. doi: 10.1136/hrt.2006.093161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messas E, Bel A, Morichetti MC, Carrion C, Handschumacher MD, Peyrard S, Vilquin JT, Desnos M, Bruneval P, Carpentier A, Menasche P, Levine RA, Hagege AA. Autologous myoblast transplantation for chronic ischemic mitral regurgitation. J Am Coll Cardiol. 2006;47:2086–2093. doi: 10.1016/j.jacc.2005.12.063. [DOI] [PubMed] [Google Scholar]
- 34.Dai W, Wold LE, Dow JS, Kloner RA. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 2005;46:714–719. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 35.Hamraoui K, Ernst SM, van Dessel PF, Kelder JC, ten Berg JM, Suttorp MJ, Jaarsma W, Plokker TH. Efficacy and safety of percutaneous treatment of iatrogenic femoral artery pseudoaneurysm by biodegradable collagen injection. J Am Coll Cardiol. 2002;39:1297–1304. doi: 10.1016/s0735-1097(02)01752-7. [DOI] [PubMed] [Google Scholar]
- 36.Urushizaki F, Yamaguchi H, Nakamura K, Sugibayashi K, Morimoto Y. Swelling and mechanical properties of poly (vinyl alcohol) hydrogels. Int J Pharmacol. 1990;58:135. [Google Scholar]
- 37.Deleted in proof.
- 38.Peppas N, Stauffer SR. Reinforced Uncrosslinked Poly (vinyl alcohol) as Potential Biomaterials. Chemical Engineering, Massachusetts Institute of Technology; Cambridge: 1973. [Google Scholar]
- 39.Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Theoretical impact of the injection of material into the myocardium: a finite element model simulation. Circulation. 2006;114:2627–2635. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- 40.Allen MJ, Schoonmaker JE, Bauer TW, Williams PF, Higham PA, Yuan H. Preclinical evaluation of a poly (vinyl alcohol) hydrogel implant as a replacement for the nucleus pulposus. Spine. 2004;29:515–523. doi: 10.1097/01.brs.0000113871.67305.38. [DOI] [PubMed] [Google Scholar]
- 41.Thompson CA, Nasseri BA, Makower J, Houser S, McGarry M, Lamson T, Pomerantseva I, Chang JY, Gold HK, Vacanti JP, Oesterle SN. Percutaneous transvenous cellular cardiomyoplasty. A novel nonsurgical approach for myocardial cell transplantation. J Am Coll Cardiol. 2003;41:1964–1971. doi: 10.1016/s0735-1097(03)00397-8. [DOI] [PubMed] [Google Scholar]
- 42.Mishra YK, Mittal S, Jaguri P, Trehan N. Coapsys mitral annuloplasty for chronic functional ischemic mitral regurgitation: 1-year results. Ann Thorac Surg. 2006;81:42–46. doi: 10.1016/j.athoracsur.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 43.Hung J, Chaput M, Guerrero JL, Handschumacher MD, Papakostas L, Sullivan S, Solis J, Levine RA. Persistent reduction of ischemic mitral regurgitation by papillary muscle repositioning: structural stabilization of the papillary muscle-ventricular wall complex. Circulation. 2007;116(Suppl I):I259–I263. doi: 10.1161/CIRCULATIONAHA.106.679951. [DOI] [PubMed] [Google Scholar]
- 44.Maselli D, Guarracino F, Chiaramonti F, Mangia F, Borelli G, Minzioni G. Percutaneous mitral annuloplasty: an anatomic study of human coronary sinus and its relation with mitral valve annulus and coronary arteries. Circulation. 2006;14:377–380. doi: 10.1161/CIRCULATIONAHA.105.609883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.