Abstract
The precedence effect refers to the fact that humans are able to localize sound in reverberant environments, because the auditory system assigns greater weight to the direct sound (lead) than the later-arriving sound (lag). In this study, absolute sound localization was studied for single source stimuli and for dual source lead-lag stimuli in 4–5 year old children and adults. Lead-lag delays ranged from 5–100 ms. Testing was conducted in free field, with pink noise bursts emitted from loudspeakers positioned on a horizontal arc in the frontal field. Listeners indicated how many sounds were heard and the perceived location of the first- and second-heard sounds. Results suggest that at short delays (up to 10 ms), the lead dominates sound localization strongly at both ages, and localization errors are similar to those with single-source stimuli. At longer delays errors can be large, stemming from over-integration of the lead and lag, interchanging of perceived locations of the first-heard and second-heard sounds due to temporal order confusion, and dominance of the lead over the lag. The errors are greater for children than adults. Results are discussed in the context of maturation of auditory and non-auditory factors.
INTRODUCTION
The present study was concerned with differences between children and adults in the ability to localize sounds when a source and a simulated echo are presented in a room. In reverberant environments sound first arrives at the listener’s ears through a direct path, which is the least disturbed path. Reflections of the sound from nearby surfaces, including walls and various objects, reach the ears with a time delay, and offer their own set of localization cues. Decades of research on this topic have shown that listeners are remarkably adept at segregating target from competing sources. In reverberant rooms, although listeners are aware of the presence of reflections, localization cues carried by reflections are de-emphasized relative to the cues carried by the source. This phenomenon is commonly attributed to auditory mechanisms that assign greater weight to the localization cues belonging to the preceding, or first-arriving sound, hence it is referred to as the precedence effect (for reviews see Blauert, 1997; Litovsky et al., 1999).
The experimental paradigm used in this study is one that has been implemented in studies on the precedence effect, which typically utilize simplified versions of source-reflection arrays, such that one source (lead) is presented from a given location, or carries a set of binaural cues presented via headphones. An unrealistic reflection (lag) is simulated whose intensity is typically the same as that of the lead. The stimulus feature that is generally varied in precedence effect studies is the time delay between the onsets of the lead and lag. At short delays the lead and lag fuse into a single auditory percept; when the delay is between 0 and 1 ms summing localization occurs, whereby both lead and lag contribute to the perceived location of the fused image. As the delay increases to 1 ms and beyond, the location of the lead dominates the perceived location of the fused auditory image, a phenomenon that has become known as localization dominance (Litovsky et al., 1999). The delay at which the lead and lag break apart into two auditory events is known as echo threshold. Another way of quantifying the extent to which the directional cues from the lag are available to the listener is to measure discrimination suppression, whereby the listener discriminates changes in the location or interaural parameters related to the lag. As delays increase, the ability of the listener to extract directional cues from the lag improves, indicating that discrimination suppression diminishes with delay, and is related to the fact that fusion is also reduced, hence the lag is more audible.
Of the precedence effect phenomena described here, localization dominance is perhaps the most relevant to real-world listening challenges. For human listeners little is known about the extent to which audible reflections in the precedence effect paradigm affect sound localization. However, behavioral studies using this paradigm have been conducted in non-human species such as owls (Spitzer and Takahashi, 2006) and cats (e.g., Dent et al., 2009), and suggest that at delays that surpass echo thresholds the locations of both the lead and lag stimuli can be perceptually resolved. These studies are important because, in addition to behavior, physiological correlates of the precedence effect have been identified in these species (e.g., Yin, 1994; Litovsky and Yin, 1998; Spitzer et al., 2004, Dent et al., 2009; for review see Litovsky and McAlpine, 2010). Findings of numerous correlates between behavior and physiology suggest that neural aspects of the precedence effect that are experienced by humans are found in responses of single neurons, even in young animals (Litovsky, 1998), and that many of the perceptual phenomena of the precedence effect can be modeled by looking at inputs to the inferior colliculus (Xia et al., 2010).
Blauert (1997) and Litovsky et al. (1999) in their reviews describe the notion that when echo threshold is initially reached, the location of the lag may be difficult to identify, but that for delays of 10–20 ms or greater listeners should be able to perceive the lead and lag each at their respective locations. Using interaural time difference (ITD) cues to simulate lead and lag locations over headphones, Litovsky and Shinn-Cunningham (2001) found that localization dominance can persist at delays that surpass echo threshold, that is, two images were reported but the perceived intracranial location of the lag was pulled toward the lead ITD. In fact, at the longest delays tested (15 ms), intracranial positions of the lead and lag deviated from the reported locations of the single source stimuli tested with the same ITDs. This finding suggests that lagging sources which are perceived as separate sounds pose a potential problem for source localization.
The present experiment thus aimed to examine the ability of children and adults to localize lead-lag stimuli that were either perceived to be fused into a single auditory event, or perceived as two auditory events, at much longer delays than previously tested (100 ms). In the latter case, by instructing listeners to report the perceived location of the first- and second-heard images, we were able to capture phenomena that encompass fusion, localization dominance and temporal order confusion. At long delays, using a discrimination paradigm, with headphone presentations of binaural click pairs, it was found that listeners may be more sensitive to the lag than the lead (Stellmack et al., 1999; Dye et al., 2006). These findings were interpreted in the context of perceptual effects that include backward recognition masking (reduced ability to localize the first stimulus once the second stimulus is heard, e.g., Massaro et al., 1976), and binaural sluggishness (a reduced ability of the auditory system to follow fast changes in the binaural parameters of incoming signals, Grantham and Wightman, 1978). These latter effects are likely to be relevant in the context of the present sound-field experiments.
While examining these effects in adult listeners has merit, we were particularly interested in developmental issues related to the precedence effect. As reviewed byLitovsky and Ashmead (1997) the precedence effect does not appear to be present in human infants at birth, although rudimentary aspects of single-source sound localization can be measured in newborn infants (Clifton et al., 1981). While the precedence effect appears at around age 5–6 months, it remains immature in childhood (Litovsky and Ashmead, 1997). As reviewed by Litovsky et al. (1999), discrimination suppression studies with adult listeners have looked at the extent to which listeners are able to extract directional cues from the lead in the presence of the lag, relative to performance with the lead alone (single-source discrimination). While adults show little effect in presence of the lag, indicating strong dominance by the first-arriving source, children show weaker dominance, with lead discrimination abilities being significantly worse than single-source discrimination (Litovsky, 1997). These developmental findings leave open the question as to whether immaturity of the precedence effect influences spatial hearing abilities in realistic tasks that measure sound localization per se. Despite a growing literature on auditory perception in children (e.g., Hall et al., 2007, 2008; Johnstone and Litovsky, 2006; Leibold and Bonino, 2009; Litovsky, 2005; Lutfi et al., 2003), little is known about emergence of spatial hearing skills, which no doubt impact children’s ability to navigate their environment, to segregate sources and to form auditory objects. The second aim of this experiment was to address this gap in knowledge by studying the precedence effect using a sound localization paradigm, and we compared these data with echo threshold data. We selected age 4–5 years, as this is the youngest age at which we could obtain reliable responses from children regarding perceived source locations using a multi-loudspeaker array.
METHODS
Participants
Nine children (5 female and 4 male) were recruited whose average age at the midpoint of testing intervals was 5.14 yrs (a range of 4.4–5.8 years). They were all enrolled in the Waisman Center Early Childhood Program which has a preschool room near the testing laboratory. Each participating child’s parents or guardians signed consent forms permitting research staff to take the child out of the classroom during specified times of the day for up to 30 min at a time, twice per week. Thus, in order to complete the entire study design each child participated in 5–10 testing sessions. Six of the children completed testing on both localization and fusion tests, while three of the children (CAA, CBR and CCY) had graduated from the program by the time fusion testing commenced and were unable to participate in that experiment. Ten adults (all female) whose average age was 22 yrs (19 to 26) also participated. Most adults were tested during a single testing session that lasted approximately 2 h (including breaks). All listeners had normal hearing, verified with pure tone audiometric thresholds <15 dB HL in each ear at standard frequencies (250, 500, 1000, 2000, 4000, 8000 Hz). In addition, on each testing day for children tympanometric measures were conducted to screen for fluctuations in middle ear pressure.
Stimuli
Stimuli were generated using MATLAB software (The Mathworks, Natick, MA) with a sampling rate of 44.1 kHz. All listeners were trained on the task prior to testing. For the purpose of training children, stimuli consisted of 10 consecutive pink noise bursts, each 25 ms in duration with 2 ms rise∕fall times, presented at a rate of 4∕s. The 10 bursts were presented so that the child would have ongoing repetitions of the stimulus, as it was determined during pilot testing that long-duration stimulus trains provided best results for children who were first learning the task. However, repeated lead-lag pairs are known to produce “buildup” of echo suppression (e.g., Freyman et al., 1991). To minimize buildup, during testing the same pink noise bursts were used, but only 3 were presented on each trial, also at a rate of 4∕s [see Fig. 1a for a schematic and figure legend for further details].
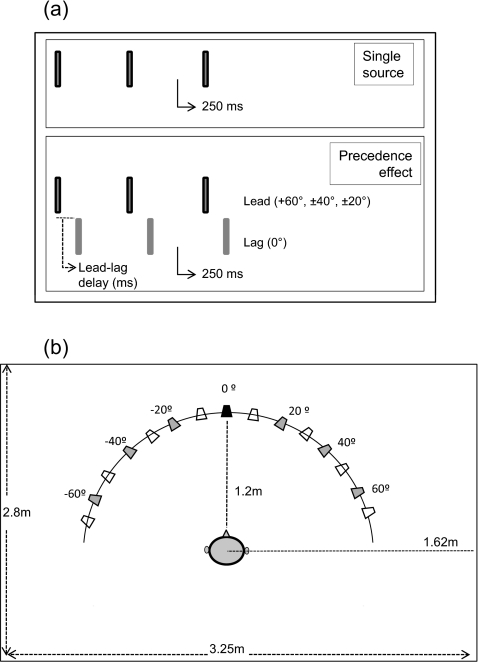
Figure 1.
Panel (a) shows a schematic representation of the stimuli. In the single source condition (top) stimuli were presented from a single loudspeaker, with an interstimulus interval of 250 ms. In the precedence effect condition (bottom) the lead stimulus location varied from among 6 possible locations, while the lag stimulus was presented from 0°. The lead-lag delay is the delay between the onsets of the lead and lag loudspeaker (see text for delay conditions). The interstimulus interval of 250 ms refers to the delay between the onsets of successive lead stimuli (or lag stimuli). Panel (b) shows a schematic of the room dimensions, and listener’s head relative to the loudspeaker array. The lag stimulus was always presented from 0° (front), indicated by the dark fill. The lead stimuli (gray fill) could be presented from one of 6 locations (±20°, ±40°, ±60°). Additional locations (unfilled) were available for responses even though no sound was emitted from those loudspeakers. Thus, responses could range from −70° to +70° in (10° increments).
During testing for both children and adults, on each trial, a new noise burst token was generated on-line, and that same burst was used for the 3 consecutive presentations. On precedence effect trials (see below) the same noise token was used for both the leading and lagging stimuli. These stimuli were chosen based on extensive pilot testing, during which we searched for stimuli that produced consistent responses on the sound localization task with single-source stimuli. The average stimulus intensity was 60 dB SPL (A weighting) as measured through a sound level meter with the microphone positioned at the location that would correspond to the center of the listener’s head. The intensity was roved over a range of 8dB (i.e., 56–64 dB) in order to minimize availability of overall level cues for localization (e.g., Grieco-Calub and Litovsky, 2010; Litovsky et al., 2006, 2009).
Testing room and equipment
Testing was conducted in a standard IAC sound booth (2.8 m×3.25 m) with reverberation time (RT60) of 250 ms. Listeners sat in the center of the room facing an array of loudspeakers positioned along the horizontal plane, at ear level for adults and approximately 10 cm above ear level for children. Fifteen loudspeakers (Cambridge SoundWorks Henry Kloss Center∕Surround IV) were placed at 10° intervals between ±70°, as illustrated in Fig. 1b. A customized MATLAB program was developed for randomization and presentation of stimuli, which was accomplished via a Tucker-Davis Technologies System 3 multiple input-output processor. Underneath each loudspeaker was placed a visual child-friendly icon. A computer monitor was placed under the front loudspeaker, and the loudspeaker arrangement with the pictures corresponding to each location was displayed on the screen, so that on each trial responses were entered via a mouse by clicking on appropriate pictures corresponding to perceived locations. Adults entered their own responses; some children were confident with the mouse and did so as well while for other children responses were entered by the experimenter.
Localization task
On this task, listeners were instructed to select a loudspeaker(s) from which they believed the sound(s) to be emanating and to thus identify the icon(s) on the computer monitor that matched the icon(s) under that loudspeaker. Two types of trials were included in this experiment: single source and lead-lag. Single source trials consisted of stimulus presentations from a single loudspeaker. Single source testing was conducted in blocks of trials during which the stimulus location varied randomly among 7 possible locations (±60°, ±40°, ±20°, 0°), with 5 repetitions per location. Each child was first trained on the task for one block of trials (35 trials; 5 repetitions×7 locations) using the 10-noise-burst stimulus. The purpose of training was to ensure that the child understood the instructions and was responding on every trial. Testing commenced with the 3-noise-burst stimuli. Each listener was tested on one block of trials (35 trials; 5 repetitions×7 locations).
Following single source testing, lead-lag testing was conducted. Lead-lag trials consisted of stimulus presentations from two loudspeakers, with a delay between their onsets. The stimuli presented first and second are henceforth referred to as the lead and lag, respectively. Testing was conducted in blocks of trials during which the delay between the lead and lag was fixed, the lag stimulus location was fixed at 0°, and the only variable was the location of the lead (±60°, ±40°, ±20°). As shown in Fig. 1, 15 loudspeakers were visible to listeners. The active ones were at ±60°, ±40°, ±20°, 0°; loudspeakers at ±70°, ±50°, ±30° provided additional response options but did not emit any sound. Each listener was tested on one block of trials per delay (30 trials; 5 repetitions×6 locations). The delay values were 5, 10, 25, 50 and 100 ms; the order of testing with these delays was randomized for each listener. All listeners except two children completed testing for each of the conditions. Two listeners left the school prior to completion (listener CCY did not complete 5 and 50 ms, and listener CFV did not complete 25 ms). During testing listeners were instructed to first determine whether they perceived one sound or two sounds. If they heard one sound they reported its location. If they heard two sounds they were instructed to decide which sound was perceived to be first and which was second. The instructions were explicit in asking that listeners report the perceived locations of the “first heard” and subsequently report the perceived location of the “second heard” sound. Similar to the single source task, locations were reported by selecting icons on the computer monitor corresponding to each perceived location. No feedback was provided regarding locations of loudspeakers emitting the stimuli. However, after each response a section of a child-friendly image was added to the screen, akin to a puzzle being progressively solved, as a means of reinforcing the child for responding. During this visual presentation, a child-friendly sound was presented to mask a low-level, unavoidable transient that occurs during switching by the multiplexer.
Fusion task
All adults were tested on the fusion task, but only 6 of the 9 children were able to return for this testing (data for listeners CAA, CBR and CCY are thus not included). Testing was conducted separately on the fusion task, to determine the delay at which each listener subjectively reported hearing two separate sounds. On this task, the lead was presented from either +40° or −40° and the lag was fixed at 0°. During training, lead-lag pairs were presented at delays ranging from 5–100 ms, and listeners were instructed to report “two” when they clearly heard two sounds, one from each loudspeaker, but to report “one” when a single sound was heard from one of the loudspeakers. During the fusion task delays were randomized throughout a block of trials, to include 5, 10, 15, 20, 25, 30 and 50 ms. For each condition (2 lead locations×7 delays) there were 5 trials. Instructions to listeners were to report whether they perceived one sound or two separate sounds, and responses were made by using the computer mouse to click on one of two icons on the computer monitor, with the numbers “1” or “2” in the center. No feedback was provided.
RESULTS
To estimate fusion echo thresholds, a logistic function was fit to each listener’s data. Regression analyses on individual listeners’ fitted data vs. raw data yielded significant results (p<0.05) for all listeners. Echo threshold was defined as the delay corresponding to 75% on the function. The average echo thresholds (±group standard deviation) were 15.2 ms (±4.9; range of 9.5–23 ms) for adults, and 24.3 ms (±13.6; range of 13 to 28) for children. Thus, there was some overlap in the echo thresholds of the two groups. A t-test for independent samples with unequal variance showed that adults had lower echo thresholds than children[t(14)=2.14, p<0.03].
The localization accuracy for single source stimuli was quantified by computing the root-mean-square (RMS) error; the mean deviation of the responses from the target locations, irrespective of the direction of the deviation. It was computed using
| (1) |
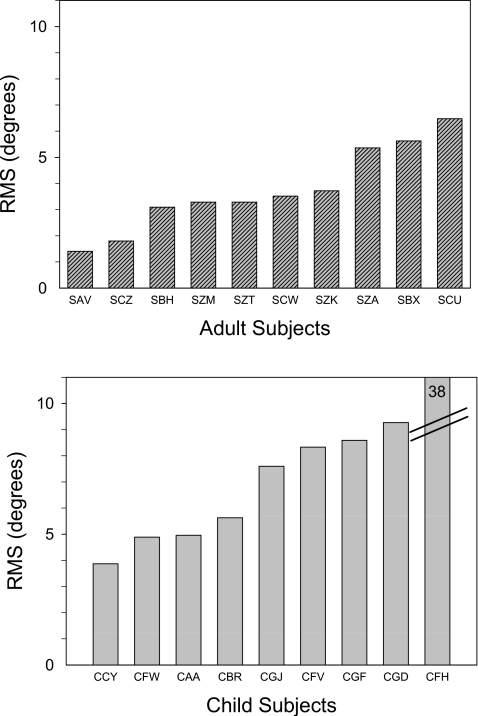
where x1=target location, x2=response location, i=incrementing trials, and n=number of trials. In Fig. 2, RMSsingle-sourcemean values (±standard deviation) for each listener in the adult (mean=3.6°±1.63°) and child (mean=10.2°±10.72°) groups are shown, rank ordered from lowest to highest. The average group mean difference was 6.4° (±2.03°). Due to a large variance in the younger group, t-test for independent samples failed normality, thus a Mann-Whitney Rank Sum test was performed, yielding a significant difference in group median values [Mann-Whitney U Statistic=10.5; T=124.5, p<0.01]. Removal of an outlier with a RMSsingle-source value of 38.3 (CFH) reduced the average for children to 6.64°, which was still significantly higher than the RMSsingle-source value for adults[t(16)=3.34,p<.01], however the mean difference between groups was very small (2.8°). It is noteworthy that single source localization abilities of 4–5-year-old children produced average errors of<10°, less than one loudspeaker location away from the targets. We conclude that single source localization is clearly emerging and reaching near-adult performance in many, but not all children of this age group.
Figure 2.
RMS localization data are shown for individual listeners from the single source condition. Adult listeners (top) and child listeners (bottom) each contributed one data point, and results within each group are rank-ordered from lowest to highest error.
For lead-lag conditions, because on many trials two sounds were heard, the analyses were aimed at quantifying the perceived locations of the first-heard and second-heard images. In the data presentation these responses are labeled as First Response and Second Response, and they are attributed to the lead and lag stimuli, respectively. There is the distinct possibility that listeners’ ability to accurately determine the temporal order of the two events was imperfect, and the data indeed suggest that temporal order confusion occurred in some conditions. Nonetheless, the question at hand was how well listeners were able to make that judgment, and to what extent this ability influenced how well they were able to identify the location(s) of what they perceived to be the “first” and “second” sounds.
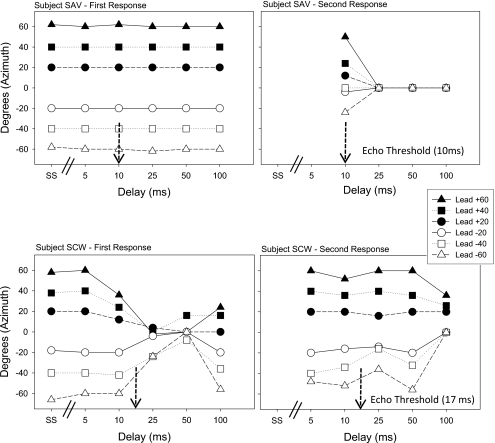
For each listener, the average reported locations for First Response and Second Response were computed at each delay. These average data are shown in Figs. 34 for two adult listeners and three child listeners, respectively. Note that error bars were omitted here, and that the delays are plotted on a categorical scale that includes single source data followed by lead-lag data with delay values arranged from smallest to largest. In each of these figures the left panels show First Response data and the right panels show Second Response data; note that the latter often lack data points at short delays, when only one sound was reported. Within each panel, points plotted along the abscissa indicate average perceived locations in lead-lag conditions (delays of 5–100 ms).
Figure 3.
“First Response” (left) and “Second Response” (right) data are shown for two adult listeners (SAV on the top and SCW on the bottom), as a function of condition∕delay (SS, 5, 10, 25, 50, 100 ms). Each panel includes data from the 6 lead- location conditions (see symbols and legend), ranging from −60° to+60°. Data are averaged across all trials for that listener∕condition (error bars are not shown). Within the lower panels, the listeners’ echo thresholds are indicated by an arrow pointing to the approximate delay corresponding to echo threshold.
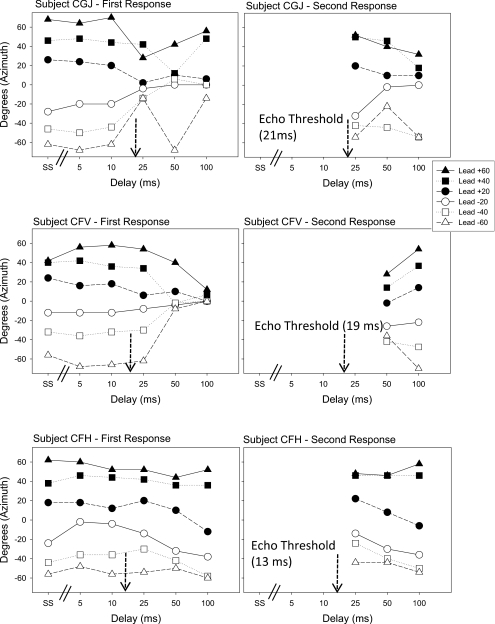
Figure 4.
Same as Fig. 3, for 3 children (listeners CGJ, CFH and CFV).
Listener SAV (Fig. 3, top) showed First Response data that were exceptionally accurate. Second Response data were available only beginning at 10 ms, due to the inaudibility of the lag at 5 ms. However, at the 10 ms delay, which coincides with this listener’s echo threshold, the perceived location of the second sound was “pulled” away from the lag location (0°) toward the lead location (more lateral locations in the room). In fact, the more lateral the lead the greater the “pulling” of the Second Response. Thus, the lead seemed to, if not dominate, at least influence, the perceived location of the lag. At a delay of 25 ms the Second Response data were aligned exactly along the lag location at 0°. Since the First Response data were aligned with the lead location (left panel), we conclude that at delays of 25 ms and longer this listener was able to identify two audible images at two different locations.
Listener SCW (Fig. 3, bottom) provides an example of a different type of response. The First Response data showed more errors in lead localization. There is a “pulling” of the response toward the lag location (0°) at 25 and 50 ms (note that echo threshold was 20 ms), and partial recovery from “pulling” at 100 ms. The bottom-right panel in Fig. 3, where Second Response data are plotted for SCW, suggests that the listener did not accurately localize the lag, even at the longest delays tested. While echo threshold reflects delays at which the lag is reported as a separate sound on majority of trials, measures of localization reflect the potential spatial hearing errors that can arise, both in terms of the lead dominating the perceived location of the lag, and of the influence exerted on the lead by the lag. Note that for this listener, the lag was never reported at its true location even at the longest delays tested. In fact, at delays of 50–100 ms two images were reported at locations that span the midrange angles (±40°).
Results from three children are shown in Fig. 4. These listeners were selected to illustrate the types of responses observed in children. These data suggest that localization dominance was fairly strong at the shorter delays (see left panels). For listeners CGJ and CFV, at longer delays two aspects of the data are noteworthy. The First Response data were “pulled” toward the lag location (0°), while the Second Response data were dominated by the respective lead locations. That is, both listeners displayed temporal order confusion, whereby the First Response was assigned to the center (lag) and the Second Response was assigned to the lateral locations (lead). Note that on the fusion task the echo threshold was 21 ms for listener CGJ and 19 ms for listener CFV; on the localization task two image reports were only observed at 50–100 ms. Listener CFH was different, however, in that this child generally localized both the “first heard” and “second heard” sounds at the lead location, suggesting extended and robust localization dominance.
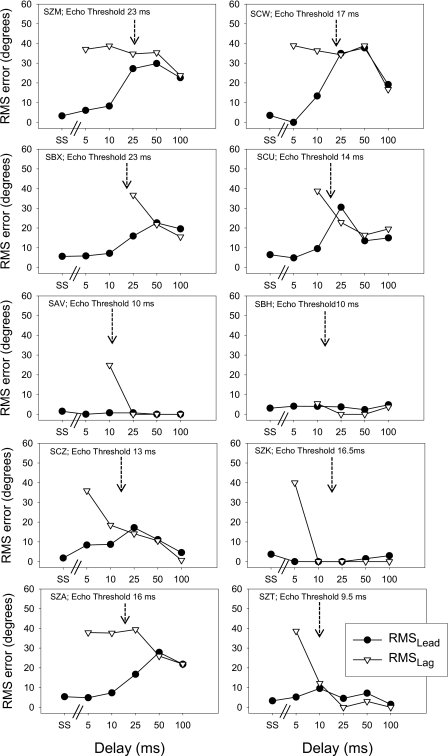
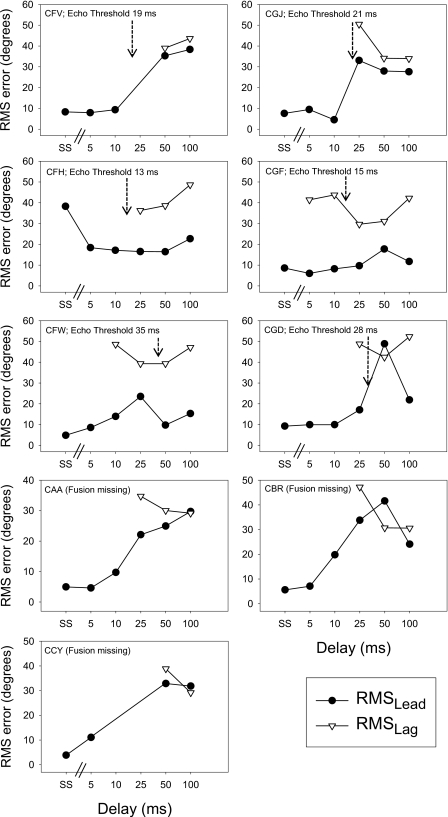
The results were next evaluated for each listener using approaches that collapsed across all lead locations, and emphasized overall localization accuracy. The assumption that is made in these analyses is that the First Response and Second Response correspond to the lead and lag stimuli, respectively. Thus, errors reflect a combination of mis-localization, and mis-attribution of sounds to the First- vs. Second-heard images due to temporal order confusion. Values of RMS errors for lead localization (termed ‘ RMSlead ’) were quantified using Eq. 1 (see above), where x1=lead location, and x2=First Response location. Similarly, values of RMS errors for lag localization (termed ‘ RMSlag ’) were quantified using Eq. 1, but with x1=lag location, and x2=Second Response location. Figures 56 show values of RMSlead and RMSlag, as a function of delay. Within each panel, listener codes are given, along with the listener’s echo threshold (not available for the last 3 children since they could not return to the laboratory for Fusion testing). In addition, single-source data are plotted as the left-most point for each listener, for comparison. The RMS value was generally small in the single source condition and at brief delays, and increased as delays increased, reflecting the localization confusion that occurred when the audible lag was localizable at a location that was different than that of the lead. The RMSlag value was larger than the RMSlead value at brief delays; the two images seem to have been temporally distinguishable and equally localizable at longer delays. Individual differences occurred in the error size however. Some listeners (e.g., SKZ, SZT) showed generally small values of RMSlead (<5°)and RMSlag (<12°) at all but the very brief delays, while other listeners (e.g., SZM, SCW) showed large (>20°)errors at delays that extended to 100 ms. The latter listeners presumably experienced localization confusion and∕or temporal order confusion, and were unable to attribute the lead and lag to their respective locations. In general, listeners with the best performance at long delays had shorter values of echo threshold (SAV, 10 ms; SBH, 10 ms; SCZ, 13 ms; SZT, 9.5 ms). But the echo threshold was not a consistent factor associated with reduced errors at long delays, as can been seen from results of SZK and SZA whose echo thresholds were both near 16 ms, but whose localization abilities differed at the long delays.
Figure 5.
Localization errors were collapsed across all lead locations, and are shown for each listener in a separate panel. Root-mean-square (RMS) values were computed using Eq. 1 (see text). RMSlead (filled symbols) indicates the average errors of the First Response relative to the location of the lead. Results are plotted as a function of stimulus condition beginning with SS for single source and all the precedence delays (5, 10, 25, 50 and 100 ms). RMSlagdata (open symbols) indicate the average errors for the Second Response relative to the location of the lag. Within each panel, listener codes are given, along with the listener’s echo threshold (see arrow).
Figure 6.
Same as Fig. 5, for child listeners.
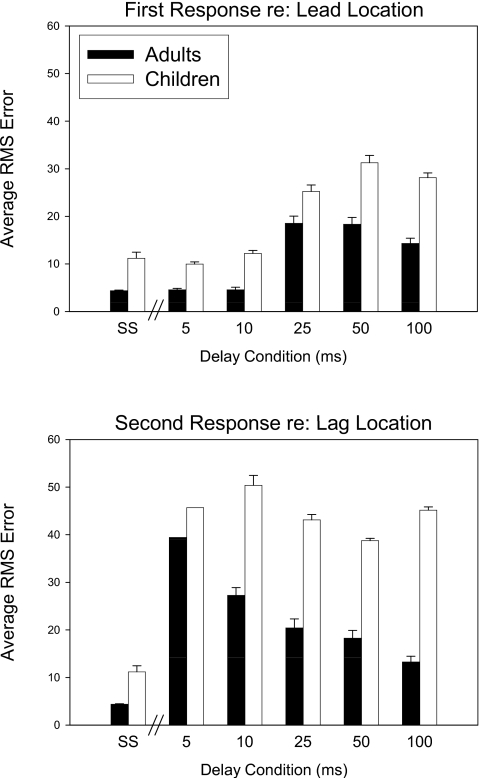
Figure 6 shows values of RMS for the children. As noted in the Methods, listener CCY was not tested at two of the delays and listener CFV at one delay. In addition, while CFH had a higher RMSsingle source value compared to the rest of the children, there was no indication that this child was an outlier when the RMSlead or RMSlag values were considered, thus all children’s data were included in the group data and analyses. In general, the RMS values for children were higher than those of adults. As in the adult data, RMSleadvalues in all but two children were most comparable to RMSsingle source values at short (e.g., <25 ms) lead-lag delays, increasing to match the values of RMSlag at longer delays. The two exceptions (listeners CFH and CGF) seemed able to localize the lead relatively well and better than the lag, at all delays. Group data are summarized in Fig. 7 where values of RMSlead (top panel) and RMSlag (bottom panel) are compared for adults and children at all delays tested, alongside with the RMSsingle source values. In the top panel, First Response RMS error values appear to increase as delays increase, and values are higher for children than adults, especially at the longer delays. In the bottom panel, Second Response RMS error values appear to decrease with longer delays, but only for adults; children’s RMS values remain high at all delays tested.
Figure 7.
Results from Figs. 56 were summarized here for adult and children. Group mean (±SD) values for RMSlead (top panel) and RMSlag (bottom panel) are compared at each stimulus condition (SS, 5, 10, 25, 50 and 100 ms) for adults and children.
The number of trials on which data were obtained for the First Response and Second Response are shown for each group in Table 1. The n values reflect the number of listeners whose data contributed to the value in each cell. The First Response numbers indicate the maximum number of trials for that condition. In some cases (e.g., 25 ms) two children were not tested, thus the total number of First Response trials was 210 (compared with 270 if all children had been tested at that delay). The number of trials for the Second Response was smaller if some children did not hear a second sound and thus did not provide a Second Response on some, or all, trials. For example at the 5 ms delay only one child provided a Second Response (note n=1), and at the 10 ms delay only two children provided a Second Response(n=2).
Table 1.
Number of trials from which localization RMS error was calculated for First Response and Second Response; n indicates number of listeners contributing to the number in each cell.
| Adults | Children | |||
|---|---|---|---|---|
| First Response | Second Response | First Response | Second Response | |
| Single | 350 (n=10) | 0 | 315 (n=9) | 0 |
| 5 ms | 300 (n=10) | 30 (n=1) | 270 (n=9) | 30 (n=1) |
| 10 ms | 300 (n=10) | 270 (n=9) | 240 (n=8) | 60 (n=2) |
| 25 ms | 300 (n=10) | 300 (n=10) | 210 (n=7) | 210 (n=7) |
| 50 ms | 300 (n=10) | 300 (n=10) | 270 (n=9) | 270 (n=9) |
| 100 ms | 300 (n=10) | 300 (n=10) | 270 (n=9) | 270 (n=9) |
Within each group, repeated measures t-test comparisons were conducted for values of RMSsingle-source vs. RMSleadand vs. RMSlag. For each comparison, data were included only from listeners for whom data were available. Scheffe’s adjustment for multiple comparisons, with a desired post-adjustment value of p<0.05, were applied for each set of analyses. These sets typically consisted of 5 comparisons (SS vs. 5, 10, 25, 50 and 100 ms), with some exceptions when very few children provided responses to both lead and lag (see below). For adults, RMSleadvalues were not different from RMSsingle-source values at delays of 5 or 10 ms, but higher at delays of 25[t(9)=2.9], 50 [t(9)=2.86] and 100 ms[t(9)=2.83]. For children, values of RMSlead were not different from RMSsingle-source at delays of 5 and 10 but significantly different at delays of 25 ms[t(8)=2.85), 50 ms [t(8)=2.9] and 100 ms[t(8)=3.08]. Thus, at delays that are greater than echo threshold, hearing the lag resulted in greater First Response localization errors. This occurred at approximately the same delays for adults and children, although, because lead-lag delays were not sampled with greater resolution, the exact relationship between echo threshold and values of RMSlead cannot be determined for either group.
For adults, values of RMSlag were not compared at a delay of 5 ms due to sparse responses; values of RMSsingle-source were significantly smaller than values of RMSlag at delays of 10 ms[t(8)=4.09], 25 ms[t(9)=2.78], 50 ms [t(6)=2.5] and 100 ms[t(6)=2.5], suggesting that adults were not able to localize the second-heard image with the same degree of accuracy as they were able to localize single source stimuli. For children, values of RMSlag were significantly higher than values of RMSsingle-source at delays of 25 ms[t(6)=4.9], 50 ms [t(8)=7.3] and 100 ms[t(8)=8.6]. Between-group comparisons were conducted on values of RMSlead at all delays. RMSleadvalues were significantly higher for children than adults at delays of 5 [t(15)=3.27] and 100 ms[t(17)=3.4], but not at the other delays. RMSlagvalues were not compared between groups at 10 ms, since only 2 children had second response data at that delay. Children had significantly higher RMSlag values than adults at 25 ms[t(13)=3.63], 50 ms [t(11)=4.2] and 100 ms[t(17)=6.7]. It is worth noting that the lag stimulus was only presented from 0° (front), so the RMSlag value only reflects the ability of the listener to identify that one source location.
These findings suggest that for both groups at the longest delays, listeners’ ability to identify the locations of the two temporally-spaced audible images was compromised. However, the fact that values of RMSlag are considerably higher than values of RMSlead suggests that the “temporal window” of the localization dominance aspect of the precedence effect are extended to much longer delays than had been previously recognized. These findings and developmental implications drawn from them are discussed below.
DISCUSSION
It is believed that the auditory system assigns greater weight to the directional cues associated with a direct sound, and reduced weight to cues associated with reflections, so that sound localization in reverberant environments may be preserved. Studies on this topic have often used simple stimuli whereby source-reflection (lead-lag) pairs are simulated under controlled conditions. The precedence effect is a catch-all term used to refer to perceptual effects associated with these experiments (Litovsky et al., 1999). Numerous studies over the years have focused on measuring the delays and conditions under which fusion of the lead and lag is weak or absent and two images are heard (Freyman et al., 1991; Litovsky and Shinn-Cunningham, 2001; Miller et al., 2009). In the present study, echo threshold values were significantly higher in the children than adults. These results are consistent with prior reports that echo thresholds are greater in children than adults (Clifton et al., 1984).
Listeners’ sensitivity to changes in the direction of the lead or lag has been studied extensively using discrimination paradigms (e.g., Freyman et al., 1991; Litovsky and Macmillan, 1994; Litovsky, 1997; Yang and Grantham, 1997; Tollin and Henning, 1998; Litovsky et al., 2000; Litovsky and Shinn-Cunningham, 2001; Miller et al., 2009). However, surprisingly little is known about the extent to which sound localization per se is affected by reflections, whether they are reported to be heard as separate sounds, or are perceptually fused with the source.
Sound localization is known to be degraded by the presence of echoes, i.e., reflections that are not heard as separate auditory events and that have diminished intensity relative to the source (Hartmann, 1983; Rakerd and Hartmann, 1985; Shinn-Cunningham et al., 2005). In the precedence effect paradigm the lag is typically presented at the same intensity as the lead, thus it is not a “reflection” in the true sense of the word. Rather, it is a delayed replica of the lead. Studies to date have utilized these types of stimuli to explore the ability of humans to localize lead-lag pairs that are presented with short delays, generally below echo threshold. Here we extended the delays to larger values, and explored the potential localization confusion that can be caused when the lag is heard. Furthermore, we utilized the localization dominance paradigm to study developmental aspects of the precedence effect, and to determine whether the precedence effect, in terms of localization dominance, operates in children similarly to adults. By extending previous investigations of the precedence effect in children and adults to a measure of localization dominance we captured a more real-world aspect of the precedence effect than has been referred to previously (e.g., Blauert, 1997; Litovsky et al., 1999), and perhaps assumed in the literature, but not explicitly studied.
The results suggest that at least some children as young as 4–5 years of age localize single source stimuli nearly with the same accuracy as adults do. Using a 15-alternative forced-choice paradigm, values of RMS errors were different in the two groups, but only marginally if an outlier in the child group was omitted. Previous studies with young children on absolute sound localization using single source sounds are sparse, but suggest a similar range of performance. Using a 9-alternative forced-choice paradigm, Van Deun et al. (2009) reported that children ages 4 and older had mean absolute errors of 5° or less for natural sounds such as a 1s bell ring. Grieco-Calub and Litovsky (2010) used a 15-alternative forced-choice paradigm, stimuli consisted of speech (spondaic words), and sound intensity was roved over an 8 dB range to minimize overall level cues. Children with an average age of 5.5 years had mean RMS errors values (±standard deviation) of 18.3° (±6.9°), which is notably higher than the mean values obtained in the present study (10.2±10.72) or in the Van Deun et al. (2009) study. The higher values of RMS seen here may be due to the fact that we used a 7-loudspeaker configuration, or more likely because the pink noise bursts used here may have been more difficult to localize. This issue would need to be further researched in order to fully account for this difference.
Under lead-lag conditions, young children, like adults, had good lead localization at short delays. The RMSlead value was similar to the RMSsingle-source value (see Fig. 7, delays of 5 and 10 ms), suggesting that the presence of the lag did not disrupt sound localization when the lag was not reported as a separate sound. In other words, the precedence effect seems to operate similarly for adults and children, both of whom were generally able to weight the lead sound more heavily in the localization process with little or no interference from the lag. Had testing ceased at delays of 10 ms, the conclusion would have been that children and adults function relatively similarly on measures of localization dominance.
Performance such as that seen in adult listener SAV (Fig. 3, top panels), where localization of the lead and lag were distinct and accurate at delays beyond echo threshold, has been reported in adults from non-human species, notably the owl (Spitzer and Takahashi, 2006). Other trends, seen in some adults who made errors, and in children, were also observed. For example, in some cases (e.g., Fig. 4, SCW delays of 25 and 50 ms; Fig. 4, CFV, 100 ms) there appeared to be an ability to generally resolve two source locations, but temporal order judgment errors between these locations commonly occurred. In other cases (e.g., Fig. 4, CFH at all delays), two sounds were heard but always attributed to the location of the lead, hence persistent localization dominance at very long delays. Finally, in other cases, (e.g., Fig. 4, CGJ, 100 ms) there was a general blur of perceived locations, where the two sources seemed to be “pulled” toward one another, without the location of either one being well resolved.
Regarding the latter case, it has been suggested that localization interference can occur among simultaneously occurring sources, even when the target and interferers consist of spectrally distinct stimuli (McFadden and Pasanen, 1976; Best et al., 2007). In the present study, it is possible that for some listeners localization errors at long delays were due to perceptual “pulling” and integration of two auditory images, despite the asynchrony in their onsets. The notion that distinct objects can be perceptually attracted toward one another in the localization process has been discussed recently in the context of perceptual grouping of sound elements (Lee et al., 2009). Similarly, the present findings are likely related to backward recognition masking (e.g., Massaro et al., 1976), or a reduced ability to localize the first stimulus once the second stimulus is heard, as has also been previously attributed to results from studies on the precedence effect at long lead-lag delays (Stellmack et al., 1999; Dye et al., 2006).
The remainder of this Discussion focuses on possible interpretations of the age differences observed here. The fact that children generally showed much greater errors in localization at long delays, as is summarized in Fig. 7, clearly shows that sound localization abilities are immature in preschool-age children when the task requires that more than one image be localized. Selective localization of the lead, given two distinct lead and lag images, is a different task than localization of a fused lead-lag image. Developmental differences on this task may have arisen as a result of age-related differences in a phenomenon known as the “buildup” of precedence (Freyman et al., 1991). Buildup is thought to occur when repeated pairs of lead-lag stimuli are presented, and the classic finding in adults is that echo threshold is increased, even with as few as three repeated stimuli such as those used here. The role of “buildup” in localization dominance has not been studied, nor has this effect been measured in children. It may be possible that age-related differences in buildup of echo suppression were partly responsible for the error types seen here, although this would need to be explored in detail with a focus on delays that surround echo threshold.
A distinct possibility to consider is that 5-year-old children are more susceptible to perceptual effects that are unrelated to the precedence effect, but that occurred here as a by-product of the experimental paradigm. One such effect is that of temporal order confusion, whereby the ability to distinguish between the “first heard” and “second heard” auditory event is poorer in children than adults. A second such effect, described above, is that of backward recognition masking (e.g., Massaro et al., 1976), or reduced ability to localize the first stimulus once the second stimulus is heard. The age-related effect seen here may also be related to the report by Hall et al. (2007) on developmental differences for measurements of the binaural temporal window, the time interval during which the auditory system integrates information related to binaural difference cues (e.g., Kollmeier and Gilkey, 1990). In children aged 5–10 yrs the integration occurs relatively late with respect to the signal, and the effects occur within 40 ms, that is a similar time window to that used here for lead-lag stimuli. As suggested by Hall et al. (2007), adults may be more adept than children at utilizing optimal temporal windows that enable them to maximize performance.
In addition to immaturity in binaural and spatial hearing abilities at this age, many other abilities remain immature into late-childhood (Lutfi et al., 2003; Hall et al., 2007, 2008; Leibold and Bonino, 2009). For example, monaural temporal resolution is immature, as noted by higher gap detection thresholds in children than in adults (Irwin et al., 1985; Wightman et al., 1989), and higher detection thresholds for pure tone signals presented in sinusoidally amplitude-modulated narrow bands of noise (Grose et al., 1993). Similarly, it has been shown that children are less sensitive to the presence of modulation than adults (Hall and Grose, 1994; Hall et al., 2008)
Finally, inattention has also been identified as a factor that limits children’s ability to extract information from auditory stimuli when more than one source is present (Lutfi et al., 2003; Leibold and Bonino, 2009). Here we posit that attentional factors specifically reduced the ability of children to perform optimally when both lead and lag stimuli were heard and the task involved reporting of two source locations in order. One possibility is that children lack experience in dual tasking and are unable to maintain focus and attention to more than one auditory object. Another possibility is that task-related, cognitive, immaturity renders the ability of children to know when to listen, and how to select each of the sources to be reported. It is difficult to determine which of these factors were more directly responsible for each of the error types observed here. The developmental findings may likely to be affected by the prolonged period of maturation that the auditory cortex undergoes throughout childhood (Ponton et al., 2000; Moore and Guan, 2001; Sussman et al., 2008). In addition, it is important to note that immature localization dominance such as that observed here may interact with children’s reduced ability to understand speech in reverberant environments (Neuman et al., 2010; Yang and Bradley, 2009) and to segregate sources in multi-talker situations (e.g., Johnstone and Litovsky, 2006).
SUMMARY AND CONCLUSIONS
-
1.
The purpose of this study was to investigate localization dominance, the measure of precedence effect that is most relevant to everyday listening because it reflects the extent to which listeners are able to localize the first-arriving sound (lead) without interference from a later-arriving sound (lag). A novel task was implemented, in 4–5 year old children and adults, whereby listeners were instructed to report how many sounds were heard, and the location of each sound.
-
2.
The lead-lag delay at which two sounds were heard, the echo threshold, was significantly longer for children (24.3±13.6 ms) than adults(15.2±4.9 ms).
-
3.
On single source localization, evaluated as baseline performance, there was clear overlap in the root-mean-square (RMS) error of the child (RMS range 3.8°–38.3°) and adult (RMS range 1.4°–6.5°) groups, although adults were significantly more accurate than children on average.
-
4.
For both age groups, the RMSlead value was similar to the RMSsingle-source value at delays of 5–10 ms, but significantly higher than the RMSsingle-source value at 25–100 ms. This finding suggests that at echo threshold, or beyond, the heard lag interferes with the listeners’ ability to identify the “first heard” sound at the location of the lead. The RMSlag value was significantly higher than the RMSlead value, suggesting that, while the “second heard” sound is clearly audible, its location is not easily identifiable and can be dominated by, or confused with, the location of the lead. Both values of RMSlead and RMSlag were larger in children than adults.
-
5.
These findings suggest that children’s ability to perform the dual source task is not fully developed, which may be attributed to a combination of auditory and non-auditory developmental effects.
ACKNOWLEDGMENTS
The authors are grateful to Eileen Storm, S. Senthilvelan and Philip Wesolek for their assistance with data analysis, and to the listeners, in particular the children who participated in the many testing sessions. This work was funded by NIH-NIDCD Grant No. 5R01DC008365 (R. Litovsky), and by core support to the Waisman Center, NIH-NICHD Grant No. 5P30HD003352 (M. Seltzer).
References
- Best, V., Gallun, F. J., Carlile, S., and Shinn-Cunningham, B. G. (2007). “Binaural interference and auditory grouping,” J. Acoust. Soc. Am. 121, 1070–1076. 10.1121/1.2407738 [DOI] [PubMed] [Google Scholar]
- Blauert, J. (1997). Spatial Hearing: The Psychophysics of Human Sound Localization, revised ed. (MIT, Cambridge, MA: ), pp. 201–271. [Google Scholar]
- Clifton, R. K., Morrongiello, B. A., and Dowd, J. M. (1984). “A developmental look at an auditory illusion: the precedence effect,” Dev. Psychobiol. 17, 519–536. 10.1002/dev.420170509 [DOI] [PubMed] [Google Scholar]
- Clifton, R. K., Morrongiello, B. A., Kulig, J. W., and Dowd, J. M. (1981). “‘Newborns’ orientation toward sound: Possible implications for cortical development,” Child Dev. 52, 833–838. 10.2307/1129084 [DOI] [PubMed] [Google Scholar]
- Dent, M. L., Tollin, D. J., and Yin, T. C. (2009). “Influence of sound source location on the behavior and physiology of the precedence effect in cats,” J. Neurophysiol. 102, 724–734. 10.1152/jn.00129.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye, R. H., Jr., Brown, C. A., Gallegos, J. A., Yost, W. A., and Stellmack, M. A. (2006). “The influence of later-arriving sounds on the ability of listeners to judge the lateral position of a source,” J. Acoust. Soc. Am. 120, 3946–3956. 10.1121/1.2372588 [DOI] [PubMed] [Google Scholar]
- Freyman, R. L., Clifton, R. K., and Litovsky, R. Y. (1991). “Dynamic processes in the precedence effect,” J. Acoust. Soc. Am. 90, 874–84. 10.1121/1.401955 [DOI] [PubMed] [Google Scholar]
- Grantham, D. W., and Wightman, F. L. (1978). “Detectability of varying interaural temporal differences,” J. Acoust. Soc. Am. 63, 511–523. 10.1121/1.381751 [DOI] [PubMed] [Google Scholar]
- Grieco-Calub, T., and Litovsky, R. (2010). “Sound localization skills in children who use bilateral cochlear implants and in children with normal acoustic hearing,” Ear Hear. In press. 10.1097/AUD.0b013e3181e50a1d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose, J. H., Hall, J. W., and Gibbs, C. (1993). “Temporal analysis in children,” J. Speech Hear. Res. 36, 351–356. [DOI] [PubMed] [Google Scholar]
- Hall, J. W., III, Buss, E., and Grose, J. H. (2007). “The binaural temporal window in adults and children,” J. Acoust. Soc. Am. 121, 401–410. 10.1121/1.2400673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, J. W., III, Buss, E., and Grose, J. H. (2008). “Comodulation detection differences in children and adults,” J. Acoust. Soc. Am. 123, 2213–2219. 10.1121/1.2839006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, J. W., and Grose, J. H. (1994). “Development of temporal resolution in children as measured by the temporal modulation transfer function,” J. Acoust. Soc. Am. 96, 150–154. 10.1121/1.410474 [DOI] [PubMed] [Google Scholar]
- Hartmann, W. M. (1983). “Localization of sound in rooms,” J. Acoust. Soc. Am. 74, 1380–1391. 10.1121/1.390163 [DOI] [PubMed] [Google Scholar]
- Irwin, R. J., Ball, A. K., Kay, N., Stillman, J. A., and Bosser, J. (1985). “The development of auditory temporal acuity in children,” Child Dev. 56, 614–620. 10.2307/1129751 [DOI] [PubMed] [Google Scholar]
- Johnstone, P. M., and Litovsky, R. Y. (2006). “Effect of masker type on speech intelligibility and spatial release from masking in children and adults,” J. Acoust. Soc. Am. 120, 2177–2189. 10.1121/1.2225416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmeier, B., and Gilkey, R. (1990). “Binaural forward and backward masking: Evidence for sluggishness in binaural detection,” J. Acoust. Soc. Am. 87, 1709–1719. 10.1121/1.399419 [DOI] [PubMed] [Google Scholar]
- Lee, A. K., Deane-Pratt, A., and Shinn-Cunningham, B. G. (2009). “Localization interference between components in an auditory scene,” J. Acoust. Soc. Am. 126, 2543–2555. 10.1121/1.3238240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold, L. J., and Bonino, A. Y. (2009). “Release from informational masking in children: Effect of multiple signal bursts,” J. Acoust. Soc. Am. 125, 2200–2208. 10.1121/1.3087435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky, R. (1997). “Developmental changes in the precedence effect: Estimates of minimal audible angle,” J. Acoust. Soc. Am. 102, 1739–1745. 10.1121/1.420106 [DOI] [PubMed] [Google Scholar]
- Litovsky, R., and Ashmead, D. (1997). “Developmental aspects of binaural and spatial hearing,” in Binaural and Spatial Hearing, edited by Gilkey R. H. and Anderson T. R. (Lawrence Earlbaum Associates, Hillsdale, NJ: ), pp. 571–592. [Google Scholar]
- Litovsky, R., and Macmillan, N. (1994). “Minimum auditory angle for clicks with simulated echoes: Effects of azimuth and standard,” J. Acoust. Soc. Am. 96, 752–758. 10.1121/1.411390 [DOI] [PubMed] [Google Scholar]
- Litovsky, R., Parkinson, A., and Arcaroli, J. (2009). “Spatial hearing and speech intelligibility in bilateral cochlear implant users,” Ear Hear. 30, 419–431. 10.1097/AUD.0b013e3181a165be [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky, R. Y. (1998). “Physiological studies of the precedence effect in the inferior colliculus of the kitten,” J. Acoust. Soc. Am. 103, 3139–3152. 10.1121/1.423072 [DOI] [PubMed] [Google Scholar]
- Litovsky, R. Y. (2005). “Speech intelligibility and spatial release from masking in young children,” J. Acoust. Soc. Am. 117, 3091–3099. 10.1121/1.1873913 [DOI] [PubMed] [Google Scholar]
- Litovsky, R. Y., Colburn, H. S., Yost, W. A., and Guzman, S. (1999). “The precedence effect. Review & tutorial paper,” J. Acoust. Soc. Am. 106, 1633–1654. 10.1121/1.427914 [DOI] [PubMed] [Google Scholar]
- Litovsky, R. Y., Hawley, M. L., Fligor, B., and Zurek, P. M. (2000). “Failure to unlearn the precedence effect,” J. Acoust. Soc. Am. 108, 2345–2352. 10.1121/1.1312361 [DOI] [PubMed] [Google Scholar]
- Litovsky, R. Y., and McAlpine, D. A., “Physiological correlates of the precedence effect and binaural masking level differences,” in Auditory Brain, Oxford Handbook of Auditory Science Vol. 2, edited by Rees A. and Palmer A. (Oxford University Press, New York, 2010), pp. 333–357. [Google Scholar]
- Litovsky, R. Y., Parkinson, A., Arcaroli, J., and Sammeth, C. (2006). “Simultaneous bilateral cochlear implantation in adults: A multicenter clinical study,” Ear Hear. 27, 714–731. 10.1097/01.aud.0000246816.50820.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky, R. Y., and Shinn-Cunningham, B. G. (2001). “Investigation of the relationship between three common measures of precedence: Fusion, localization dominance and discrimination suppression,” J. Acoust. Soc. Am. 109, 346–358. 10.1121/1.1328792 [DOI] [PubMed] [Google Scholar]
- Litovsky, R. Y., and Yin, T. C. T. (1998). “Physiological studies of the precedence effect in the inferior colliculus of the cat: I. Correlates of psychophysics,” J. Neurophysiol. 80, 1302–1316. [DOI] [PubMed] [Google Scholar]
- Lutfi, R. A., Kistler, D. J., Oh, E. L., Wightman, F. L., and Callahan, M. R. (2003). “One factor underlies individual differences in auditory informational masking within and across age groups,” Percept. Psychophys. 65, 396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro, D. W., Cohen, M. M., and Idson, W. L. (1976). “Recognition masking of auditory lateralization and pitch judgments,” J. Acoust. Soc. Am. 59, 434–441. 10.1121/1.380887 [DOI] [PubMed] [Google Scholar]
- McFadden, D., and Pasanen, E. G. (1976). “Lateralization at high frequencies based on interaural time differences,” J. Acoust. Soc. Am. 59, 634–639. 10.1121/1.380913 [DOI] [PubMed] [Google Scholar]
- Miller, S., Litovsky, R. Y., and Kluender, K. (2009). “Predicting echo thresholds from speech onset characteristics,” J. Acoust. Soc. Am. 125, EL134–EL140. 10.1121/1.3082261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, J. K., and Guan, Y. L. (2001). “Cytoarchitectural and axonal maturation in human auditory cortex,” J. Assoc. Res. Otolaryngol. 2, 297–311. 10.1007/s101620010052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman, A. C., Wroblewski, M., Hajicek, J., and Rubinstein, A. (2010). “Combined effects of noise and reverberation on speech recognition performance of normal-hearing children and adults,” Ear Hear. 31, 336–344. 10.1097/AUD.0b013e3181d3d514 [DOI] [PubMed] [Google Scholar]
- Ponton, C. W., Eggermont, J. J., Kwong, B., and Don, M. (2000). “Maturation of human central auditory system activity: Evidence from multi-channel evoked potentials,” Clin. Neurophysiol. 111, 220–236. 10.1016/S1388-2457(99)00236-9 [DOI] [PubMed] [Google Scholar]
- Rakerd, B., and Hartmann, W. M. (1985). “Localization of sound in rooms, II: The effects of a single reflecting surface,” J. Acoust. Soc. Am. 78, 524–533. 10.1121/1.392474 [DOI] [PubMed] [Google Scholar]
- Shinn-Cunningham, B. G., Kopco, N., and Martin, T. J. (2005). “Localizing nearby sound sources in a classroom: Binaural room impulse responses,” J. Acoust. Soc. Am. 117, 3100–3115. 10.1121/1.1872572 [DOI] [PubMed] [Google Scholar]
- Spitzer, M. W., Bala, A. D., and Takahashi, T. T. (2004). “A neuronal correlate of the precedence effect is associated with spatial selectivity in the barn owl’s midbrain,” J. Neurophysiol. 92, 2051–2070. 10.1152/jn.01235.2003 [DOI] [PubMed] [Google Scholar]
- Spitzer, M. W., and Takahashi, T. T. (2006). “Sound localization by barn owls in a simulated echoic environment,” J. Neurophysiol. 95, 3571–3584. 10.1152/jn.00982.2005 [DOI] [PubMed] [Google Scholar]
- Stellmack, M. A., Dye, R. H., Jr., and Guzman, S. J. (1999). “Observer weighting of interaural delays in source and echo clicks,” J. Acoust. Soc. Am. 105, 377–87. 10.1121/1.424555 [DOI] [PubMed] [Google Scholar]
- Sussman, E., Steinschneider, M., Gumenyuk, V., Grushko, J., and Lawson, K. (2008). “The maturation of human evoked brain potentials to sounds presented at different stimulus rates,” Hear. Res. 236, 61–79. 10.1016/j.heares.2007.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollin, D. J., and Henning, G. B. (1998). “Some aspects of the lateralization of echoed sound in man. I: Classical interaural delay-based precedence,” J. Acoust. Soc. Am. 104, 3030–3038. 10.1121/1.423884 [DOI] [PubMed] [Google Scholar]
- Van Deun, L., van Wieringen, A., Van den Bogaert, T., Scherf, F., Offeciers, F. E., Van de Heyning, P. H., Desloovere, C., Dhooge, I. J., Deggouj, N., De Raeve, L., and Wouters, J. (2009). “Sound localization, sound lateralization, and binaural masking level differences in young children with normal hearing,” Ear Hear. 30, 178–190. 10.1097/AUD.0b013e318194256b [DOI] [PubMed] [Google Scholar]
- Wightman, F., Allen, P., Dolan, T., Kistler, D., and Jamieson, D. (1989). “Temporal resolution in children,” Child Dev. 60, 611–624. 10.2307/1130727 [DOI] [PubMed] [Google Scholar]
- Xia, J., Brughera, A., Colburn, H. S., and Shinn-Cunningham, B. (2010). “Physiological and psychophysical modeling of the precedence effect,” J. Assoc. Res. Otolaryngol. 11, 495–513. 10.1007/s10162-010-0212-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, W., and Bradley, J. S. (2009). “Effects of room acoustics on the intelligibility of speech in classrooms for young children,” J. Acoust. Soc. Am. 125, 922–933. 10.1121/1.3058900 [DOI] [PubMed] [Google Scholar]
- Yang, X., and Grantham, D. W. (1997). “Echo suppression and discrimination suppression aspects of the precedence effect,” Percept. Psychophys. 59, 1108–1117. [DOI] [PubMed] [Google Scholar]
- Yin, T. C. (1994). “Physiological correlates of the precedence effect and summing localization in the inferior colliculus of the cat,” Neuroscience 14, 5170–5186. [DOI] [PMC free article] [PubMed] [Google Scholar]