Abstract
The detectability of a 10-ms tone masked by a 400-ms wideband noise was measured as a function of the delay in the onset of the tone compared to the onset of the noise burst. Unlike most studies like this on auditory overshoot, special attention was given to signal delays between 0 and 45 ms. Nine well-practiced subjects were tested using an adaptive psychophysical procedure in which the level of the masking noise was adjusted to estimate 79% correct detections. Tones of both 3.0 and 4.0 kHz, at different levels, were used as signals. For the subjects showing overshoot, detectability remained approximately constant for at least 20–30 ms of signal delay, and then detectability began to improve gradually toward its maximum at about 150–200 ms. That is, there was a “hesitation” prior to detectability beginning to improve, and the duration of this hesitation was similar to that seen in physiological measurements of the medial olivocochlear (MOC) system. This result provides further support for the hypothesis that the MOC efferent system makes a major contribution to overshoot in simultaneous masking.
INTRODUCTION
Under certain stimulus conditions, the detectability of a tonal signal presented soon after the onset of a burst of masking noise can be considerably worse than when the presentation of the tone is delayed. This effect has been called overshoot or the temporal effect, and it has been the object of considerable research since its discovery by Zwicker (1965a, 1965b) and Elliott (1965). The optimal conditions for overshoot include: tonal signals only a few milliseconds in duration, tonal signals relatively high in frequency, wideband maskers of moderate spectrum level, and signals having delays of just a few milliseconds being compared to those having delays of about 150 ms or more (e.g., Bacon, 1990; Bacon and Smith, 1991; Wright, 1995, 1996, 1997). Historically, the explanations for overshoot appealed to short-term neural adaptation or short-term depletion of neurotransmitter substance (Smith and Zwislocki, 1975; Smith, 1979; Westerman and Smith, 1984), but von Klitzing and Kohlrausch (1994) suggested that the olivocochlear efferent system might be involved, and considerable research since then has supported this suggestion (Strickland, 2001, 2004, 2008; Keefe et al., 2003, 2009; Walsh et al., 2010a, 2010b; also see Kawase et al., 1993).
Guinan and colleagues have used a version of the stimulus-frequency otoacoustic emission (SFOAE) to study the behavior of the medial olivocochlear (MOC) efferent system in humans (e.g., Guinan, 2006; Guinan et al., 2003; Backus and Guinan, 2006). Among their numerous findings are: the MOC system is more strongly activated by noise bands of moderate width than by tones or sounds with narrow bandwidths; the onset of the MOC response is not seen immediately upon presentation of the eliciting noise band but exhibits a time lag (a “hesitation”) of about 25 ms; and once activated, the MOC system causes Guinan’s SFOAE measure to increase in magnitude with a fast time constant of about 70 ms.
Recently, Walsh et al. (2010a, 2010b) reported that a nonlinear version of the SFOAE (called the nSFOAE) demonstrates numerous characteristics similar to the SFOAE measured by Guinan and also similar to overshoot measured behaviorally in the same ears. Namely, the nSFOAE increased in magnitude as the presentation of a brief, high-frequency probe tone was increasingly delayed following the onset of a long-duration, wideband noise, and that rising, dynamic response also existed when the noise was low-passed below the signal frequency but not when the noise was band-passed or high-passed. These, and other, parallels with behavioral overshoot suggested that aspects of cochlear micromechanics play a major role in the existence of behavioral overshoot; that is, (for some listeners) behavioral overshoot appears to be an obligatory consequence of the functioning of the human cochlea rather than the result of some form of neural adaptation.
Of special interest here is the fact that the nSFOAE response of Walsh et al. (2010a) often demonstrated a “hesitation” of about 25 ms before beginning to increase, much like the “onset delay” seen by Backus and Guinan (2006) for their SFOAE measure. If the nSFOAE response truly is tapping aspects of cochlear behavior that are related to the mechanisms responsible for behavioral overshoot, then behavioral overshoot ought to contain a hesitation like that seen in the physiology. Curiously little psychophysical evidence is available on this matter. Investigators studying overshoot typically have used only one value of signal delay in the region of interest here and one other delay in the range of about 150–250 ms, and even when more numerous values of signal delay were tested, few were in the region of interest here. That is, the literature provides little evidence on whether behavioral overshoot shows hesitation, because the relevant range of signal delays has not been thoroughly investigated. The few relevant reports are discussed in Section 4 below. Hill et al. (1997) reviewed the literature on the time elapsing before a change was seen in various physiological measures, including OAEs, after presumed activation of the olivocochlear efferent system. Most of the reported latencies ranged from about 5–40 ms (with one being 140 ms), depending upon the specific measure, the eliciting stimulus, the procedure, and the species. These times are in the same general range as the values of hesitation seen by Guinan and colleagues and Walsh et al.
The use of the term “hesitation” here is not meant to suggest that there is a time lag in the activation of the mechanism(s) underlying the physiological or behavioral response. Rather, “hesitation” simply denotes the time lag between the onset of the activating sound and the beginning of a change in the physiological or behavioral data. Presumably, the underlying mechanism is triggered immediately by the sound, but its effects require time to be evident.
Because the MOC system appears to be heavily involved in behavioral overshoot, because MOC-related measurements often contain a hesitation of about 25 ms, and because past investigators of behavioral overshoot typically did not explore in detail the first 30 ms or so of signal delay, we designed a study to determine if signal detectability in a behavioral overshoot task remains relatively constant (shows a hesitation) over a range of short delays in signal onset, as the physiological, OAE measures suggest.
METHODS
General
Two crews of listeners were tested with slightly different stimuli. All members of a crew were tested simultaneously when possible. Six listeners served in both crews, and three others served only on one. The primary reason for testing the second crew was that the masker levels needed by the first crew were weaker than are commonly viewed as optimal for producing overshoot (see below). Additionally, we were able to verify the generality of the initial findings by testing the second crew at a different signal frequency as well as with a stronger signal and masker.
Subjects
For the first crew, three females (aged 21–27) and five males (aged 20–21) were hired. The second crew consisted of three females (aged 25–27) and four males (aged 20–21); two of the females and all four of the males also served on the first crew. All of the subjects in both crews had dozens of hours of previous listening experience on overshoot and other auditory tasks.
All subjects had audiometrically normal hearing sensitivity (≤15 dB Hearing Level) in both ears for the standard audiometric frequencies between 250 and 8000 Hz, and normal middle-ear function as measured by a clinical audiometric screening device (Auto Tymp 38, GSI, Inc.). All subjects were paid for their participation. Both crews were tested on tasks other than hesitation, and those results will be reported elsewhere. About five weeks elapsed between the testing of the first and second crews.
Procedures
The procedures used to collect overshoot data were described in Walsh et al. (2010b). Briefly, the individual trials were two-interval, two-alternative forced choice; the signal level was fixed and the masker level was adjusted using a three-up∕one-down decision rule that estimates the level required for 79% correct decisions (Levitt, 1971); the step size was 2 dB; there were 50 trials per block; multiple subjects were tested simultaneously; the trial lengths were fixed, not self-paced; the headphones were TDH-39s in circumaural cushions; and only the right ear was stimulated. Each trial had a 1-s response interval during which the subject had to respond, or else that trial was ignored for that subject. At least the first two reversals of each block of trials were discarded, and the mean of the final even number of reversals was taken as the estimate of sensitivity. Blocks of trials having fewer than 45 responses, fewer than four remaining reversals, or standard deviations of the reversals greater than 3.5 dB were discarded. For the first crew, three or four usable blocks were collected for each condition of listening for each subject; for the second crew, between 8 and 12 usable blocks were collected for each condition.
The masking noise was 400 ms in duration and was gated with a 2-ms rise∕fall time; the noise was wideband (0.1–6.0 kHz) and a new sample was generated for every observation interval of every trial; the signal was a 10-ms tone burst, gated with a rise∕fall time of 5 ms (no steady-state segment); the signals and noises were generated using a sampling rate of 50 kHz and 16-bit resolution; and the time between observation intervals was 500 ms in order to allow the auditory system to “reset” (see McFadden, 1989).
For the first crew, the signal was 4.0 kHz and 60 dB SPL at maximum amplitude; for the second crew, the signal was 3.0 kHz and 70 dB in level. For the first crew, the signal delays ranged from 3 to 45 ms in 3-ms increments, plus 100, 150, and 200 ms. For the second crew, the signal delays ranged from 3 to 45 ms in 6-ms increments, plus 100, 150, and 200 ms.
The signal delay was constant for all trials in a block. The same value of delay was tested on four consecutive blocks. The data for the different delays were collected in a pseudorandom order that differed across signal frequency, and to some extent across individual subjects (e.g., when makeup sessions were required). Prior to each set of four blocks, the subjects were told only whether the signal would appear near the beginning or in the middle of the noise burst. Again, all subjects were highly practiced at overshoot and several other psychophysical tasks.
The Institutional Review Board at The University of Texas at Austin approved this research protocol, and informed consent was obtained from all subjects prior to the study.
RESULTS
For both signal frequencies, the detectability of the signal (and thus the magnitude of the overshoot) did remain approximately constant for delay values ranging from 3 to about 30 ms, beyond which detectability began to improve slowly (masker level increased). Thus, these behavioral data contain a hesitation similar to that observed in physiological measures of SFOAEs (Backus and Guinan, 2006; Walsh et al., 2010a, 2010b).
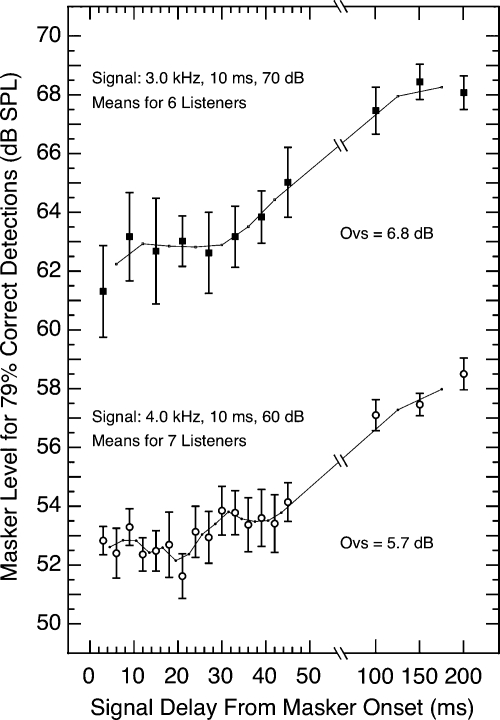
To illustrate how the detectability of the tone changed across signal delay, the noise levels required for the criterion level of masking were averaged across subjects for each value of delay tested, and those means are shown in Fig. 1. At the bottom of Fig. 1 are the data for the first crew (4.0-kHz signal at 60 dB), and at the top are those for the second crew (3.0-kHz signal at 70 dB). For each set of data, a two-point running average also is shown (solid lines). The mean values of overshoot (Ovs) shown are the differences in masker level required for signal delays of 200 and 3 ms; we presume that the small values obtained are primarily attributable to subject sampling. Note that more blocks were collected from each subject for the 3.0-kHz signal than for the 4.0-kHz signal. The error bars in Fig. 1 reveal that for both frequencies the individual differences were greater for the short-delay than for the long-delay conditions; that same effect also was evident within subjects (see below). The appearance of a second plateau in the 4.0-kHz data is not evident in the data for any of the individual subjects (see below); rather, it is an artifact of the averaging process. The data from subject NN were excluded from Fig. 1 because he showed minimal overshoot (see below).
Figure 1.
Overall level of the masker needed for constant detectability of the 10-ms tonal signal as a function of the delay in the signal’s onset following the onset of the wideband masking noise (400 ms in duration). The data at the top are for a 3.0-kHz signal of 70 dB SPL; the data at the bottom are for a 4.0-kHz signal of 60 dB SPL. Each point is a mean of six (top) or seven (bottom) subjects; five subjects were common to the two sets of data; the flags indicate standard errors of the mean; and the short line segments illustrate the running average across successive pairs of delay values. For clarity, the abscissa spacing is different for the two ranges of values of signal delay. “Ovs” designates the difference between the 200- and 3-ms means (the overshoot). The corresponding data for individual subjects are shown in Figs. 23. The data for subject NN were excluded from both the 3.0- and 4.0-kHz plots because he showed minimal overshoot.
Visual inspection of the 3.0-kHz data in Fig. 1 suggests that detectability remained about the same for signal delays ranging from 3 ms to about 30 ms, and then began to improve, but statistical analyses failed to confirm this apparent improvement. Repeated-measures analyses of variance (ANOVA) with signal delay as the single factor were applied to different ranges of signal delay. When the range was 3–45 ms, there was no statistically significant effect(F=1.52, p=0.19). That is, the apparent improvement beyond about 30 ms could not be confirmed statistically with the Ns and the individual differences that exist in these data (see below). When the ANOVA was recalculated after including one additional value of delay (100 ms), the outcome was significant(F=3.76, p=0.002). When corresponding analyses were performed on the 4.0-kHz data, the same pattern of outcomes was observed.
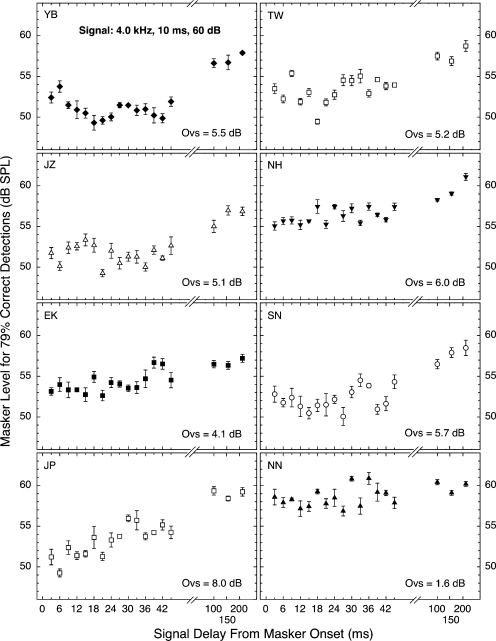
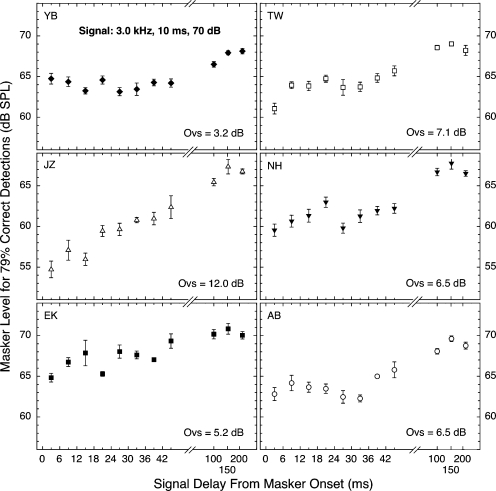
The individual data upon which Fig. 1 was based are presented in Figs. 23, for the first and second crews, respectively. The top five panels in each figure are for subjects who served in both crews. Each panel of each figure contains an estimate of overshoot magnitude (again calculated as masker level required at 200 ms minus masker level required at 3 ms). As can be seen in Fig. 2, subject NN had essentially no overshoot at 4.0 kHz; the same was true at 3.0 kHz, and for this reason, NN was excluded from Figs. 13.
Figure 2.
Overall level of the wideband masking noise needed for 79% correct detections of a 4.0-kHz, 10-ms tone presented at various delays after masker onset. Each panel contains the data for a single subject. The flags indicate standard errors of the mean. “Ovs” designates the difference between the 200- and 3-ms means (the overshoot). Because subject NN had minimal overshoot (bottom right panel), his data were excluded from Figs. 13. Each data point was based on three or four blocks of 50 trials each.
Figure 3.
Overall level of the wideband masking noise needed for 79% correct detections of a 3.0-kHz, 10-ms tone presented at various delays after masker onset. Each panel contains the data for a single subject. Note the different range of ordinate values for the middle pair of panels. The flags indicate standard errors of the mean. “Ovs” designates the difference between the 200- and 3-ms means (the overshoot). Each data point was based on 8–12 blocks of trials.
Surely the most obvious characteristic of the individual data in Figs. 23 is the variability, both within and across subjects. This has been reported for overshoot data since the discovery of the effect (e.g., Zwicker, 1965a, 1965b; Elliott, 1965; McFadden, 1989; Champlin and McFadden, 1989; Schmidt and Zwicker, 1991; Bacon and Smith, 1991; Wright, 1995, 1996; Strickland, 2004). Even with this variability, however, the data for most of the subjects generally followed the trend shown by the across-subject means in Fig. 1. Namely, detectability generally remained reasonably constant over a range of short signal delays and then began to improve (stronger maskers required) toward the eventual maximum. Thus, overshoot did not begin to decline immediately with increasing signal delay, but showed a hesitation. Some apparent exceptions to this general pattern can be seen. The data for both subject JP in Fig. 2 and subject JZ in Fig. 3 appear to be continually rising over the range of short delays studied. We are doubtful that this pattern would survive more intensive study, in part because the data for subject JZ at the other signal frequency were noticeably more typical (flatter) than those at 3.0 kHz. Another exception is that subject TW (Fig. 3) did exhibit a range of relatively constant detectability, but only after an initial step up from poorer detectability. Only research on additional subjects can determine whether these individualistic patterns reflect interesting differences in the physiological mechanisms contributing to overshoot. For the present, we believe that the average data of Fig. 1 are more informative about the issue of hesitation than are the individual data of Figs. 23.
The signal level used for the first crew (60 dB SPL) was chosen with the expectation that most listeners would require masking noises of about 60–65 dB in overall level (about 25 dB spectrum level), which is in the range generally regarded to be optimal for overshoot (Bacon, 1990; Overson et al., 1996). That also matches the spectrum level used for the majority of our nSFOAE measurements. However, our subjects generally were less sensitive than expected, so the masker levels necessary for 79% correct detections uniformly fell below the optimal range, and overshoot magnitudes (see Fig. 2) were smaller than commonly is seen at 4.0 kHz. Consequently, the signal level was increased to 70 dB for the second crew of listeners, and the masker levels increased accordingly; however, the magnitudes of overshoot did not increase much for most of the six subjects who served in both crews. We have no explanation for this. Nonetheless, the presence of hesitation was evident both in the mean data (Fig. 1) and generally in the data for most of our individual subjects (Figs. 23).
DISCUSSION
The outcome here of primary interest is that the magnitude of behavioral overshoot did not begin to decrease immediately as signal delay was increased from masker onset. Rather, for both test frequencies and both signal levels, the detectability of the signal remained approximately constant for at least 30 ms before beginning to improve toward its eventual maximum at about 150–200 ms. This hesitation is quite similar to that observed by Backus and Guinan (2006) for a measure of the SFOAE, also by Walsh et al. (2010a) for a nonlinear version of the SFOAE, and by numerous other investigators using an array of measures, stimuli, and species (see Hill et al., 1997). The existence of a hesitation in both the behavioral and physiological measures strengthens the argument that these physiological measures are tapping mechanisms that are relevant to the processing underlying behavioral overshoot. Specifically, these parallels strengthen the suggestion that the actions of the MOC efferent system are a major contributor to overshoot, as originally proposed by von Klitzing and Kohlrausch (1994) and later elaborated by Strickland (2004, 2008), Keefe et al. (2009), and Walsh et al. (2010a, 2010b).1
The available evidence suggests that the noise masker activates the MOC efferent system, which increases the amount of inhibition on the outer hair cells, thereby altering the local micromechanics of the cochlea in a way that apparently diminishes the ability of the noise to mask the signal (Kawase et al., 1993). Because overshoot-like effects can be seen with tonal maskers (e.g., Bacon and Viemeister, 1985; Bacon and Moore, 1986) even though tones are relatively ineffective at activating the MOC system (Guinan, 2006; Walsh et al., 2010a, 2010b), there must be mechanisms other than the MOC system also contributing to behavioral overshoot in some listening situations [arguments for multiple mechanisms were made by Wright (1995, 1996, 1997) and Scharf et al. (2008)], and hesitation may be absent or different in those situations. Nonetheless, the existence of hesitation in behavioral overshoot using stimuli that also lead to hesitation in nSFOAEs, as were used here, does strengthen the proposed link between overshoot and the MOC system.
Prior to the suggestion from von Klitzing and Kohlrausch (1994) that overshoot is largely attributable to activation of the efferent system, the prevailing explanation for overshoot appealed to neural adaptation (Smith and Zwislocki, 1975; Smith, 1979; Westerman and Smith, 1984). However, neural adaptation failed to account for certain facts of behavioral overshoot that are less of a problem for the efferent explanation (see discussions by Bacon and Healy, 2000; Strickland, 2001): (1) the magnitude of behavioral overshoot (in humans) often greatly exceeds the 3–5 dB adaptations predicted from the responses of primary auditory fibers (of anesthetized laboratory animals) (see Bacon and Moore, 1986); (2) behavioral overshoot is under the control of frequency regions adjacent to the signal frequency (e.g., McFadden, 1989; Bacon and Smith, 1991; Schmidt and Zwicker, 1991; Overson et al., 1996), and even can be observed with maskers having no energy at the signal frequency (Strickland, 2004); (3) behavioral overshoot can be observed using sounds in the contralateral ear, at least in some subjects (Turner and Doherty, 1997; Bacon and Liu, 2000). Nonetheless, some form of neural adaptation may be contributing to overshoot in some listening situations along with the contribution made by the MOC system. (Indeed, some of the marked individual differences seen in overshoot eventually may prove to be attributable to the balance of the contributions from different mechanisms present in individual listeners.) Note that distinguishing between a “pure” neural adaptation and the effects of the efferent system is not simple. The firing rate of a primary auditory neuron will decline after activation of the efferent system, but there is a time lag of a few tens of milliseconds before the decline begins (Wiederhold and Kiang, 1970), which is reminiscent of the presumably MOC-based hesitation that is the topic of this report.
Previous findings
A few previous studies also have employed multiple signal delays in the range from 0 to about 25 ms:
Zwicker (1965a) reported approximately constant overshoot for three signal delays of 5 ms or shorter, but the overshoot for delays of 10 and 20 ms was clearly smaller (his Fig. 3), unlike our present finding. Zwicker’s signal was a 5.0-kHz tone of 2-ms duration, his masker was wideband noise, and the psychophysical method was adjustment. Zwicker (1965b) also showed little evidence of hesitation beyond about 10 ms (his Figs. 2 and 6).
The figures of Elliott (1965) are difficult to read in the region of short delays, and it is not clear how many short delays were tested, but there appears to be little evidence for hesitation (her Figs. 2–5). The signal was a 1.0-kHz tone of 2-ms duration, the masker was wideband, and the psychophysical method was adjustment.
Miśkiewicz et al. (2006) varied signal delay in small steps using lowpass, highpass, and bandpass precursors, always with a bandpass masker. The signal was a 5.0-kHz tone of 2-ms duration, and the psychophysical method was adaptive forced-choice. The overshoot obtained with the highpass precursor does appear to be reasonably constant from 0 to about 6 ms of delay but it declined substantially by 22 ms of delay, unlike the present results. Other aspects of the Miśkiewicz et al. data also are at odds with our findings; for example, for Miśkiewicz et al., the highpass precursor produced more overshoot than the lowpass precursor whereas we have seen the opposite effect (Walsh et al., 2010b). Also, for many of the Miśkiewicz et al. conditions, the masker was only 30 ms in duration, which creates the possibility of offset effects on the tonal signal (see Wright, 1995, 1997).
Neither Bacon and Viemeister (1985) nor Bacon and Moore (1986) systematically explored the short-delay region, but the few short-delay values they did collect offered little evidence for a hesitation preceding the inevitable improvement in detection associated with long signal delays. Their signals were a 1.0-kHz tone of 20-ms duration, their maskers were tones either at or slightly above the signal frequency, and their psychophysical methods were adaptive forced-choice. These failures to see evidence for hesitation may be attributable to their use of tonal maskers because tones are less effective than noise bands at activating the mechanism responsible for producing dynamic changes in the SFOAE and nSFOAE responses (e.g., Guinan, 2006; Walsh et al., 2010a, 2010b). Also, their 20-ms signal constituted a substantial fraction of the presumed hesitation period. Formby et al. (2000) also tested a large number of signal delays, but they used a relatively narrowband noise for their masker, again meaning that the MOC efferent system may not have been effectively activated. Finally, Roverud and Strickland (2010) used a 6-ms signal with some conditions mimicking short signal delays, and did observe evidence of hesitation in some subjects in some conditions; these investigators also used tones for both maskers and signals.
So, prior to this study, the research literature was equivocal about the existence of hesitation in behavioral overshoot. As noted, the studies discussed differed from ours in such parameters as signal duration, signal frequency, masker complexity, and psychophysical procedure. Perhaps one or more of these eventually will account for the various differences in past outcomes. Nonetheless, at least with the combination of acoustic stimuli and psychophysical method we used, hesitation was commonly seen in our subjects.2
ACKNOWLEDGMENTS
This work was supported by a research grant awarded to D.M. by the National Institute on Deafness and other Communication Disorders (NIDCD; RO1 DC000153). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCD or the National Institutes of Health. Two anonymous reviewers and the associate editor provided helpful comments on an earlier version of this report.
Footnotes
When comparing the durations of hesitation we measure behaviorally and physiologically (Walsh et al., 2010a, 2010b), it is important to note that our nSFOAE responses were obtained with long-duration signals and were analyzed using 20-ms windows, meaning that the physiological data plotted at, say, 25 ms actually represented the average response magnitude over the range from 25 to 45 ms. Thus, our physiological measures are inherently underestimates of the duration of hesitation. The measure used by Backus and Guinan (2006) does not involve a similar analysis window.
One implication of our finding hesitation with short signal delays is that the change in tuning reported by Strickland (2001) as a function of time since masker onset also ought to show hesitation; that is, the sharp tuning observed with a signal delay of 2 ms ought to persist for another 20 ms or so before beginning to decline toward the ultimate, broader value seen with signal delays of about 200 ms.
References
- Backus, B. C., and Guinan, J. J., Jr. (2006). “Time-course of the human medial olivocochlear reflex,” J. Acoust. Soc. Am. 119, 2889–2904. 10.1121/1.2169918 [DOI] [PubMed] [Google Scholar]
- Bacon, S. P. (1990). “Effect of masker level on overshoot,” J. Acoust. Soc. Am. 88, 698–702. 10.1121/1.399773 [DOI] [PubMed] [Google Scholar]
- Bacon, S. P., and Healy, E. W. (2000). “Effects of ipsilateral and contralateral precursors on the temporal effect in simultaneous masking with pure tones,” J. Acoust. Soc. Am. 107, 1589–1597. 10.1121/1.428443 [DOI] [PubMed] [Google Scholar]
- Bacon, S. P., and Liu, L. (2000). “Effects of ipsilateral and contralateral precursors on overshoot,” J. Acoust. Soc. Am. 108, 1811–1818. 10.1121/1.1290246 [DOI] [PubMed] [Google Scholar]
- Bacon, S. P., and Moore, B. C. J. (1986). “Temporal effects in simultaneous pure-tone masking: Effects of signal frequency, masker∕signal frequency ratio, and masker level,” Hear. Res. 23, 257–266. 10.1016/0378-5955(86)90114-0 [DOI] [PubMed] [Google Scholar]
- Bacon, S. P., and Smith, M. A. (1991). “Spectral, intensive, and temporal factors influencing overshoot,” Q. J. Exp. Psychol. 43A, 373–399. [DOI] [PubMed] [Google Scholar]
- Bacon, S. P., and Viemeister, N. F. (1985). “The temporal course of simultaneous tone-on-tone masking,” J. Acoust. Soc. Am. 78, 1231–1235. 10.1121/1.392891 [DOI] [PubMed] [Google Scholar]
- Champlin, C. A., and McFadden, D. (1989). “Reductions in overshoot following intense sound exposures,” J. Acoust. Soc. Am. 85, 2005–2011. 10.1121/1.397853 [DOI] [PubMed] [Google Scholar]
- Elliott, L. L. (1965). “Changes in the simultaneous masked threshold of brief tones,” J. Acoust. Soc. Am. 38, 738–746. 10.1121/1.1909798 [DOI] [PubMed] [Google Scholar]
- Formby, C., Sherlock, L. P., and Ferguson, S. H. (2000). “Enhancement of the edges of temporal masking functions by complex patterns of overshoot and undershoot,” J. Acoust. Soc. Am. 107, 2169–2187. 10.1121/1.428498 [DOI] [PubMed] [Google Scholar]
- Guinan, J. J., Jr. (2006). “Olivocochlear efferents: Anatomy, physiology, function, and the measurement of efferent effects in humans,” Ear Hear. 27, 589–607. 10.1097/01.aud.0000240507.83072.e7 [DOI] [PubMed] [Google Scholar]
- Guinan, J. J., Jr., Backus, B. C., Lilaonitkul, W., and Aharonson, V. (2003). “Medial olivocochlear efferent reflex in humans: Otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs,” J. Assoc. Res. Otolaryngol. 4, 521–540. 10.1007/s10162-002-3037-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, J. C., Prasher, D. K., and Luxon, L. M. (1997). “Latency of contralateral sound-evoked auditory efferent suppression of otoacoustic emissions,” Acta Oto-Laryngol. 117, 343–351. 10.3109/00016489709113405 [DOI] [PubMed] [Google Scholar]
- Kawase, T., Delgutte, B., and Liberman, M. C. (1993). “Antimasking effects of the olivocochlear reflex. II. Enhancement of auditory-nerve response to masked tones,” J. Neurophysiol. 70, 2533–2549. [DOI] [PubMed] [Google Scholar]
- Keefe, D. H., Schairer, K. S., Ellison, J. C., Fitzpatrick, D. F., and Jesteadt, W. (2009). “Use of stimulus-frequency otoacoustic emissions to investigate efferent and cochlear contributions to temporal overshoot,” J. Acoust. Soc. Am. 125, 1595–1604. 10.1121/1.3068443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe, D. H., Schairer, K. S., and Jesteadt, W. (2003). “Is there an OAE correlate to behavioral overshoot?,” Assoc. Res. Otolaryngol. Abstr. 26, 397. [Google Scholar]
- Levitt, H. (1971). “Transformed up-down methods in psychoacoustics,” J. Acoust. Soc. Am. 49, 467–477. 10.1121/1.1912375 [DOI] [PubMed] [Google Scholar]
- McFadden, D. (1989). “Spectral differences in the ability of temporal gaps to reset the mechanisms underlying overshoot,” J. Acoust. Soc. Am. 85, 254–261. 10.1121/1.397732 [DOI] [PubMed] [Google Scholar]
- Miśkiewicz, A., Buus, S., and Florentine, M. (2006). “Effect of masker-fringe onset asynchrony on overshoot,” J. Acoust. Soc. Am. 119, 1331–1334. 10.1121/1.2162175 [DOI] [PubMed] [Google Scholar]
- Overson, G. J., Bacon, S. P., and Webb, T. M. (1996). “The effect of level and relative frequency region on the recovery of overshoot,” J. Acoust. Soc. Am. 99, 1059–1065. 10.1121/1.415232 [DOI] [PubMed] [Google Scholar]
- Roverud, E., and Strickland, E. A. (2010). “The time course of cochlear gain reduction measured using a more efficient psychophysical technique,” J. Acoust. Soc. Am. In press. [DOI] [PMC free article] [PubMed]
- Scharf, B., Reeves, A., and Giovanetti, H. (2008). “Role of attention in overshoot: Frequency certainty versus uncertainty,” J. Acoust. Soc. Am. 123, 1555–1561. 10.1121/1.2835436 [DOI] [PubMed] [Google Scholar]
- Schmidt, S., and Zwicker, E. (1991). “The effect of masker spectral asymmetry on overshoot in simultaneous masking,” J. Acoust. Soc. Am. 89, 1324–1330. 10.1121/1.400656 [DOI] [PubMed] [Google Scholar]
- Smith, R. L. (1979). “Adaptation, saturation, and physiological masking in single auditory-nerve fibers,” J. Acoust. Soc. Am. 65, 166–178. 10.1121/1.382260 [DOI] [PubMed] [Google Scholar]
- Smith, R. L., and Zwislocki, J. J. (1975). “Short-term adaptation and incremental responses in single auditory-nerve fibers,” Biol. Cybern. 17, 169–182. 10.1007/BF00364166 [DOI] [PubMed] [Google Scholar]
- Strickland, E. A. (2001). “The relationship between frequency selectivity and overshoot,” J. Acoust. Soc. Am. 109, 2062–2073. 10.1121/1.1357811 [DOI] [PubMed] [Google Scholar]
- Strickland, E. A. (2004). “The temporal effect with notched-noise maskers: Analysis in terms of input-output functions,” J. Acoust. Soc. Am. 115, 2234–2245. 10.1121/1.1691036 [DOI] [PubMed] [Google Scholar]
- Strickland, E. A. (2008). “The relationship between precursor level and the temporal effect,” J. Acoust. Soc. Am. 123, 946–954. 10.1121/1.2821977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, C. W., and Doherty, K. A. (1997). “Temporal masking and the ‘active process’ in normal and hearing-impaired listeners,” in Modeling Sensorineural Hearing Loss, edited by Jesteadt W. (Erlbaum, Hillsdale, NJ: ), pp. 387–396. [Google Scholar]
- von Klitzing, R., and Kohlrausch, A. (1994). “Effect of masker level on overshoot in running- and frozen-noise maskers,” J. Acoust. Soc. Am. 95, 2192–2201. 10.1121/1.408679 [DOI] [PubMed] [Google Scholar]
- Walsh, K. P., Pasanen, E. G., and McFadden, D. (2010a). “Properties of a nonlinear version of the stimulus-frequency otoacoustic emission,” J. Acoust. Soc. Am. 127, 955–969. 10.1121/1.3279832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, K. P., Pasanen, E. G., and McFadden, D. (2010b). “Overshoot measured physiologically and psychophysically in the same human ears,” Hear. Res. 268, 22–37 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman, L. A., and Smith, R. L. (1984). “Rapid and short-term adaptation in auditory-nerve responses,” Hear. Res. 15, 249–260. 10.1016/0378-5955(84)90032-7 [DOI] [PubMed] [Google Scholar]
- Wiederhold, M. L., and Kiang, N. Y. S. (1970). “Effects of electric stimulation of the crossed olivocochlear bundle on single auditory-nerve fibers in the cat,” J. Acoust. Soc. Am. 48, 950–965. 10.1121/1.1912234 [DOI] [PubMed] [Google Scholar]
- Wright, B. A. (1995). “Detectability of simultaneously masked signals as a function of signal bandwidth for different signal delays,” J. Acoust. Soc. Am. 98, 2493–2503. 10.1121/1.413280 [DOI] [PubMed] [Google Scholar]
- Wright, B. A. (1996). “Correlated individual differences in conditions used to measure psychophysical suppression and signal enhancement,” J. Acoust. Soc. Am. 100, 3295–3303. 10.1121/1.417213 [DOI] [PubMed] [Google Scholar]
- Wright, B. A. (1997). “Detectability of simultaneously masked signals as a function of masker bandwidth and configuration for different signal delays,” J. Acoust. Soc. Am. 101, 420–429. 10.1121/1.417987 [DOI] [PubMed] [Google Scholar]
- Zwicker, E. (1965a). “Temporal effects in simultaneous masking by white-noise bursts,” J. Acoust. Soc. Am. 37, 653–656. 10.1121/1.1909389 [DOI] [PubMed] [Google Scholar]
- Zwicker, E. (1965b). “Temporal effects in simultaneous masking and loudness,” J. Acoust. Soc. Am. 38, 132–141. 10.1121/1.1909588 [DOI] [PubMed] [Google Scholar]