Abstract
Background and Purpose
RTOG 0933 is a phase II clinical trial of hippocampal avoidance during whole-brain radiotherapy (HA-WBRT) to prevent radiation-induced neurocognitive decline. By quantifying baseline incidence of perihippocampal or hippocampal metastases, we sought to estimate the risk of developing metastases in the hippocampal avoidance region (the hippocampus plus 5mm margin).
Materials/Methods
Patients with ≤10 brain metastases treated at two separate institutions were reviewed. Axial images from pre-treatment, post-contrast MRIs were used to contour each metastasis and hippocampus according to a published protocol. Clinical and radiographic variables were correlated with perihippocampal metastasis using a binary logistical regression analysis, with two-sided p < 0.05 for statistical significance.
Results
1133 metastases were identified in 371 patients. Metastases within 5mm of the hippocampus were observed in 8.6% of patients (95% CI 5.7–11.5%) and 3.0% of brain metastases. None of the metastases lay within the hippocampus. A 1-cm3 increase in the aggregate volume of intracranial metastatic disease was associated with an odds ratio of 1.02 (95% CI 1.006–1.034, p = 0.003) for the presence of perihippocampal metastasis.
Conclusion
With an estimated perihippocampal metastasis risk of 8.6%, we deem HA-WBRT safe for clinical testing in patients with brain metastases as part of RTOG 0933.
Keywords: Brain metastases, Whole-brain radiotherapy, Neurocognitive function, Hippocampal avoidance, RTOG 0933
Introduction
RTOG 0933 is a phase II clinical trial that aims to test the hypothesis that avoiding the hippocampus during whole-brain radiotherapy (HA-WBRT) for patients with brain metastases may delay or reduce the onset, frequency, and/or severity of neurocognitive function (NCF) decline, without compromising intracranial disease control. The trial involves a planned statistical comparison of phase II NCF outcomes to those of the WBRT alone arm of a recent phase III trial of WBRT with or without motexafin gadolinium [1, 2]. After developing appropriate treatment planning techniques, testing them in phantoms and conducting initial in-house piloting, we have developed multiple intensity-modulated radiotherapy techniques for HA-WBRT [3, 4]. These techniques allow for the conformal avoidance of the hippocampus plus a 5mm margin, which accounts for systematic setup error and dose falloff between whole brain clinical target volume and the hippocampus.
Before initiating HA-WBRT clinically, we sought to estimate the risk of disease progression within the hippocampal avoidance region to determine a reliable first estimate of the safety profile of our intervention. Theoretically, the optimal approach to assessing this risk would entail observation of patients with brain metastases treated with HA-WBRT and followed with sequential imaging. Given the absence of such a dataset currently, we instead opted to retrospectively review two separate institutional databases, one from the University of Wisconsin and the other from Rush University Medical Center. Both databases contain patients with brain metastases and pre-treatment MRI scans and permit an analysis of baseline incidence of perihippocampal metastases. Our analyzed cohort involves 371 patients with 1133 brain metastases, representing the most comprehensive analysis of the estimate of perihippocampal brain metastases to our knowledge. Our aims were to 1) quantify the percentage of patients with metastases located within the hippocampal avoidance region and 2) identify clinical and radiographic variables that correlate with the presence of metastases within the hippocampal avoidance region. These data would then enable us to estimate the risk of radiographically evident perihippocampal disease progression after HA-WBRT and identify which patients would be at increased risk.
Methods and Materials
371 consecutive patients who were treated in the Radiation Oncology Clinics of two separate institutions (Rush University Medical Center and the University of Wisconsin Comprehensive Cancer Center) were retrospectively reviewed. Patients with up to ten brain metastases were included. We excluded patients with more than ten metastases, as they constituted a very uncommon subgroup. Demographic data including age, gender, histology, and Recursive Partitioning Analysis (RPA) class [5], were collected. All patients were treated in each institution either with WBRT, stereotactic radiosurgery (SRS), or an SRS boost followed by WBRT. T1-weighted, postcontrast axial MR image sets obtained prior to cranial irradiation were retrieved and reviewed for each patient. Each image set was imported to the Pinnacle treatment planning system (Philips Radiation Oncology Systems, Fitchburg, WI) for contouring.
Axial images were used to contour the hippocampus as well as each metastasis. The anatomic boundaries of the hippocampus were identified according to a previous protocol [4, 6]. The hippocampus was identified by the gray matter within the following anatomic boundaries: the anterior boundary includes the entire hippocampal head bounded by the temporal horn. The hippocampus was delineated from the amygdala by either the uncal recess of the temporal horn, the thin line formed by the alveus, or a line formed from the most anterior portion of the temporal horn straight medially to the ambient cistern. The lateral boundary is served by the cerebrospinal fluid (CSF) in the temporal horn. Similarly, the medial boundary extends to the CSF in the uncal and ambient cisterns. Posteriorly, the hippocampal tail was outlined to the crus of the fornix.
After the hippocampus was outlined, the resulting regions of interest were expanded to create three-dimensional envelopes surrounding the hippocampus at distances of 5, 10, and 15 mm. All enhancing metastases were contoured and the volume of each lesion, its location within the brain parenchyma, and distance from the hippocampus were recorded. The distance was recorded as <5 mm, 5 to <10 mm, 10 to <15 mm, and ≥15 mm from the hippocampus.
The number of metastases within 5mm from the hippocampus was correlated with the following clinical and radiographic variables: age, sex, primary histology, RPA class, aggregate number of intracranial metastases, and aggregate volume of intracranial metastases. A binary logistical regression model using a backward step-wise approach was developed, and a two-sided p value < 0.05 was used for statistical significance. For the 265 patients with data collected on extracranial metastatic sites, a separate but similar regression model was created using number of extracranial metastatic sites as an additional variable.
Results
Patient Characteristics
Demographic and disease-specific data on 371 patients are presented in Table 1. The majority of patients had non-small-cell lung cancer (NSCLC) (42%); more than half were female (56%); and, most were in RPA class 2 (84%). A total of 1133 metastases were identified in the 371 patients analyzed. This yielded a mean of 3.1 metastases per patient, with a median volume per metastasis of 0.52 cm3 and a median aggregate volume of intracranial metastasis of 4.9 cm3. The majority of metastases were observed in the outer cortex of the frontal (24.6%), parietal (15.6%), temporal (16.0%) and occipital (14.9%) lobes and the cerebellum (15.2%).
Table 1.
Patient characteristics.
| Number | Percent (%) | |
|---|---|---|
| Primary Site | ||
| NSCLC | 155 | 41.8 |
| SCLC | 38 | 10.2 |
| Breast | 63 | 17.0 |
| Melanoma | 47 | 12.7 |
| Renal | 15 | 4.0 |
| Other | 53 | 14.3 |
| Age | ||
| >60 | 184 | 49.6 |
| ≤60 | 187 | 50.4 |
| Gender | ||
| Male | 164 | 44.2 |
| Female | 207 | 55.8 |
| RPA | ||
| I | 33 | 8.9 |
| II | 310 | 83.6 |
| III | 28 | 7.5 |
|
No. of Extracranial Metastatic Sites * |
||
| 0 | 89 | 36.6 |
| 1 | 85 | 35.0 |
| 2 | 38 | 15.6 |
| ≥3 | 31 | 12.8 |
Abbreviations: NSCLC, non-small-cell lung cancer, SCLC, small cell lung cancer, RPA, recursive partition analysis score (5).
Data on extracranial metastases collected only on 243 patients.
Distance from Hippocampus
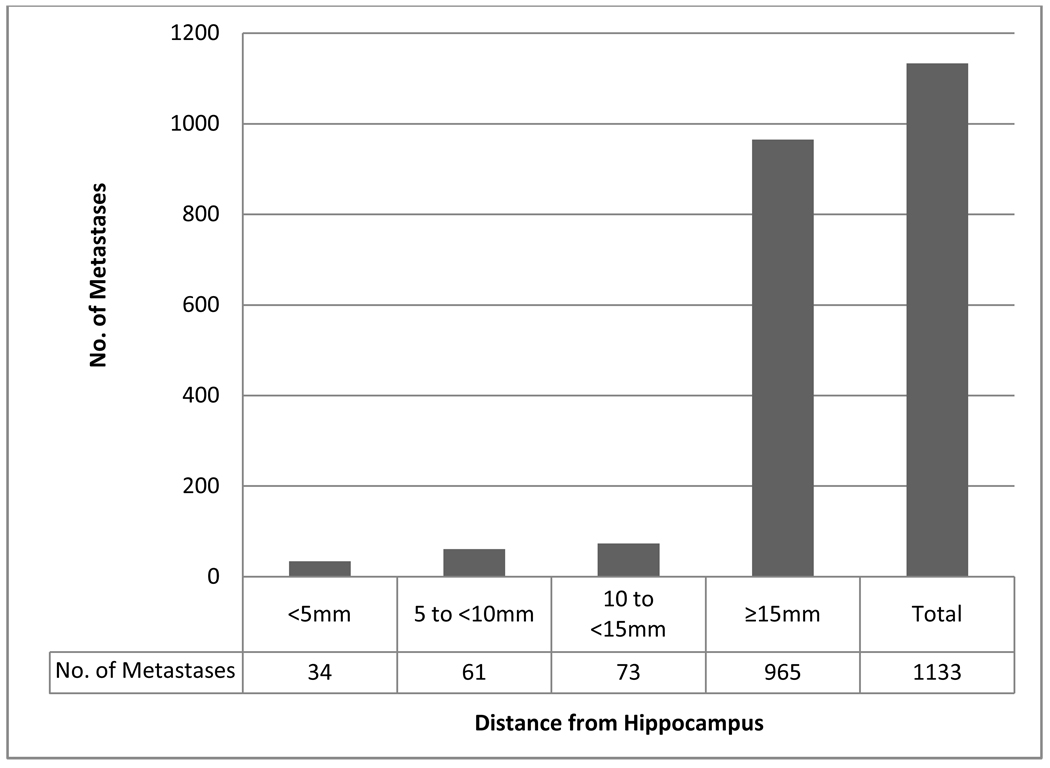
Table 2 lists patients according to the perihippocampal distance of the metastasis closest to the hippocampus, stratified by primary histology, aggregate number of intracranial metastases, and aggregate volume of intracranial metastases. Thirty-two of 371 patients had a metastasis within 5 mm of the hippocampus, yielding an incidence of 8.6%, with a 95% confidence interval of 5.7–11.5%. No patient had a metastasis within the hippocampus. Figure 1 delineates perihippocampal distance of each brain metastasis. Of the 1133 brain metastases reviewed, none of the metastases lay within the hippocampus, and 34 (3.0%) of the metastases lay within 5mm of the hippocampus.
Table 2.
Patients according to distance from the hippocampus of the closest metastasis.
| <5mm | 5 to <10mm | 10 to <15mm | ≥15mm | |
|---|---|---|---|---|
| Primary Site | ||||
| NSCLC | 14 (9.0%) | 20 (12.9) | 22 (14.2) | 99 (63.9) |
| SCLC | 4 (10.5) | 5 (13.2) | 10 (26.3) | 19 (50) |
| Breast | 4 (6.3) | 9 (14.3) | 11 (17.5) | 39 (61.9) |
| Melanoma | 7 (14.9) | 5 (10.6) | 3 (6.4) | 32 (68.1) |
| Renal | 0 | 0 | 2 (13.3) | 13 (86.7) |
| Other | 3 (5.7) | 6 (11.3) | 6 (11.3) | 38 (71.7) |
| Aggregate Number of Metastases | ||||
| 1 | 10 (6.7%) | 10 (6.7) | 11 (7.4) | 118 (79.2) |
| 2 | 7 (8.9) | 9 (11.4) | 14 (17.7) | 49 (62.0) |
| 3 | 7 (15.9) | 5 (11.4) | 7 (15.9) | 25 (56.8) |
| ≥4 | 8 (8.1) | 21 (21.2) | 22 (22.2) | 48 (48.5) |
| Aggregate Volume of Metastases | ||||
| ≤ Median* | 9 (4.9%) | 15 (8.1) | 23 (12.4) | 138 (74.6) |
| > Median* | 23 (12.4) | 30 (16.1) | 31 (16.7) | 102 (54.8) |
| Total | 32 (8.6%) | 45 (12.1) | 54 (14.5) | 240 (64.7) |
Abbreviations: NSCLC, non-small-cell lung cancer, SCLC, small cell lung cancer.
Median aggregate volume of intracranial metastases = 4.9 cm3
Percent of patients within each row given parenthetically.
Figure 1. Perihippocampal distance of brain metastases.
Of the 1133 brain metastases reviewed, 34 (3%) of them lay within 5mm of the hippocampus. However, none of the metastases lay within the hippocampus.
Correlation of Clinical and Radiographic Factors with Metastasis within the Hippocampal Avoidance Region
A binary logistic regression model for the incidence of brain metastasis within 5 mm of the hippocampus was created using age, sex, RPA class, primary histology, and aggregate number and volume of intracranial metastases (Table 3). Only the aggregate volume of intracranial metastases was a statistically significant predictor for the risk of perihippocampal disease, with an odds ratio of 1.02 (95% confidence interval 1.006–1.034, p = 0.003) for every 1-cm3 increase in the aggregate volume of intracranial metastatic disease. Aggregate intracranial disease volumes of 1-cm3, 10-cm3, and 35-cm3 were associated with estimated incidences of perihippocampal metastasis of 6.4%, 7.5%, and 11.8%, respectively.
Table 3.
Binary logistic regression analysis for incidence of metastases within 5mm of the hippocampus
| Variable | Odds Ratio |
95% CI | P value |
|---|---|---|---|
| Age | 0.98 | 0.94–1.01 | 0.131 |
| Sex | 0.97 | 0.42–2.20 | 0.934 |
| RPA (Class I as Reference) | 0.691 | ||
| Class II | 0.96 | 0.25–3.69 | |
| Class III | 1.41 | 0.32–6.18 | |
| Primary Histology (NSCLC as Reference) |
0.76 | ||
| SCLC | 0.91 | 0.26–3.27 | |
| Breast | 0.52 | 0.14–1.88 | |
| Melanoma | 1.46 | 0.50–4.22 | |
| Renal Cell | 0.00 | N/A* | |
| Other | 0.58 | 0.15–2.20 | |
| Aggregate Number of Intracranial Metastases |
1.05 | 0.89–1.24 | 0.60 |
| Aggregate Volume of Intracranial Metastases |
1.02 | 1.006–1.034 | 0.003 |
None of the patients with renal cell carcinoma had a metastasis within 5mm of the hippocampus.
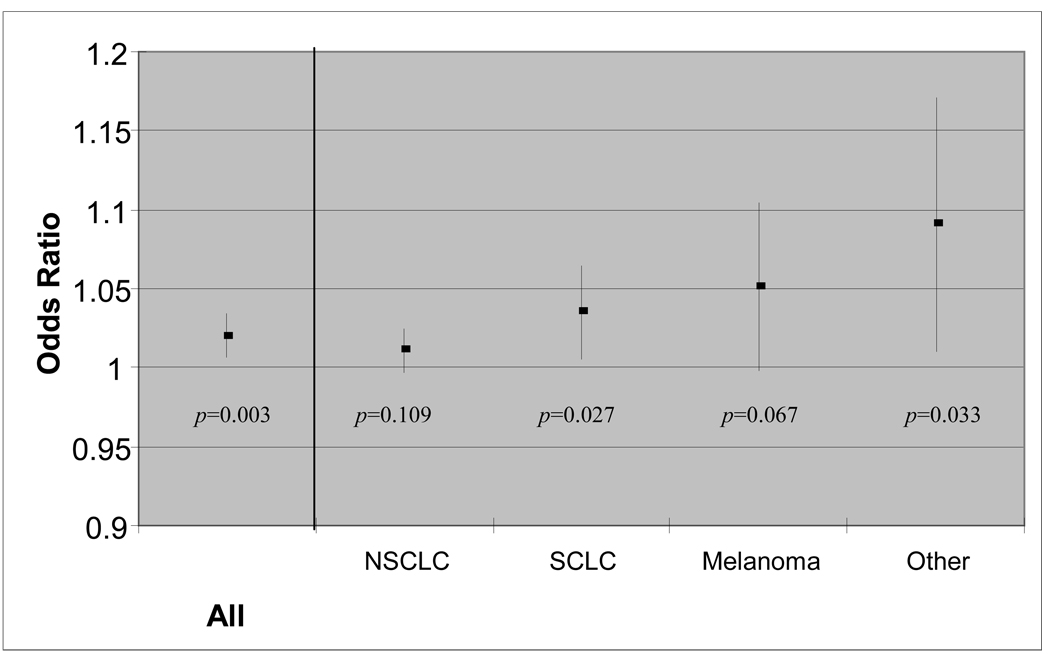
Aggregate intracranial metastatic volume was observed to be predictive for increased risk of perihippocampal metastases for all primary histologies, except breast cancer and renal cell carcinoma (Figure 2). A trend to significance was observed for non-small cell lung cancer (p = 0.109) and melanoma (p = 0.067); statistical significance was observed for small cell lung cancer (p = 0.027) and other malignancies (p = 0.033). Statistical significance was not observed for breast cancer, and none of the 15 patients with renal cell carcinoma demonstrated a perihippocampal metastasis. 265 patients had data on the number of extracranial metastatic sites at presentation. Binary logistic regression analysis restricted to these patients, with number of extracranial metastatic sites included in the model, again demonstrated aggregate volume of intracranial disease to be the only statistically significant variable (p < 0.001).
Figure 2. Odds ratio (with 95% confidence interval) of perihippocampal metastasis according to aggregate volume of intracranial metastases.
Binary logistic regression analysis was performed for all patients and for patients stratified by primary histology. A trend towards significance was observed for patients with non-small-cell lung cancer (NSCLC) (p=0.109) and melanoma (p=0.067). Statistical significance was observed for all patients (p=0.003) and for patients with small cell lung cancer (SCLC) (p=0.027) and malignancies other than NSCLC, SCLC, melanoma, breast cancer, or renal cell cancer (p=0.033). Patients with renal cell carcinoma were not included in this figure, as none of them had a metastasis within 5 mm of the hippocampus. Patients with breast cancer were not included in this figure, as statistical significance was not observed. Abbreviations: NSCLC, non-small-cell lung cancer, SCLC, small cell lung cancer
Discussion
Based on this comprehensive analysis of 371 patients with 1133 brain metastases at presentation, we observed brain metastases within 5mm of the hippocampus in 8.6% of patients, with an estimated upper limit of the 95% confidence interval of 11.5%. Of the 1133 brain metastases reviewed, 3.0% lay within 5mm of the hippocampus. However, none of the metastasis lay within the hippocampus.
From this, we conclude that 91.4% of newly diagnosed patients will be eligible for HA-WBRT of brain metastases. Although response rates after WBRT without hippocampal avoidance vary, complete or partial responses have been documented in more than 60% of patients in randomized controlled studies conducted by the RTOG, with intracranial disease control observed in approximately 50% of patients at 6 months [7]. It is currently not possible to provide a direct estimate of the risk of developing a metastasis after HA-WBRT, since such a comprehensive dataset does not exist. However, if we assume that the risk of developing subsequent brain metastasis in the hippocampal avoidance region scales in the same proportion as that at presentation, we can conclude that a patient treated with HA-WBRT will derive 91.4% of the relative benefit of WBRT in terms of radiographically evident intracranial lesions, with a lower 95% confidence limit of 88.5%. This modest increase in risk of intra-cranial progression with hippocampal avoidance may be partially compensated by the possibility of salvage with radiosurgery, which remains to be validated. Should salvage radiosurgery be used for a perihippocampal recurrence, we expect that given the very steep radiation dose falloff with stereotactic radiosurgery, only some but not all of the potential neurocognitive benefit of hippocampal avoidance will be lost.
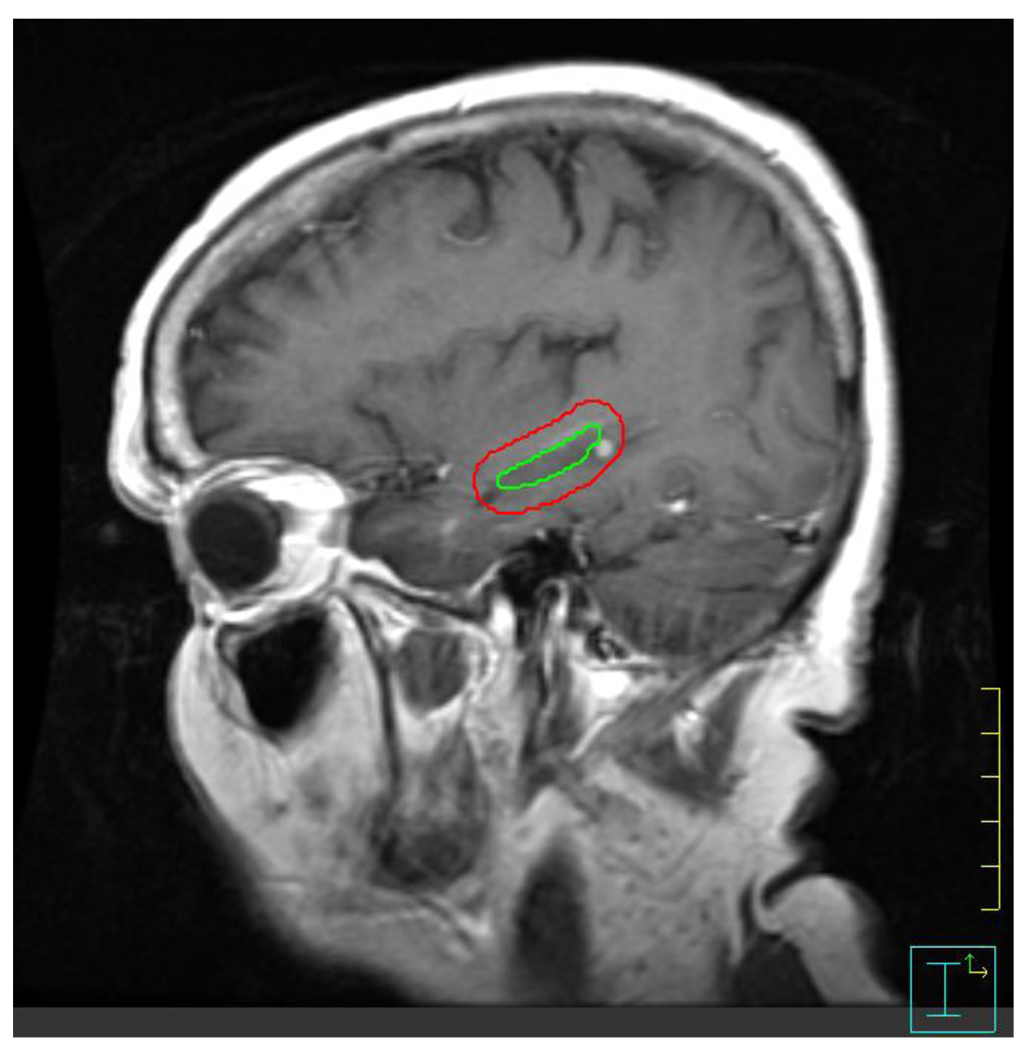
Importantly, we observed that none of the 1133 brain metastases reviewed in this analysis lay within the hippocampus. The identification of a hippocampal metastasis depends on the anatomic definition of the hippocampus, which can be susceptible to inter-observer variability. Figure 3 provides examples of our approach to hippocampal contouring and of perihippocampal metastases. Our approach to hippocampal contouring is based on a prior published protocol for manual hippocampal contouring [4, 6], as well as an unpublished population-based hippocampal template that we have developed through the deformable co-registration of 100 manual hippocampal contours. In addition, our approach to hippocampal contouring will be adopted in the RTOG 0933 phase II clinical trial of HA-WBRT through credentialing and quality assurance review to be conducted centrally by our research group [4]. As a result, we deem our findings to be a reliable first estimate of the likelihood of hippocampal and perihippocampal disease progression after HA-WBRT.
Figure 3. Perihippocampal metastasis.
Contrast-enhanced T1 sagittal images of a patient who has a metastasis within 5mm of the hippocampus, which represents the hippocampal avoidance volume during HA-WBRT. The green contour represents the hippocampal contour; the red contour represents the hippocampal avoidance region. 32 of 371 patients had metastasis within the hippocampal avoidance volume. Zero patients had a metastasis within the hippocampus, itself.
In addition, we observe that the aggregate volume of intracranial metastatic disease predicts for risk of metastasis within 5 mm of the hippocampus. Though binary logistic regression analysis demonstrates statistical significance, the clinical significance of this predictive relationship is relatively small, with an odds ratio of 1.02. That is, each cubic centimeter increase in intracranial metastatic volume increases the odds of a perihippocampal metastasis by a factor of 1.02. Based on this model, aggregate intracranial metastatic volume would need to equal or exceed 35 cubic cm before reaching the upper limit of our estimated 95% confidence interval for risk of perihippocampal disease progression. Of the 371 patients who presented with up to 10 brain metastases, 25 (6.7%) were observed to have an aggregate intracranial metastatic volume equal to or larger than 35 cubic cm.
We have previously described a similar analysis in a smaller dataset, involving 100 patients, and observed 8% of patients to have a perihippocampal metastasis at presentation [4, 6]. The upper limit of the 95% confidence interval in that study was 15.2%. We sought to expand our analysis in order to provide a more accurate estimate of the upper limit of the 95% confidence interval and thus the safety profile of HA-WBRT. Between the two studies, the similar percentage of patients with perihippocampal metastasis (8.6% on this review; 8% on the prior review) and similar rate of brain metastases within 5mm of the hippocampus (3.0% on this review; 3.3% on the prior review) point to the reproducibility of our methodology in reviewing two separate institutional databases. By enlarging our patient database, we have reduced the standard error and, in so doing, improved the accuracy of our risk estimate. Based on these data, we conclude that HA-WBRT is safe for clinical testing. Through the RTOG (RTOG 0933), we have developed a multi-institutional phase II clinical trial of HA-WBRT in patients with brain metastases (Table 4). This trial has been approved by the Division of Cancer Prevention at the National Cancer Institute and is scheduled to open in 2010.
Table 4.
Study schema for RTOG 0933 phase II trial of hippocampal avoidance during WBRT (HA-WBRT) for brain metastases.
| For Patients with MRI Evidence of Brain Metastasis Within 1 Month of WBRT | |||
|---|---|---|---|
|
R E G I S T E R1 |
Within 2 Weeks Prior to Treatment | Radiation Therapy | Follow Up 3 |
|
WBRT with Hippocampal Avoidance using IMRT (30 Gy in 10 Fractions) |
|
|
Institutions must be credentialed by the RTOG prior to enrolling patients. To receive credentialing, institutions must partake in a pilot training project on hippocampal contouring and treatment-planning for hippocampal avoidance during whole-brain radiotherapy.
Prior to treatment, all hippocampal contours and HA-WBRT treatment plans will be centrally reviewed for quality assurance. Refinements will be made as needed, and the treatment plans will be returned to the treating physician within 3 business days of submission.
Follow up will occur every 2 months for the first 6 months after treatment, then every three months until death.
Abbreviations: NCF, neurocognitive function. HA-WBRT, hippocampal avoidance during whole-brain radiotherapy. IMRT, intensity-modulated radiotherapy.
Conclusion
If we assume that the risk of development of a subsequent brain metastasis within the hippocampal avoidance region scales in the same proportion as that at presentation, we conclude that a patient treated with HA-WBRT will derive 91.4% of the relative benefit of WBRT in terms of prevention of the emergence of radiographically visible intracranial lesions, with a lower 95% confidence limit of 88.5%. Intracranial volume of metastatic disease, but not number of metastases, predicts for increased risk of developing perihippocampal metastases. However, the clinical significance of this relationship is relatively small, with an odds ratio of 1.02. Based on this safety profile, we deem HA-WBRT safe for clinical testing in patients with brain metastases as part of RTOG 0933, a phase II clinical trial scheduled to open in 2010.
Acknowledgements
We would like to thank Sayana Thomas, M.D., and Benjamin T. Gielda, M.D., for their technical contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Notification: None
References
- 1.Mehta MP, Rodrigus P, Terhaard CH, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21:2529–2536. doi: 10.1200/JCO.2003.12.122. [DOI] [PubMed] [Google Scholar]
- 2.Mehta MP, Shapiro WR, Glantz MJ, et al. Lead-in phase to randomized trial of motexafin gadolinium and whole-brain radiation for patients with brain metastases: centralized assessment of magnetic resonance imaging, neurocognitive, and neurologic end points. J Clin Oncol. 2002;20:3445–3453. doi: 10.1200/JCO.2002.07.500. [DOI] [PubMed] [Google Scholar]
- 3.Gutierrez AN, Westerly DC, Tome WA, et al. Whole brain radiotherapy with hippocampal avoidance and simultaneously integrated brain metastases boost: a planning study. Int J Radiat Oncol Biol Phys. 2007;69:589–597. doi: 10.1016/j.ijrobp.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gondi V, Tolakanahalli R, Mehta M, et al. Hippocampal-sparing whole-brain radiotherapy: A "how-to" technique, utilizing helical tomotherapy and LINAC-based intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010 doi: 10.1016/j.ijrobp.2010.01.039. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 6.Ghia A, Tome WA, Thomas S, et al. Distribution of brain metastases in relation to the hippocampus: implications for neurocognitive functional preservation. Int J Radiat Oncol Biol Phys. 2007;68:971–977. doi: 10.1016/j.ijrobp.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24:1295–1304. doi: 10.1200/JCO.2005.04.6185. [DOI] [PubMed] [Google Scholar]