Abstract
The production of reactive oxygen species (ROS) in a highly regulated fashion is a hallmark of members of the NADPH oxidase (Nox) family of enzymes. Nox enzymes are present in most eukaryotic groups such as the amebozoid, fungi, algae and plants, and animals, where they are involved in seemingly diverse biological processes. However, a comprehensive survey of Nox functions throughout biology reveals common functional themes. Noxes are often activated in response to stressful conditions such as nutrient starvation, physical damage or pathogen attack. Although the end result varies depending on the organism and tissue, Nox-produced ROS mediates the response to the adverse stimuli, such as innate immunity responses in plant and animals or cell differentiation in Dictyostelium, fungi and plants. These responses involve ROS-mediated signaling mechanisms occurring at intracellular or cell-to-cell levels, and sometimes involve cell wall or extracellular matrix cross-linking. Indeed, Noxes are involved in local and systemic signaling from plants to fish, and in cross-linking of the plant hair-cell wall, synthesis of the nematode cuticle and formation the sea urchin fertilization envelope. The extensive use of Nox enzymes in biology to regulate cell-to-cell signaling and morphogenesis suggest that additional functions in mammalian signaling and development remain to be discovered.
Introduction
Nox and Duox enzymes are membrane flavocytochromes that catalyze the NADPH-dependent reduction of molecular oxygen to generate superoxide and/or hydrogen peroxide. For the first 25 years of the field, however, the only known mammalian NADPH-oxidase was the phagocyte NADPH-oxidase. Also referred to as the respiratory burst oxidase, the enzyme uses Nox2 (a.k.a. gp91phox) as its catalytic subunit. The enzyme is activated in neutrophils and other inflammatory cells upon exposure to microbes or inflammatory products, resulting in the generation of high levels of superoxide, with secondary production of hydrogen peroxide, hydroxyl radical and, in the presence of myeloperoxidase, HOCl. Together, this witches brew of radicals and oxidants function (along with other non-oxidative mechanisms) to kill or damage invading microbes. The importance of the Nox2 system in innate immunity is illustrated by the genetic condition Chronic Granulomatous Disease, wherein affected patients suffer frequent and severe infections, often resulting from normally innocuous microbes such as the common mold Aspergillus niger.
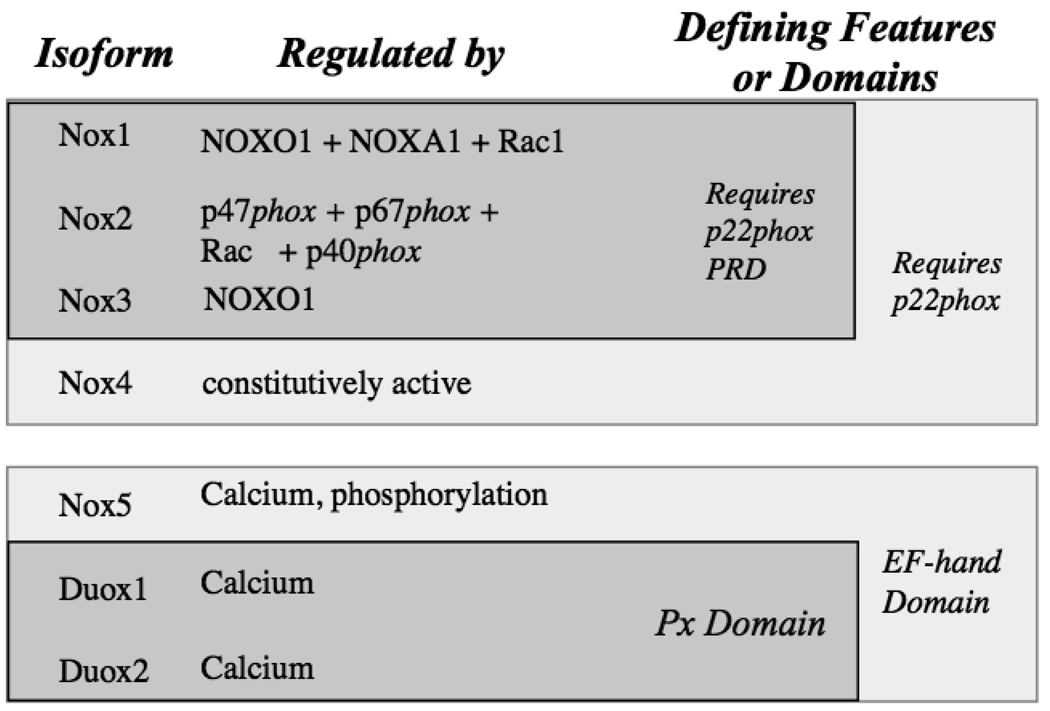
Around the turn of the 21st century, it was discovered that Nox enzymes in mammals represent a family of homologous enzymes consisting in humans of 7 gene products (6 in rodents) plus additional splice variants, indicated in Fig. 1. These can be organized into two broad classes: the p22phox-requiring Nox enzymes (Nox1, Nox2, Nox3 and Nox4), and the Ca2+-regulated Nox enzymes (Nox5, Duox1 and Duox2). In addition, they can be grouped into three sub-families based on their domain structure. Nox1, Nox2, Nox3 and Nox4 consist solely of the catalytic subunit, which is made up of an N-terminal transmembrane domain that binds two heme groups plus a C-terminal dehydrogenase domain that binds FAD and NADPH. All four bind to the small membrane associated subunit p22phox, which both stabilizes the flavocytochrome and provides a binding site for regulatory subunits in the case of Nox1, Nox2 and Nox3 (but not Nox4). Nox5 is the sole representative of the second group: this enzyme contains an EF-hand containing Ca2+-binding domain N-terminal to the catalytic domain, and is regulated by Ca2+ and one protein kinase C. Duox1 and Duox2 build on the Nox5 structure in that they have an additional domain that is homologous to heme-containing peroxidases at their extreme N-termini; this is then linked via an additional transmembrane α-helix to the Nox5-like structure. Like Nox5, Duox enzymes are activated by Ca2+.
Fig. 1.
Regulation and Defining Features of Human Nox/Duox Homologues
The realization that the Nox enzymes in mammals represent a family members of which are widely expressed in many tissues has raised important questions about their normal biological roles. Although it has been tempting to try to assign immunity-based functions based upon analogy to the phagocyte NADPH oxidase, their typically considerably lower expression levels and lower output of ROS raises questions about whether they function in an analogous manner. A growing literature focusing on signaling roles of ROS has supported a role of Nox-derived ROS in various signaling processes. While gene-deleted or mutant mice have in some cases been informative (for example, in demonstrating that Nox3 plays a key role in otolith formation in the inner ear), some knockout mice fail to demonstrate an obvious phenotype, perhaps due to isoform redundancy or adaptation. In addition, in most of the published animals, the knockout has been expressed in all tissues, making it impossible to delineate tissue- or cell-specific phenotypes.
With this in mind, the present review explores Nox enzymes throughout biology, reviewing biological functions in these simpler systems, and attempts to determine whether common functional themes exist. Recently, we reviewed the occurrence of Nox enzymes in biology [1], comparing 105 Nox sequences. These occur in plants and algae, fungi, amoeba, nematode worms, echinoderms, urochordates, insects, fish, reptiles, birds and mammals, and can be organized into seven distinct sub-families based on sequence similarities of the catalytic domains. The enzymes have not been reported in prokaryotes, but rather evolved more-or-less at the same time as single cell eukaryotes, predating multicellularity by some 1.4 billion years. Some of these non-mammalian systems ---- for example, C. elegans and Drosophila ---, have experimental advantages in comparison with mammalian systems, in that they express a small number of Nox enzymes thereby reducing the likelihood of redundant functions. In addition, the use of these model organisms is extremely powerful with regard to the availability of elegant genetic tools, allowing for example exploration of the biological function of a given gene at a particular stage in development and in a specific tissue. The following sections are organized phylogenetically, more-or-less according to the evolutionary distance from mammals, starting with plants and ending with fish. The final sections attempt to discern common themes or patterns that are present in many species.
Plants
The first plant Nox gene was identified in rice as a homolog of mammalian gp91phox (Nox2) and was named rbohA for respiratory burst oxidase homolog [2]. A single plant species contains multiple rboh genes, as for example the 10 Atrboh genes seen in Arabidopsis thaliana, suggesting multiple functions and/or localizations in the plant. Characterization of rbohA in A. thaliana showed that RbohA was localized at the plasma membrane and predicted two EF-hand Ca2+ binding domains within the RbohA N-terminal region [3], a characteristic that is shared by all plant Noxes. Indeed, all members of the Rboh family show a domain structure similar to that of mammalian Nox5 rather than Nox2. Like Nox5, Rboh activity is regulated by Ca2+, and is independent of regulatory subunits, which are missing in plants. Some plant Nox enzymes are synergistically activated by Ca2+ and phosphorylation, as shown for AtrbohD in a heterologous expression system [4]. In addition, Rbohs are regulated directly or indirectly by Rac GTPases. OsRac1 activates ROS production in rice [5], and binding to the N-terminal Ca++-binding domain of OsRbohB was demonstrated by yeast two-hybrid, pull-down experiments, NMR titration and in vivo FRET microscopy [6]. The binding interface was recently identified using the crystal structure of the EF-hand domain [7]. The relationship between binding and activation, to our knowledge, has not been demonstrated. A role for Rac in the localization of a plant Nox has been shown (see below) and might account at least in part for effects of Rac on activity. Diverse mechanisms including binding of specific phospholipids [8] and binding of chaperone or partner proteins [9, 10] regulate the localization of mammalian Nox enzymes. Therefore, binding alone is an insufficient criterion to prove direct activation, and additional studies will be of interest to clarify whether Rac effects on Rboh activity are direct or indirect.
ROS in plants were at first viewed as constitutive by-products of both photosynthesis and respiration. However, regulated production of ROS was seen during the early stages of the plant defense response, which can be triggered by infection or physical damage [11]. This “oxidative burst” has been studied in many plant species using mainly biochemical approaches. A link between Nox enzymes and the oxidative burst became apparent when Nox homologues were discovered in the plant genome [2]. Of the 10 Atrboh genes in A. thaliana (AtrbohA-J), only four, AtrbohB, AtrbohC, AtrbohD and AtrbohF, have been studied in detail. Using mutants carrying inactivating insertions at the AtrbohD and AtrbohF genes, it was shown that AtrbohD is required for ROS production in response to pathogens, while AtrbohF is important for the programmed cell death that occurs when a potential pathogen is recognized during the “hypersensitive response”, a process during which rapid plant cell death in and around the site of infection together with cross-linking of plant cell wall proteins contains and encapsulates the invader, preventing the spread of an infection [12]. Some of the AtrbohD and AtrbohF mutant phenotypes were enhanced in the double AtrbohD AtrbohF mutant, suggesting a partial overlap in plant Nox functions [13].
Notably, AtrbohD and AtrbohF mediate the production of ROS, sometimes in different cell types and with different outcomes. Both genes are expressed in the guard cells that form the stomatal pores in the leaf epidermis. The plant hormone abscisic acid induces the closing of the stomata, as a way to reduce water loss, using cytosolic Ca2+ as second messenger [14–16]. AtrbohD AtrbohF double mutants were impaired in abscisic acid-dependent responses, including stomatal closing, ROS production, cytosolic Ca2+ increases and activation of plasma membrane Ca2+-permeable channels in guard cells. The fact that external H2O2 rescued Ca2+ channel activation and stomatal closing in AtrbohD AtrbohF mutants provided further evidence for the function of ROS in abscisic acid signal transduction, and established the critical role of AtrbohD and AtrbohF [17].
AtrbohD (but not AtrbohF or AtrbohC) also plays a critical role in a systemic stress response in plants involving ROS signaling over extended distances. Systemic acquired resistance (SAR) is a process that involves the activation of adaptive mechanisms throughout the plant in response to local environmental stresses such as wounding, heat, cold, high-intensity light, and salinity. Taking advantage of the fact that the Zat12 gene [18] is rapidly induced in response to stress signals, the Zat12 promoter fused to a luciferase reporter gene was used [19] to monitor and image signal transmission in transgenic plants. The authors found that mechanical wounding induced both local and systemic expression of luciferase, indicating the transmission of a signal moving at a rate of 8.4 cm per minute in the up-and-down directions. In contrast, the signal travelled at less than 6% of this rate in rbohD defective plants. Signal propagation was interrupted by pre-treatment with catalase or diphenylene iodonium (DPI) at locations distant from the initiation site, indicating that signal propagation requires H2O2 along its path and that RbohD plays an essential role in this process. The speed of this signaling mechanism suggests active propagation, rather than simple diffusion, as diagrammed in Fig. 2, left panel.
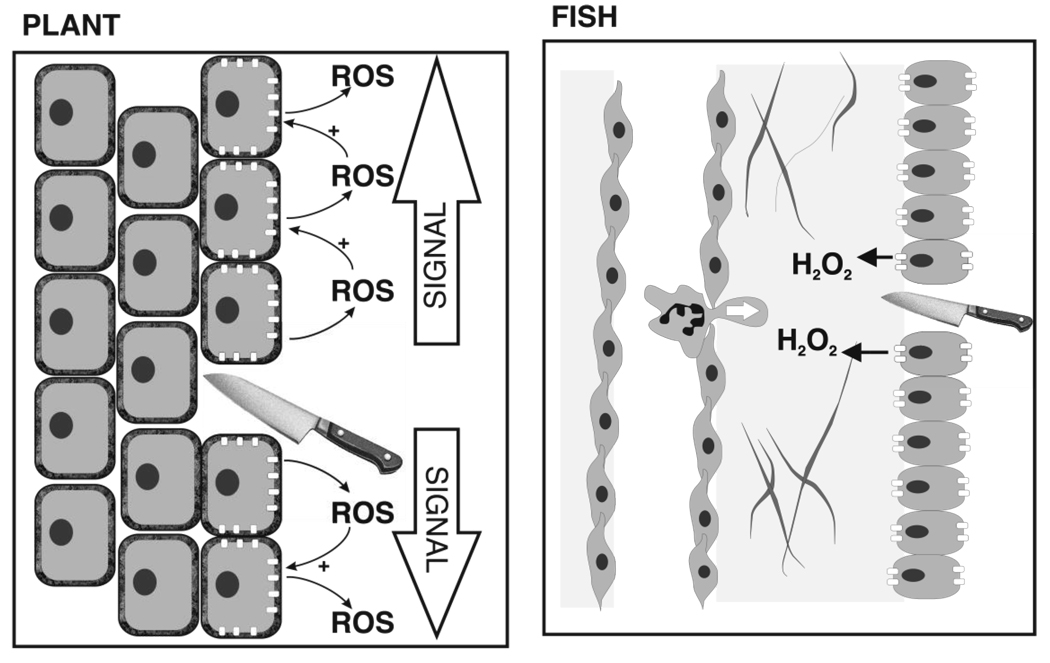
Fig. 2. Local and Systemic Cell-to-Cell Signaling by Nox Enzymes in Plant and Fish in Response to Cell or Tissue Damage.
The left panel depicts systemic signaling by RbohD-catalyzed production of ROS in plants. According to this model, cell damage initiates activation of RbohD at the wound site, and the signal is then propagated systemically by ROS-induced activation of RbohD (indicated by white boxes) in neighboring cells, so that diffusion does not diminish the concentration of ROS. The right-hand panel depicts H2O2 signaling in fish in response to wounding, which activates the calcium-dependent Duox (indicated by small white boxes), resulting in a gradient of H2O2 around the wound site. H2O2 then acts both directly to sterilize the area around the wound, and as a chemotactic factor to recruit neutrophils to the region.
Nox enzymes also play a role in plant growth and morphology. Root hairs are elaborated from certain root epithelial cells, providing a large surface area for water absorption. Elongation of roots and root hairs are often used as a model system for investigating plant cell growth patterns. The analysis of a root hair defective mutant (rhd2) led to the conclusion that the NADPH-oxidase AtrbohC is required for root elongation [20], a process that requires establishment of a calcium gradient at the root tip. Using RHD2/AtrbohC tagged with GFP, it was shown that this plasma membrane-localized Nox becomes restricted to the cells that form root hairs, accumulating at the sites of hair growth [21]. The localization depends on the GTPase ROP2 (a plant isolog of Rac) and the Rho GTP-dissociation inhibitor SCN1. Mutation of AtrbohC in the first or second EF-hand motifs, predicted to affect Ca2+ binding, decreased ROS production in heterologous expression systems, and these alleles failed to complement the rhd2 mutant in vivo. Evidence for a second level of Ca2+ regulation was obtained from experiments showing Ca2+-dependent phosphorylation of RHD2/AtrbohC in vitro, and defective ROS production was seen when the enzyme, mutated in two conserved putative phosphorylation sites, was expressed in human cells. It was proposed that root shape is determined by the apical localization of RHD2/AtrbohC, and that a positive feedback loop exists in which ROS produced by RHD2/AtrbohC activates Ca2+ channels, as occurs in other cell-types [15, 17], thereby sustaining the activation of this calcium/phosphorylation-dependent Nox enzyme [21].
Regarding plant root hair growth, recent imaging studies [22] imply a more complicated interplay among Rboh activity, ROS, Ca2+, and pH[22]. pH was previously shown to regulate cell wall properties, weakening their structure and allowing polar growth in areas of alkalinization [23, 24]. In root hairs in A. thaliana, periods of growth were associated with oscillations in localized extracellular ROS and pH (indicated in Fig. 3 lower right), with decreased root tip ROS and increased pH corresponding to periods of growth. Under growth conditions, ROS, although absent at the apex of the root tip, was produced abundantly in the sheath immediately surrounding the tip. In contrast ROS was uniformly seen around the entire root hair during periods of quiescence. The production of ROS required the NADPH oxidase RHD2/AtrbohC. Application of exogenous ROS resulted in elongation arrest, while the scavenging of ROS led to tip bursting, caused by non-localized or unregulated cell expansion. The authors proposed that ROS functions to modulate the physical properties of the cell wall, stabilizing the structure surrounding the growing root tip during the growth phase, allowing directed growth exclusively at the apex of the root tip where the elevated pH weakens the cell wall structure. In this context, it is of interest that Nox/Duox enzymes in other systems support the peroxidase-dependent formation of tyrosine-tyrosine and other cross-links, thereby stabilizing extracellular matrix structures. Similarly, plant cell walls contain similar cross-linked tyrosine and lysine residues, which stabilize the structure of extensins, hydroxyproline-rich glycoproteins of the cell wall [25–28], and these provide a likely target for AtrbohC-dependent reactions. Thus, at least one developmental role for Nox enzymes root tip growth in plants involves the stabilization of the root hair structure, probably via tyrosine cross-linking and/or similar cross-linking reactions as indicated in Fig. 3.
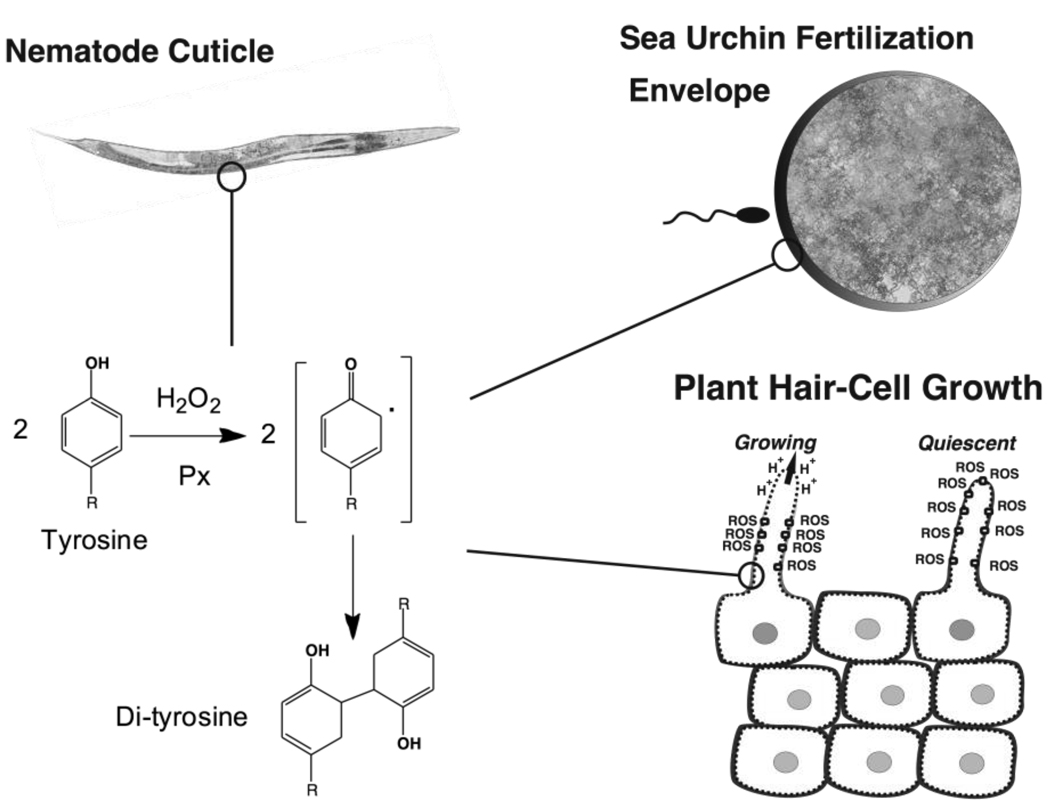
Figure 3. Universality of tyrosine cross-linking among diverse phyla.
Shown are examples of tyrosine cross-linking in diverse organisms including nematodes, sea urchin eggs, and plants. The reaction requires H2O2 and a peroxidase, which may be either part of a Duox enzyme or may be a separately encoded peroxidase (Px). Also shown in plants is the distribution of ROS (reactive oxygen species) and protons in growing versus quiescent root hairs.
Algae
Algae represent a diverse group of photosynthetic unicellular and multicellular eukaryotic organisms that include organisms ranging from phytoplankton to seaweed such as kelp, but in modern classifications exclude cyanobacteria, which are considered to be prokaryotic. In the red alga C. crispus, the Nox homolog Ccrboh was originally identified as a gene encoding a Nox2-like respiratory burst oxidase homolog [29]. In contrast to other Nox homologues, the predicted sequence of Ccrboh contains an insertion of ~300 amino acids located between two subregions of the NADPH binding site. This large loop is predicted to encode four additional transmembrane-spanning domains suggesting that the NADPH-binding domain is anchored to the membrane via this region. The Ccrboh sequence also allowed the identification of additional homologues in EST and genome databases from unicellular red and diatom algae [29], which according to a molecular taxonomy analysis are grouped within the NOXD family [1]. Investigation of these enzymes using biochemical and/or genetic approaches has not been reported and will be of considerable interest.
The catalytic domains of algae NOXD are similar to fungal NOXC and to animal NOX5, but in contrast to these, NOXD members lack EF-hand domains, suggesting that if they are regulated by calcium, they must utilize an independent calcium-binding protein rather than a fused calcium-binding domain. In addition, genes encoding homologs of the Nox regulatory subunits p22phox, p47phox, p67phox and p40phox are absent in genomes from algae, and the genome analysis of the diatom Thalassiosira pseudonana [30], a unicellular brown algae, lacks members of the RHO subfamily of small GTPases. Thus, neither phox-like subunits nor small GTPases regulate NOXD members.
Little is known about the function of alga Nox, although circumstantial evidence suggests a role for some NOXD type enzymes in adaptation to stress and/or host defense. The red macroalga Chondrous crispus produces an oxidative burst in response to extracts from a pathogenic green alga, and inhibition by diphenylene iodonium suggested the participation of a Nox-like enzyme [31]. In addition,Ccrboh was induced during infection by pathogenic alga and in response to the stress signals methyl jasmonate and oxidized fatty acids, consistent with a role in this process.
Amebozoa
Regulated production of superoxide is seen during early multicellular development in the slime mold Dictyostelium discoideum [32]. The presence of three Nox genes (noxA, noxB and noxC) as well as homologues of p22phox and p67phox in D. discoideum [32, 33] [34] suggested the involvement of Noxes in this process. [34] While NoxA and NoxB and p22phox and p67phox homologues show similarities to the mammalian Nox2 system, no homologs of p47phox and p40phox were detected in the D. discoideum genome [34].. Rac likely participates, since Dictyostelium p67phox and Rac1A–C show conservation in the amino acid residues that are important in mammals for the binding of p67phox to Rac2. [34]NoxC contains 2 N-terminal EF-hand motifs and therefore its structure is similar to Nox5.
Dictyostelium undergo a developmental process in response to the environmental stress of an exhausted food supply in which ameboid free-living single cells aggregate, responding to a c-AMP signal to form a multicellular stage, ultimately maturing to form a stalked fruiting body and asexual spores. Nox-derived ROS participate in this process: the scavenging of superoxide, either pharmacologically or by overexpressing superoxide dismutase, inhibited aggregation and arrested development [32]. The inactivation of noxA, noxB, noxC or p22phox also arrested development and prevented formation of asexual spores, but did not affect viability, growth or phagocytosis of bacteria by the ameboid form [34]. NoxA-C are sequentially expressed during development, suggesting a programmed sequence of ROS-regulated events related to development.[34]
Fungi
The phylogenetic analysis of nox genes in fungi showed that unicellular and some dimorphic fungi lack nox genes, while other fungi contain one, two or three genes (noxA-noxC), depending on the species [33, 35]. According to their structure, Nox encoded by these genes have been grouped into the NoxA/NoxB and the NoxC/NoxD subfamilies of NADPH oxidases [1]. Members of the NoxA/NoxB subfamily show structures related to the subunit-regulated Nox enzymes such as mammalian Nox2, but NoxB members contain an additional short N-terminal extension not present in the NoxA group. NoxC members contain an N-terminal putative Ca2+-binding EF-hand-containing domain [33, 35] and therefore are structurally more related to D. discoideum NoxC, plant Rbohs and mammalian Nox5. NoxA and NoxB are commonly present in different fungal species where they play roles in cell differentiation, growth and pathogenicity. NoxC is less common and its function has only been analyzed in Podospora anserina, where its mutation results in no detectable phenotype [36].
Members of the NoxA/B family are regulated by the fungal ortholgs of p67phox and Rac, but orthologs of p47phox, p40phox and p22phox are not present in the fungal genome. Fungal homologs of p67phox (NoxR, NOR-1) and Rac (RacA, Rac1) have been related to Nox function in different fungi using genetic approaches. Epichloë festucae noxR [37], racA [38] and noxA [38] mutants show similar phenotypes. The deletion of nor-1 in Neurospora crassa results in nox-1 plus nox-2 phenotypes, indicating that NOR-1 is required to activate both NOX-1 and NOX-2 at different developmental stages [39]. Mutation of noxR in Aspergillus nidulans [40] results in a phenotype like that seen with noxA inactivation [33]. Likewise, noxR mutant phenotypes indicate that NoxR regulates Nox activity in Botrytis cinerea [41] and P. anserina [36]. As with binding of mammalian Rac1/2 to p67phox, a physical interaction between E. festucae NoxR and RacA was observed using yeast two-hybrid and heterologous immunoprecipitation experiments [37]. The significance of this binding was tested in genetic experiments by showing that expression of a NoxR protein with a point mutation in the predicted RacA binding site of NoxR failed to complement a noxR null mutant [37]. Based on fungal NoxR structural analysis, Kawahara and Lambeth [42] suggested that orthologs of the yeast Bem1 scaffold protein might interact with NoxR and regulate fungal NoxA/B. However, in contrast to noxA mutants, bemA-deleted mutants in A. nidulans are able to develop sexual fruiting bodies [43], suggesting that BemA is dispensable for NoxA function. Other proposed candidates meeting criteria as a binding partner for NoxR include a Cdc24 ortholog and CBSn [42]; to our knowledge, these have not been investigated for their possible role in NoxA/B function. The above data suggest that a somewhat simplified regulatory subunit system exists for NoxA/B proteins, and that the p47phox-like organizer protein binding to a p22phox-docking protein was a later evolutionary adaptation that functioned to increase the efficiency of binding among other subunits. This idea is consistent with studies [44, 45] showing that showed that human neutrophil p47phox is dispensable for catalytic activity in an in vitro Nox2 system when high concentrations of p67phox are present.
In fungi, high levels of ROS have been related to ageing [46] and asexual differentiation [47], the latter consistent with an earlier proposal that cell differentiation in fungi and other eukaryotes is a response to a hyperoxidant state [35, 48]. A direct link between Nox-derived ROS and cell differentiation was first established by Lara-Ortiz [33], who demonstrated that in A. nidulans NoxA was necessary for both the production of ROS and the differentiation of fruiting bodies, while derepression of noxA resulted in premature and increased sexual development. Indeed, the presence of nox genes in fungi correlates with the ability to develop multicellular fruiting bodies [33, 35]. The significance of this correlation was strengthened by the fact that in P. anserina [49] and N. crassa [39], the disruption of nox1 (a NoxA homolog) prevents sexual development and in both fungi, the inactivation of nox2 (a NoxB homologue) results in production of sexual spores that are non-viable or unable to germinate [39, 49]. Interestingly, B. cinerea NoxA and NoxB are also required for the formation of sclerotia, which are multicellular survival structures. In this fungus, sclerotia formation is a prerequisite for sexual development [41].
In plant-interacting fungi, Nox enzymes are involved in regulating complex interactions between the fungus and the plant that determine whether the fungus will be pathogenic or will coexist with its host. In the endophytic fungus E. festucae, mutants in which the noxA gene is deleted grow normally in culture but show increased hyphal branching in its plant host, changing the interaction from mutualistic to antagonistic [38]. In the plant pathogen Magnaporthe grisea Nox1 and Nox2 are independently required for plant penetration [50], while in Botrytis cinerea NoxA and NoxB are both needed for pathogenicity, with the double mutant showing additive effects [41]. In the plant pathogen Claviceps purpurea, nox1 mutants show decreased germination and, while being able to penetrate the host epidermis, are impaired in the colonization of the plant ovarian tissue [51]. These results suggest that inhibitors directed at specific fungal Nox enzymes could be beneficial to prevent certain fungal infections in plants. Recently, Nox function was related to cellulose degradation in the saprophytic fungus P. anserina [36]. nox1-, nox2-, nox1, nox2- and noxR- null mutants failed to develop cellular structures associated with the penetration of cellophane membranes. In addition, PaNox1 mutants degraded cellulose more efficiently than the wild type strain and showed increased expression of genes involved in cellulose breakdown, suggesting that Nox1 functions to transcriptionally repress the production of cellulolytic enzymes.
An additional role of Nox has been observed in N. crassa, where NOX-1 is not only essential for fruiting body formation, but is also required for normal elongation of aerial and vegetative hyphae [39]. This suggests that ROS is involved in hyphal growth and raises an interesting parallel with the role of ROS-derived Nox in plant hair-cell growth [20–22] (see Fig. 3).
C. elegans
The genome of the nematode worm C. elegans shows two Duox-like genes predicted to encode hypothetical proteins known as Ce-Duox1 and Ce-Duox2 [52]. The chromosomal location of Duox2 is immediately distal to Duox1 at the end of the chromosome, suggesting a recent gene duplication. While both genes are predicted to encode a full-length homolog of the human enzyme, several studies have failed to detect expression of Duox2 [52, 53] and animals expressing a deletion of the gene region encoding Duox2 show no phenotype [54]. [52, 53][54] Together, these data may indicate that Ce-Duox2 is a pseudogene, and that C. elegans expresses Ce-Duox1 as its sole functional Nox enzyme.
Immunochemical staining revealed strong expression of Ce-Duox1 in hypodermal cells [52], the outermost layer of cells that surround the worm. Hypodermal cells directly underlie the cuticle, the hard exoskeletal-like structure that provides both protection and an osmotic barrier for the animal. The hypodermal cells play a key role in secreting proteins including collagen that form the cuticle. Although staining was not seen in other regions of the worm, studies described below imply that Ce-Duox1 must also be expressed in the gut epithelium. Therefore, from a topological point of view, Ce-Duox1 appears to be expressed in cells that form the boundary between the worm and its external environment.
Studies of Ce-Duox1 in C. elegans provided the first example of the biological function of a defined Nox enzyme in the modification of the extracellular matrix [52]. Ce-Duox1 functions in the biogenesis of cuticle, and more specifically, in the cross-linking of tyrosine residues to join cuticle proteins, resulting in the hardening and stabilization of the cuticle structure. The hallmark of this process is the generation of di- and tri-tyrosine residues that can be identified upon hydrolysis of cuticle proteins. The role of Ce-Duox1 in this process was defined by using RNA interference to knock down the expression of Ce-Duox1 [52]. This resulted in translucent worms often showing blistered or fragmented cuticle. The cuticle from affected animals showed almost a complete absence of di- and tri-tyrosine residues, and the expressed, purified peroxidase homology domain from Ce-Duox1 catalyzed the H2O2-dependent generation of di- and tri-tyrosine from tyrosine [52][55]. It was later found that point mutations in the peroxidase domain, while they did not affect H2O2 generation, caused cuticle defects in worms [54], and these same mutations diminished both heme binding and tyrosine cross-linking activity by the expressed peroxidase domain (Meitzler and Ortiz de Montellano, personal communication). These data confirmed the previously proposed role for the Ce-Duox peroxidase domain in tyrosine cross-linking [52]. C. elegans also secretes several peroxidases, and one of these, MoLT-7 also catalyzes tyrosine cross-linking of cuticle proteins [56]. Like Ce-Duox1, MoLT-7 mutants showed cuticle-defective phenotypes and the two enzymes appeared to cooperate in proper extracellular matrix formation. Therefore, Ce-Duox1 functions in cuticle biogenesis by providing a source of hydrogen peroxide which is then used by both its own peroxidase domain and by Molt-7 for the formation of di- and tri-tyrosine cross-links of cuticle proteins (see Fig. 3 and Fig. 4).
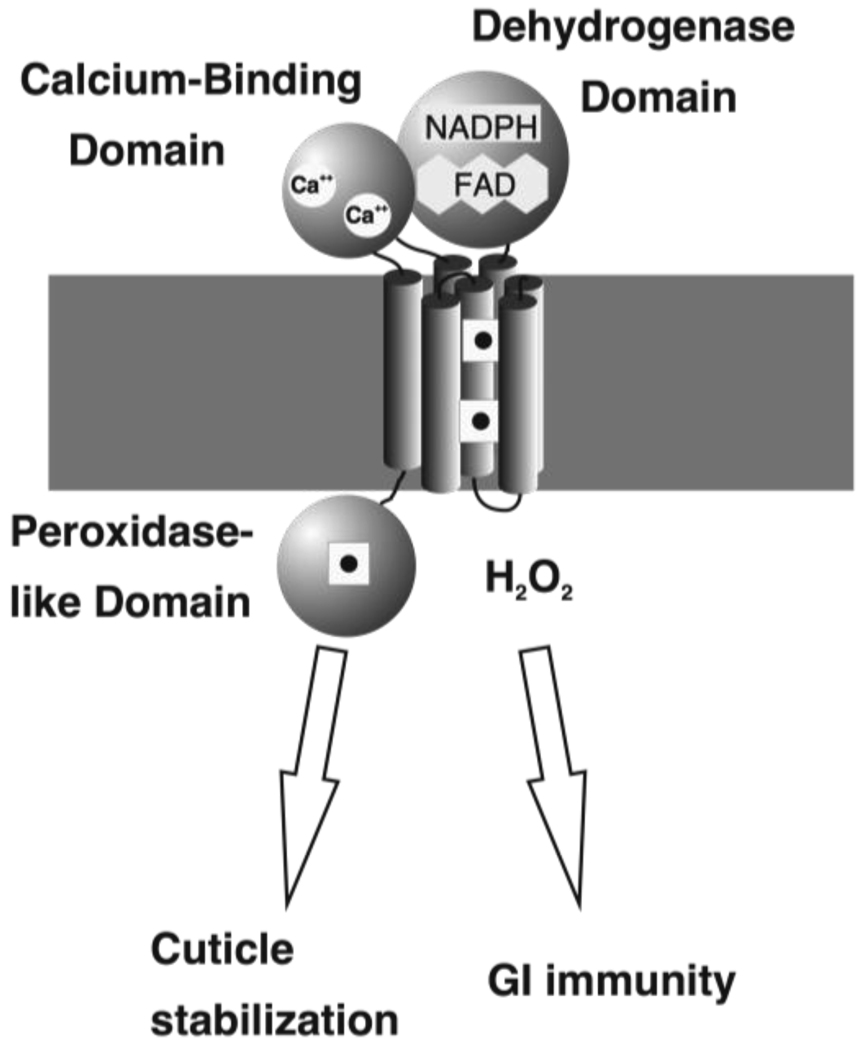
Fig. 4. Biological roles for ROS-generating and Peroxidase-like domains of C. elegans Duox1.
Duox in nematode worms functions in both gastrointestinal immunity and in cuticle stabilization, the latter via tyrosine cross-linking as shown in Fig. 3. Various domains and prosthetic groups are indicated, including the peroxidase-homology domain, which contains a heme group (square with dark center). A point mutation in the peroxidase domain inactivates the cuticle stabilization (producing the blister phenotype), but does not affect GI immunity, indicating that the immunity function does not require a functional peroxidase domain.
In addition to a role in cuticle biogenesis, Ce-Duox1 also plays an important role in host defense. When exposed to pathogenic bacteria, C. elegans worms show increased production of ROS [54]. When Ce-Duox1 expression was generally decreased using RNAi, the increased production of ROS was blunted. Moreover, RNAi animals showed increased lethality when exposed to a pathogenic bacterium. Point mutations in the peroxidase domain failed to affect pathogen susceptibility, suggesting that the main function of Ce-Duox1 in innate immunity relates to its generation of ROS and does not result solely from disruption of the cuticle barrier. Consistent with this interpretation, antioxidants increased the sensitivity of the nematode to infection. In addition, decreasing the expression of Ce-Duox1 in either the hypodermis or intestine resulted in a shortened lifespan in worms grown on E. coli. The data imply that Duox1 functions as an important component of the worm’s innate immune response. While it is tempting to speculate that Ce-Duox1-generated ROS functions in a manner analogous to that in neutrophils (i.e., effecting direct damage to microbial biomolecules), the actual role of ROS in this system is not yet clearly defined since the ROS might also act as a signal to regulate other defense pathways involved in the innate immune response to pathogens.
The functions of Ce-Duox1 in C. elegans echo those of some Nox enzymes in mammalian systems. Nox enzymes provide H2O2 as a substrate for peroxidases that then carry out various peroxidase-dependent enzymatic reactions. For example, the Nox2 system of phagocytes generates the weakly bactericidal H2O2 that is then used as a substrate by myeloperoxidase to generate the more potently microbicidal HOCl [57]. Similarly, Duox in the mammalian gastrointestinal system, bronchi, and salivary glands generate H2O2 that is then used as a substrate by secreted lactoperoxidase, providing a host defense mechanism for wet epithelium [58]. Duox enzymes in the thyroid generate H2O2 as a substrate for thyroid peroxidase [59, 60] which catalyzes the cross-linking of tyrosine residues in the extracellular protein thyroglobulin and their iodination, part of the pathway to form thyroid hormone; this series of reactions that can be considered to be modifications of extracellular matrix analogous to the modification of cuticle by Ce-Duox1.
Insects
Insects encode several types of Nox enzymes. The genome of the fruit fly Drosophila melanogaster encodes two calcium-regulated Nox enzymes: a Nox5 homolog termed d-Nox (d for Drosophila) and a Duox known as d-Duox. In addition to Nox enzymes containing calcium-binding domains, the malaria mosquito Anopheles gambiae as well as Aedes aegypti, the principal vector of yellow and dengue fevers, also encode a unique Nox gene, “Nox-mosquito” or NoxM [1], which has only the Nox domain and no calcium-binding domain and is closely related to the Nox4 sub-group.
The function of d-Duox and d-Nox in Drosophila has been explored using double stranded hairpin RNA, driven by a GAL4-UAS binary system. The widespread availability of various GAL4 drivers allows knockdown of a given transcript in a variety of tissues and at many stages in development, providing a great deal of experimental flexibility and allowing, for example, bypass of early lethality. Using this system, a variety of interesting phenotypes were generated [[61] and Ritsick and Lambeth, unpublished]. General suppression of d-Duox resulted in arrest and death at the second instar stage, suggesting a role in molting. A small percentage of animals survived but did not grow further, resulting in stunted adults with defective wings. When d-Duox was selectively suppressed in wing imaginal discs, there were defects in wing inflation as well as extreme fragility of wings. While the latter phenotype was not explored, we speculate that it might have resulted in defective tyrosine cross-linking of wing proteins, which might be expected to result in an easily damaged wing. Suppression of d-Nox expression resulted in sterile females with massively enlarged abdomens (see below). In addition, there were defects in head morphology, including an enlarged head and defective structure of the mouthpart known as the clypeus.
The female sterility phenotype was explored in an in-depth study [61] and was found to result from defective calcium signaling in ovarian smooth muscle, resulting in defective smooth muscle contraction. The enlarged abdomen was a consequence of the accumulation of viable mature eggs, which were not deposited outside of the female. Dysfunction of smooth muscle was identified as the culprit, since selective expression of dsRNA in smooth muscle fully recapitulated the phenotype. The dysfunction was traced specifically to a dysfunction in agonist-stimulated muscle contraction that resulted from a markedly diminished calcium flux in response to a hormone implicated in egg laying. The defective calcium response was not restored by calcium or hormone alone, but the combination resulted in full recovery of the response. Therefore, H2O2 derived from d-Duox functions as a co-signal (along with a second signal such as IP3) activating a calcium channel and resulting in smooth muscle contraction. Other morphological phenotypes may also arise from the same mechanism; for example at hatching (eclosion), the fly head enlarges by filling with fluid helping to rupture the egg case. Defective smooth muscle contraction may result in a failure to pump fluids back out of the head after eclosion (Ritsick and Lambeth, unpublished). The above study provides a clear example of a signaling role for Nox-generated ROS, and allows explanation of a morophological/functional phenotype that results from defective signal transduction.
Distinct roles for d-Duox in gut immunity were defined recently in Drosophila [62] and in the mosquito Anopheles gambiae [63]. d-Duox was identified as the major source of ROS in the Drosophila intestine, and both ROS levels and d-Duox expression were stimulated following exposure to bacteria. In addition to being induced, the calcium-dependent d-Duox is also activated by pathogens using a pathway involving Gαq activation of phospholipase Cβ, resulting in IP3 release and a calcium flux [64]. A complex signaling network composed of both positive and negative elements control both the expression and activity of d-Duox, resulting in elevated H2O2 in response to pathogenic bacteria, but suppressing H2O2 levels when only commensal bacteria are present. Specific knockdown using RNAi in the gut but not hemocytes resulted in an inability to produce ROS as well as a marked increase in mortality upon exposure to pathogenic bacteria [62]. Both H2O2 levels and survival were partially reversed by knocking down the expression of catalase, an enzyme that metabolizes H2O2. The authors also showed that the peroxidase-like domain of d-Duox displayed significant antimicrobial effects but only in the presence of H2O2 and chloride, suggesting that like myeloperoxidase in higher organisms, the d-Duox peroxidase domain produces the bactericidal HOCl. Markers of oxidative damage in the ingested bacteria supported the idea that the bactericidal role of d-Duox is due to direct ROS-dependent oxidative damage rather than to a signaling role for ROS in innate immunity (although this might have been occurring also). Interestingly, the reintroduction of both fly and human d-Duox (but not d-Duox lacking the peroxidase domain) attenuated infection-induced mortality. The restoration of function by the human Duox1 is not easily explained, since this enzyme lacks critical amino acid residues in the Px domain that are proposed to be important for high level peroxidase activity [55]; in addition, the latter group failed to detect heme binding human Duox1 peroxidase domain protein overexpressed in insect cells. However, in unpublished studies, we found that expressed human Duox1 peroxidase domain binds heme and possesses a modest peroxidase activity (Innoe, Smith and Lambeth, unpublished data). These discrepancies will require additional investigation. Nevertheless, the above studies strongly support a general role for d-Duox (including its peroxidase-like domain) in innate immunity in the gut of Drosophila, similar to a proposed role for Duox enzymes that are expressed in the GI tract of mammals [58, 65].
In Anopheles gambiae, Duox plays an additional and possibly opposite role in innate immunity [63] by cooperating with a secreted peroxidase to generate di-tyrosine cross-links in the GI system; this process generates a cross-linked network of proteins that serve as a permeability barrier to microbial immune elicitors that would otherwise trigger epithelial immunity and microbicidal responses. While this prevents triggering of the immune response by commensal microbes, the authors also suggest that this mechanism provides a protected environment in the midgut in which malarial parasites can develop. Thus, roles of Duox enzymes in gut immunity in insects appear to be complex.
Echinodermata
Sea urchins express at least two Nox enzymes: Nox-U1 [66] is an ancestor of Nox1, Nox2 and Nox3, while Udx1 (short for Urchin duox1) is a member of the Duox sub-family. In addition, ancestors of both p47phox and p67phox are present, whereas an ancestor of p22phox has not yet been identified [42]. While there appears to be no functional information available for Nox-U1, Udx1 plays a dual role in both egg fertilization and development of early stage embryos.
During fertilization, Udx-1 functions to prevent polyspermy and to protect the fertilized egg. Fusion of the sperm with the egg triggers the “cortical reaction”, wherein vesicles fuse with the plasma membrane, adding to the membrane and releasing their contents into the extracellular space. The increased membrane surface causes the glycocalyx or vitelline membrane to detach and become elevated above the egg membrane surface, forming a histologically distinct “fertilization envelope”. Contents of the cortical granules including ovoperoxidase then combine with and alter the vitelline membrane in a “hardening” reaction involving peroxidase-mediated tyrosine cross-linking between envelope proteins. The latter utilizes hydrogen peroxide, generated from O2 in a respiratory burst [67, 68]. The hardened fertilization envelope provides a physical barrier that prevents polyspermy (i.e., the fertilization of an egg by more than one sperm) and protects the developing fertilized egg/embryo. In addition to the physical barrier, H2O2 itself is toxic to sperm and may guard against polyspermy prior to hardening of the fertilization envelope.
The H2O2-generating enzyme responsible for the sea urchin respiratory burst was identified as Udx-1. Early experiments showed that preparations of membranes from Strongylocentrotus purpuratus eggs produced H2O2 (but not detectable superoxide) when stimulated with calcium plus ATP [69], the latter suggestive of the participation of a kinase. The kinase was later identified as protein kinase C [70]. Wessel and colleagues [71] measured an increase in H2O2 production to around 60 nM following fertilization or calcium ionophore. Subcellular fractionation showed that the activity was associated with the plasma membrane. Udx-1 (a Duox) was later identified from the Sea Urchin Genome project, and antibodies were used to localize it to the cell surface as well as to an internal pool associated with yolk-enriched fractions. Antibodies to the extracellular domain of the enzyme inhibited H2O2 generation, as did antibodies to the DH domain when injected into the egg. Furthermore, inhibition of enzymatic activity prevented tyrosine cross-linking of the fertilization envelope. In addition to perhaps functioning along with secreted peroxidases in the cross-linking reaction, the authors suggested that the peroxidase domain might act as a catalase to degrade H2O2 before it could enter and damage the oocyte; this concept, while attractive, has not been tested.
An ovoperoxidase homolog is present in many invertebrates and peroxidase activity and cortical granules are seen in species including fish and mammals, suggesting that some aspects of a conserved mechanism are present in other species [71]. To our knowledge, the occurrence of a respiratory burst or the expression of Nox/Duox enzymes in mammalian eggs has not been reported, making the significance of this mechanism in mammalian fertilization unclear.
Udx-1 also plays a role in development post fertilization. Unlike proteins like ovoperoxidase that participate exclusively in the cortical reaction, Udx1 transcripts are expressed in all stages of oogenesis and early development through at least the 60-cell stage [72]. Expression was highest in apical membranes and occurred in epithelial cells following gastrulation. When Udx1 is inhibited with diphenylene idodonium, all embryos were arrested at their treated stage, resulting in abnormal embryos. Defective embryos were partially rescued with added H2O2 pointing to this reactive oxygen species as a developmental signal [72]. Inhibition of Udx-1 with antibody resulted in delayed cleavage (cytokinesis), identifying Udx-1 as the source of the signal. A role for Udx-1 in adult was not investigated, but its localization in epithelial cells following gastrulation might suggest a role in host defense, similar to that in C. elegans. This occurrence and function of Udx-1 in adult animals remains an area for future investigation.
Urochordates
The genome of the sea squirt Ciona intestinalis --- among the most primitive of chordates ---- encodes a Nox2-like common ancestor of Nox1, Nox2 and Nox3, along with the first appearance in evolution of Nox4. In addition to the Nox2-like enzyme, Ciona also possesses homologues of p22phox, p67phox, and p47phox, but not p40phox [42]. Based on expression of Nox2 and its presumed regulatory subunits in blood cells, the system was proposed to have a host defense function analogous to that in mammalian phagocytes [73], although this was not tested. Nox4 was expressed in blood cells and in the endostyle, a ciliated, mucous-secreting groove that aids in transporting food to the esophagus. The endostyle has iodine-concentrating and peroxidase activities, is capable of iodination reactions, and is considered to be homologous to the follicular thyroid of higher organisms [74]. This raises the interesting possibility that Nox4 functions in a manner analogous to Duox in mammalian thyroid, providing H2O2 substrate for secreted peroxidases that catalyze iodination reactions. Its location guarding the entrance to the alimentary tract might also suggest an anti-microbial function for Ciona Nox4. To our knowledge, these interesting possibilities have not been investigated.
Fish
The genome of zebrafish encodes Nox1, Nox2, Nox4, Nox5 and a single Duox. In addition, zebrafish have the gene for p22phox, those for the regulators of Nox1 (NOXO1 and NOXA1), and those for Nox2 (p47phox, p67phox, and p40phox). While the functions of most of these have not been investigated in fish, Duox has recently been found to play a surprising role in wound healing and chemotaxis of neutrophils. When the epithelial layer is disrupted by physical trauma in fish or man, polymorphonuclear leukocytes are rapidly recruited to the wound. These cells prevent the entry of microorganisms into the damaged site, remove cellular debris, and helps to coordinate wound closure. Niethammer et al. [75] showed that in fish, the initial chemoattractant signal is H2O2. Using a larval zebrafish tail-fin wound model the investigators visualized H2O2 production, using a genetically encoded, fluorescent H2O2 sensor protein. This animal model has the experimental advantage of being transparent, allowing visualization of both the fluorescent sensor protein and also neutrophils expressing a transgenic fluorescent protein. The gradient of H2O2 was highest at the wound margin and extended 200 micrometers from the wound, far enough to reach nearby blood vessels. H2O2 levels at the wound margin reached concentrations of 0.5 – 50 uM, and the gradient was established by 10 min, resulting in recruitment of PMNs shortly thereafter. This process is summarized in Fig. 2. This initial H2O2 may also serve a dual function to sterilze the wound prior to the arrival of professional phagocytes. Duox was identified as the source of the H2O2, based on antisense RNA experiments. Complete suppression with antisense to Duox caused a developmental defect characterized by cell death primarily in the head region. This was partly rescued by p53 knockdown, which allowed survival and permitted observation of a significant decrease in wound-induced H2O2 generation and subsequent leukocyte infiltration. Thus, Nox enzymes play a dual role in innate immunity following wounding in fish. Duox responds immediately with the output of H2O2, which may not only sterilize the wound site directly, but also serves as a chemotactic signal to recruit neutrophils. Once these cells arrive (about 10 minutes), they phagocytose invading microbes. Although to our knowledge it has not been tested in fish, the Nox2 system presumably then functions to kill the engulfed microbes by classical oxidative killing mechanisms.
This scenario provides a fascinating paradigm involving the cooperation of two or more different Nox enzymes in the innate immune response in mammals: that is, Nox or Duox enzymes residing in tissues may serve to generate primary (H2O2) and/or secondary (e.g., H2O2-triggered cytokines) signals that then recruit phagocytes and repair cells to an area where damage or infection has occurred. In support of this idea, H2O2 was previously shown to be chemotactic towards both human PMNs and vascular smooth muscle cells, so it seems reasonable to propose such a function. Duox and Nox enzymes are expressed in mammalian gut and pulmonary epithelial cells, and might have a similar function in these tissues, both in directly sterilizing affected areas, in recruiting Nox2-expressing inflammatory cells to fight infection, and in recruiting nearby fibroblasts or epithelial cells to repair the damaged area. Thus, studies in fish suggest that non-myeloid Nox enzymes may function in the earliest stages of inflammation and repair, prior to the arrival of professional phagocytes.
Overarching biological themes and implications (and some speculations) for the functions of Nox enzymes in mammals
Stress Response
Consideration of the above reveals that one of the most frequent and general processes in which Nox enzyme participate can be considered to be a “stress response”, which can include a response to noxious stimuli such as toxins or harsh physical conditions, physical damage to tissue or cells, and invasion by foreign organisms. The end response of the cells or tissues varies depending on the organism and tissue. In plants, AtrbohD and AtrbohF mediate stomatal closure, helping tissues retain water in response to drought conditions. AtrbohD also mediates a systemic response to a wound, helping to protect the plant against additional injury. AtrbohF initiates a program of cell death and encapsulation in leaves surrounding an invading pathogen, sacrificing part of itself to deprive the invader of viable tissue in which to propagate and thereby preventing spread of the infection. In Dictyostelium, the environmental stress is the depletion of the food supply, resulting in the Nox-dependent differentiation to generate a spore-producing fruiting body. Sporulation then allows the next generation to survive and prosper when the food supply returns. Similar arguments can be made for the role of Nox enzymes in fruiting body development in fungi. In addition, Nox enzymes in fungi may participate in generating a hyperoxidant state to trigger cell differentiation. In fish, Nox enzymes participate in the response to wounding, generating H2O2, which not only is likely to sterilize the wound site, but also serves as a chemoattractant signal to recruit neutrophils to the breach. We suggest that many of the roles of Nox enzymes in higher organisms can be interpreted in terms of a local or systemic stress response, for example, to tissue damage, toxins and infection, and that much of the Nox-dependent pathology [76–78] that is seen in higher organisms results from a persistently activated stress response involving chronic Nox-dependent ROS production. For example, the fibrotic response [77, 79] that is associated with pulmonary fibrosis, cirrhosis of the liver, and diabetic nephropathy might be interpreted in this context. Likewise, tissue destructive chronic inflammatory conditions such as atherosclerosis and neurodegenerative diseases can be considered in this light.
Innate Immunity
Invasion of tissue by microbes (and sometimes foreign substances such as asbestos) trigger a series responses aimed at ridding the organism of these invaders. For historical reasons, the archetype for a role for Nox enzymes in this process is the mammalian phagocyte NADPH oxidase, which kills invading organisms by virtue of its robust generation of reactive oxygen species. However, as described above, in addition to direct chemical damage, other Nox enzyme–dependent processes that involve signal transduction, adaptive changes in cells or tissues (e.g. involving apoptosis or differentiation), peroxidase-dependent cross-linking of extracellular matrix, etc., can and do exert protective effects against invading microbes, and it is not always easy to differentiate these protective effects from the more violent full-scale assault on microbes that occurs in mammalian phagocytes. The extent to which the phagocyte model should be projected onto these other systems is therefore not clear, and it is perhaps more informative to consider several roles for ROS when considering innate immune mechanisms. As discussed above, plants utilize Nox enzymes to cross-link cell wall proteins and to initiate a program of localized cell death, to wall off and isolate potential pathogens. In C. elegans and insects, Duox enzymes participate in host defense in the gastrointestinal system, and in the latter system, the presence of oxidative damage in the ingested bacteria suggests a role analogous to that seen in phagocytes. In fish, Duox-derived H2O2 initially serves as a chemoattractant signal to recruit circulating phagocytes to a wound, where presumably the fish phagocyte Nox2 functions in a “conventional” microbicidal oxidative burst. Hence, Nox enzymes cooperate in the fish innate immune response both to generate ROS signals and to produce cytotoxic oxidants. Nox enzymes are also activated and/or induced in wound sites in mammals, and this, coupled with the observed ability of H2O2 to function as a chemoattractant for human neutrophils, suggests that this mechanism will also function in mammals. In addition, dityrosine cross-links, formed by H2O2-dependent cross-linking of extracellular matrix proteins, are observed in inflamed mammalian tissues where they participate in wound repair and possibly encapsulate certain organisms such as the tuberculosis bacterium. Thus, the multiple roles of Nox/Duox enzymes in innate immune mechanisms throughout biology suggest that Nox/Duox mechanisms in mammalian innate immunity should also be considered in this broader context.
Development and Morphogenesis
Nox enzymes frequently participate in giving form to an organism, although the mechanisms are not always clear. As discussed below, these mechanisms may include stabilization of extracellular matrix or cell walls by cross-linking and/or signaling to trigger cell differentiation, cell replication, apoptosis or combinations of these processes. Examples include the differentiation of Dictyostelium from its single cell amoeboid form to its multicellular fruiting body form, as well as differentiation of fungi resulting in fruiting body development. In addition to cell division and differentiation, this process seems likely to involve modification of extracellular matrix structures, as exemplified by plant root hair growth (see Fig. 3) wherein Nox-dependent ROS participates in stabilizing the cell wall in non-growing areas, while allowing directed growth in areas where ROS is low. Among more complex animals, insect Duox permits development beyond the second larval instar stage and also participates in wing development, although in neither case has the mechanism been elucidated. The participation of Nox enzymes in mammalian development has not been extensively investigated, and may be difficult to observe because the presence of multiple isoenzymes which may have redundant or overlapping functions. However, one example in mammals is provided by the differentiation of cardiac embryonic stem cells into spontaneously beating cardiomyocytes, which is prevented by oxygen scavenging and by down-regulation of Nox4 [80]. The widespread use of Nox enzymes in biology to regulate development and morphogenesis suggests that additional functions in mammalian development remain to be discovered.
Intracellular signaling
An important component of the mechanism of many of the above biological effects involves ROS-mediated signal transduction within the cell. While H2O2 is usually considered as the proximate signaling species, based on inhibition of a given process by catalase, the kinetics of H2O2 reaction with various amino acid residues has been found to be too slow to account for its signaling role [81]. It has therefore been suggested that the ultimate signaling species is a non-enzymatic product of H2O2 (e.g., a lipid peroxide), or that H2O2 serves as a substrate for a peroxidase resulting in the production a more powerful oxidant such as HOCl or a protein oxidation product such as dityrosine. Alternatively, another H2O2-reactive enzyme such as a peroxiredoxin may mediate signaling. Regardless of the final signal, a great deal of evidence indicates that Nox-derived ROS functions in signal transduction. One of the clearest examples in which the causal chain of events has been established linking Nox/ROS-mediated signal transduction and regulation of a physiological process is the ROS-dependent regulation in fly smooth muscle of agonist-stimulated calcium signaling, a process detailed above that triggers smooth muscle contraction and egg laying. A variety of proteins in mammals have been demonstrated to be targets of Nox/ROS signal transduction. These include ion transporters, enzymes (e.g., protein tyrosine phosphatases) and transcription factors (e.g., NF kappa-B) [82]. For example, the reversible Nox/ROS-dependent oxidation of a catalytic site cysteine in protein tyrosine phosphatase 1B and other tyrosine phosphatases [83, 84] inhibits catalytic activity, allowing protein tyrosine phosphate to accumulate and activate signaling cascades. Through these targets, Nox/Duox enzymes are thought to participate in regulating a variety of cellular processes.
Cell-to-cell and propagated signaling
Most reactive oxygen species have short lifetimes and do not readily penetrate the cell membrane. The negatively charged superoxide, for example, fails to cross membranes and readily dismutates, both spontaneously and, in the presence of superoxide dismutase, the latter at a near diffusion limited rate. Hydroxyl radical reacts rapidly and indiscriminately with most biomolecules, and hence does not survive diffusion past a very short distance. These factors limit the effective radius of many reactive oxygen species. In contrast, H2O2 readily penetrates the cell membrane and can diffuse many cell radii, making it a candidate for cell-to-cell signaling [81]. This extracellular signaling role has been directly demonstrated in fish wounding, as diagrammed in Fig. 2, described in detail above. It seems likely, given the physical and chemical properties of H2O2, that this cell-to-cell signaling mechanism will have been retained in mammals during evolution. It is unlikely, however, that H2O2 will survive long enough to function as an endocrine mediator, so its roles are more likely to be local within a tissue or region, as in the case of wound healing.
In addition to a diffusion-limited role in local signaling, H2O2 in plants provides a paradigm in which the signal can be propagated to distant cells without loss in signal strength, as in Fig. 2. This can be considered analogous to nerve signal propagation through depolarization, because the signal is re-initiated at various points as it passes through a structure. In plants, this allows the organism to respond systemically to localized damage or microbial invasion. Whether such propagated Nox signaling functions in the animal kingdom is unknown, but it is instructive to ask whether positive feedback loops exist which might enable such mechanisms in animals. For calcium regulated Nox enzymes, animal and plant models provide several examples in which calcium activates a Nox enzyme, producing H2O2, which then sensitizes calcium channels to other local signals such as proctolin in fly ovary. This suggests a mechanism in which H2O2 acts not only locally, but also likely diffuses to neighboring cells, triggering calcium fluxes and resulting in local cell-to-cell signal propagation as in Fig. 2, left panel. Similarly, Clark and colleagues [85, 86] have shown that both human Nox2 and Nox5 are activated by H2O2 in a c-Abl tyrosine kinase-dependent manner. Hence, the signaling machinery exists among animal Nox enzymes by which a locally activated Nox should be able to propagate Nox activation locally or systemically in such a way that the H2O2 signal spreads, exceeding what would be possible from H2O2 diffusion alone. To our knowledge, this concept has not been explored in mammalian systems, but in our opinion, seems likely to be present, for example in lung or intestinal epithelia, and might, for example, allow the spread to nearby cells of an innate immune response, for example when localized damage or infection is encountered. Pathologically, this sort of mechanism might propagate an inflammatory response, resulting in tissue damage out of proportion to the initial insult, as for example occurs during certain types of infections such as the H5N1 and 1918 influenzas [87].
Modifications of the extracellular matrix
In addition to signaling, many of the Nox/Duox-dependent biological processes described above modify extracellular proteins such as those of cell wall or extracellular matrix. Although the most commonly described protein modification is tyrosine cross-linking, there are many other types of oxidative cross-links as well as oxidative modifications of proteins that have been observed, summarized in [52]. For example, a type of cross-link is formed from the deamination of protein lysyl ε-amino groups to form lysyl aldehydes, which then react with nearby amino groups [88]. Such reactions require not only H2O2 generated by the Nox/Duox enzyme, but also the cooperation of a peroxidase, which is provided either by the peroxidase domain of the Duox itself (as in C. elegans) or by an independent peroxidase, which is secreted extracellularly. The Nox/Duox-dependent cross-linking of tyrosine residues has been well characterized in C. elegans, plant, and sea urchin oocyte, as shown in Fig. 3, and seems likely to function in a variety of other organisms, for example in fungi during fruiting body development, Dictyostelium sporulation and structural stabilization of the wings of fly.
The occurrence of di-tyrosine or other oxidatively generated cross-links in mammalian tissues is less well documented. The best studied of these is an analogous process in mammalian thyroid gland that results in the production of thyroid hormone. In a series of Duox dependent reactions, certain tyrosine residues in the extracellular matrix protein thyroglobulin are cross-linked and iodinated in reactions that requires Duox-generated H2O2 along with thyroid peroxidase. Proteolytic cleavage of this unusual iodinated di-tyrosine residue from thyroglobulin then frees the active thyroid hormone. The occurrence of di-tyrosine in mammal has been observed primarily in inflamed tissues including atherosclerotic plaques, where it is produced by the combined action of phagocytic Nox2 and myeloperoxidase [89]. Outside of these two examples, there is little documentation of the occurrence of dityrosine as a normal constituent of cells or tissues, possibly because in organisms lacking cell walls or exoskeletons, dityrosine is produced in smaller amounts for more specialized purposes. Consistent with this idea, dityrosine residues have been observed in a transporter in synaptic vesicles, resulting in transporter oligomerization [90]. Formation of the oligomer was found to affect both its localization and its function. Whether a Nox/Duox system is responsible for this functionally important post-translational modification has not, to our knowledge, been explored. Therefore, we suggest that Nox/Duox-supported oxidative cross-linking and/or modification of specific functional proteins is likely to be biologically important. Due to far smaller quantities of these modified amino acids than are seen in plants, insects and nematodes, however, detection and functional investigation of such modifications is likely to require the study of individual functional proteins in genetically modified animals, for example, in which specific Nox enzymes are deleted.
Concluding Remarks
Noxes have been retained throughout eukaryotic evolution. Nox regulation and Nox multiplicity allows for the choreographed production of ROS in time and space, allowing its use for a variety of coordinated functions. In diverse biological systems and even within the same organism, Nox enzymes play seemingly diverse roles, but several common themes can be discerned. These include killing pathogens, triggering apoptotic processes, protein cross-linking of the extracellular matrix, induction and maintenance of development and morphogenesis, and intra- and inter-cellular signaling. ROS signaling roles can occur through the specific oxidation of redox sensor proteins or lipids that include or are linked to signaling pathways, ion channels, and transcription factors. The precise mechanisms by which Nox-derived ROS regulate these pathways provide an important area for future investigation.
Acknowledgments
This work was supported by NIH grants CA084138, CA105116 and EHS grant ES011163 to JDL, and PAPIIT-UNAM IN201709 and CONACYT 49667Q to JA.
Abbreviations
- Nox
NADPH oxidase
- Duox
dual oxidase
- NADPH
nicotinamide adenine dinucleotide phosphate (reduced)
- ROS
reactive oxygen species
- Px
peroxidase
- PRD
proline-rich domain
- Ce
C. elegans
- NOXO1
Nox organizer protein 1
- NOXA1
Nox activator protein 1
- PMN
polymorphonuclear leukocytes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kawahara BT, Quinn MT, Lambeth JD. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol. 2007;7:109. doi: 10.1186/1471-2148-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groom QJ, Torres MA, Fordham-Skelton AP, Hammond-Kosack KE, Robinson NJ, Jones JD. rbohA, a rice homologue of the mammalian gp91phox respiratory burst oxidase gene. Plant J. 1996;10:515–522. doi: 10.1046/j.1365-313x.1996.10030515.x. [DOI] [PubMed] [Google Scholar]
- 3.Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C. A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell. 1998;10:255–266. doi: 10.1105/tpc.10.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogasawara Y, Kaya H, Hiraoka G, Yumoto F, Kimura S, Kadota Y, Hishinuma H, Senzaki E, Yamagoe S, Nagata K, Nara M, Suzuki K, Tanokura M, Kuchitsu K. Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J Biol Chem. 2008;283:8885–8892. doi: 10.1074/jbc.M708106200. [DOI] [PubMed] [Google Scholar]
- 5.Ono E, Wong HL, Kawasaki T, Hasegawa M, Kodama O, Shimamoto K. Essential role of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci U S A. 2001;98:759–764. doi: 10.1073/pnas.021273498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong HL, Pinontoan R, Hayashi K, Tabata R, Yaeno T, Hasegawa K, Kojima C, Yoshioka H, Iba K, Kawasaki T, Shimamoto K. Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell. 2007;19:4022–4034. doi: 10.1105/tpc.107.055624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oda T, Hashimoto H, Kuwabara N, Akashi S, Hayashi K, Kojima C, Wong HL, Kawasaki T, Shimamoto K, Sato M, Shimizu T. Structure of the N-terminal regulatory domain of a plant NADPH oxidase and its functional implications. J Biol Chem. 285:1435–1445. doi: 10.1074/jbc.M109.058909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawahara T, Lambeth JD. Phosphatidylinositol (4,5)-bisphosphate Modulates Nox5 Localization via an N-Terminal Polybasic Region. Mol Biol Cell. 2008;19:4020–4031. doi: 10.1091/mbc.E07-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luxen S, Noack D, Frausto M, Davanture S, Torbett BE, Knaus UG. Heterodimerization controls localization of Duox-DuoxA NADPH oxidases in airway cells. J Cell Sci. 2009;122:1238–1247. doi: 10.1242/jcs.044123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakano Y, Banfi B, Jesaitis AJ, Dinauer MC, Allen LA, Nauseef WM. Critical roles for p22phox in the structural maturation and subcellular targeting of Nox3. The Biochemical journal. 2007;403:97–108. doi: 10.1042/BJ20060819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doke N. Generation of superoxide by potato tuber protoplasts during the hypersensitive response to hyphal cell wall components of Phytophthora infestans and specific inhibition of the reaction by suppressors of hypersensitivity. Physiol Plant Pathol. 1983;23:359–367. [Google Scholar]
- 12.Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- 13.Torres MA, Dangl JL, Jones JD. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci U S A. 2002;99:517–522. doi: 10.1073/pnas.012452499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAinsh MR, Brownlee C, Hetherington AM. Visualizing Changes in Cytosolic-Free Ca2+ during the Response of Stomatal Guard Cells to Abscisic Acid. Plant Cell. 1992;4:1113–1122. doi: 10.1105/tpc.4.9.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder JI, Hagiwara S. Repetitive increases in cytosolic Ca2+ of guard cells by abscisic acid activation of nonselective Ca2+ permeable channels. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:9305–9309. doi: 10.1073/pnas.87.23.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. Embo J. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell. 2005;17:268–281. doi: 10.1105/tpc.104.026971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal. 2009;2:ra45. doi: 10.1126/scisignal.2000448. [DOI] [PubMed] [Google Scholar]
- 20.Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, Davies JM, Dolan L. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 21.Takeda S, Gapper C, Kaya H, Bell E, Kuchitsu K, Dolan L. Local positive feedback regulation determines cell shape in root hair cells. Science. 2008;319:1241–1244. doi: 10.1126/science.1152505. [DOI] [PubMed] [Google Scholar]
- 22.Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S. Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci U S A. 2007;104:20996–21001. doi: 10.1073/pnas.0708586104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bibikova TN, Jacob T, Dahse I, Gilroy S. Localized changes in apoplastic and cytoplasmic pH are associated with root hair development in Arabidopsis thaliana. Development. 1998;125:2925–2934. doi: 10.1242/dev.125.15.2925. [DOI] [PubMed] [Google Scholar]
- 24.Cosgrove DJ. Enzymes and other agents that enhance cell wall extensibility. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:391–417. doi: 10.1146/annurev.arplant.50.1.391. [DOI] [PubMed] [Google Scholar]
- 25.Fry SC. Isodityrosine, a new cross-linking amino acid from plant cell-wall glycoprotein. The Biochemical journal. 1982;204:449–455. doi: 10.1042/bj2040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Held MA, Tan L, Kamyab A, Hare M, Shpak E, Kieliszewski MJ. Di-isodityrosine is the intermolecular cross-link of isodityrosine-rich extensin analogs cross-linked in vitro. The Journal of biological chemistry. 2004;279:55474–55482. doi: 10.1074/jbc.M408396200. [DOI] [PubMed] [Google Scholar]
- 27.Brady JD, Sadler IH, Fry SC. Di-isodityrosine, a novel tetrametric derivative of tyrosine in plant cell wall proteins: a new potential cross-link. The Biochemical journal. 1996;315(Pt 1):323–327. doi: 10.1042/bj3150323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brady JD, Sadler IH, Fry SC. Pulcherosine, an oxidatively coupled trimer of tyrosine in plant cell walls: its role in cross-link formation. Phytochemistry. 1998;47:349–353. doi: 10.1016/s0031-9422(97)00592-x. [DOI] [PubMed] [Google Scholar]
- 29.Herve C, Tonon T, Collen J, Corre E, Boyen C. NADPH oxidases in Eukaryotes: red algae provide new hints! Curr Genet. 2006;49:190–204. doi: 10.1007/s00294-005-0044-z. [DOI] [PubMed] [Google Scholar]
- 30.Montsant A, Allen AE, Coesel S, De MA, A F, Mangogna M, Siaut M, Heijde M, Jabbari K, Maheswari U, Rayko E, Vardi A, Apt KE, Berges JA, Chiovitti A, Davis AK, Thamatrakoln K, Hadi MZ, Lane TW, Lippmeier JC, Martinez D, Parker M-S, Pazour GJ, Saito MA, Rokhsar DS, Armbrust EV, Bowler C. Identification and comparative genomic analysis of signaling and regulatory components in the diatom Thalassiosira pseudonana. Journal of Phycology. 2007;43:585–604. [Google Scholar]
- 31.Bouarab K, Potin P, Correa J, Kloareg B. Sulfated Oligosaccharides Mediate the Interaction between a Marine Red Alga and Its Green Algal Pathogenic Endophyte. Plant Cell. 1999;11:1635–1650. doi: 10.1105/tpc.11.9.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bloomfield G, Pears C. Superoxide signalling required for multicellular development of Dictyostelium. J Cell Sci. 2003;116:3387–3397. doi: 10.1242/jcs.00649. [DOI] [PubMed] [Google Scholar]
- 33.Lara-Ortiz T, Riveros-Rosas H, Aguirre J. Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol Microbiol. 2003;50:1241–1255. doi: 10.1046/j.1365-2958.2003.03800.x. [DOI] [PubMed] [Google Scholar]
- 34.Lardy B, Bof M, Aubry L, Paclet MH, Morel F, Satre M, Klein G. NADPH oxidase homologs are required for normal cell differentiation and morphogenesis in Dictyostelium discoideum. Biochim Biophys Acta. 2005;1744:199–212. doi: 10.1016/j.bbamcr.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Aguirre J, Rios-Momberg M, Hewitt D, Hansberg W. Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol. 2005;13:111–118. doi: 10.1016/j.tim.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Brun S, Malagnac F, Bidard F, Lalucque H, Silar P. Functions and regulation of the Nox family in the filamentous fungus Podospora anserina: a new role in cellulose degradation. Mol Microbiol. 2009;74:480–496. doi: 10.1111/j.1365-2958.2009.06878.x. [DOI] [PubMed] [Google Scholar]
- 37.Takemoto D, Tanaka A, Scott B. A p67Phox-like regulator is recruited to control hyphal branching in a fungal-grass mutualistic symbiosis. Plant Cell. 2006;18:2807–2821. doi: 10.1105/tpc.106.046169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka A, Christensen MJ, Takemoto D, Park P, Scott B. Reactive oxygen species play a role in regulating a fungus-perennial ryegrass mutualistic interaction. Plant Cell. 2006;18:1052–1066. doi: 10.1105/tpc.105.039263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cano-Dominguez N, Alvarez-Delfin K, Hansberg W, Aguirre J. NADPH oxidases NOX-1 and NOX-2 require the regulatory subunit NOR-1 to control cell differentiation and growth in Neurospora crassa. Eukaryot Cell. 2008;7:1352–1361. doi: 10.1128/EC.00137-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semighini CP, Harris SD. Regulation of apical dominance in Aspergillus nidulans hyphae by reactive oxygen species. Genetics. 2008;179:1919–1932. doi: 10.1534/genetics.108.089318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segmüller N, Kokkelink L, Giesbert S, Odinius D, van Kan J, Tudzynski P. NADPH oxidases are involved in differentiation and pathogenesis in Botrytis cinerea. MPMI. 2008;21:808–819. doi: 10.1094/MPMI-21-6-0808. [DOI] [PubMed] [Google Scholar]
- 42.Kawahara T, Lambeth JD. Molecular evolution of Phox-related regulatory subunits for NADPH oxidase enzymes. BMC Evol Biol. 2007;7:178. doi: 10.1186/1471-2148-7-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leeder AC, Turner G. Characterisation of Aspergillus nidulans polarisome component BemA. Fungal Genet Biol. 2008;45:897–911. doi: 10.1016/j.fgb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Freeman JL, Lambeth JD. NADPH oxidase activity is independent of p47phox in vitro. The Journal of biological chemistry. 1996;271:22578–22582. doi: 10.1074/jbc.271.37.22578. [DOI] [PubMed] [Google Scholar]
- 45.Koshkin V, Lotan O, Pick E. The cytosolic component p47phox is not a Sine Qua Non participant in the activation of NADPH oxidase but is required for optimal superoxide production. Journal of Biological Chemistry. 1996;271:30326–30329. doi: 10.1074/jbc.271.48.30326. [DOI] [PubMed] [Google Scholar]
- 46.Osiewacz HD, Scheckhuber CQ. Impact of ROS on ageing of two fungal model systems: Saccharomyces cerevisiae and Podospora anserina. Free Radic Res. 2006;40:1350–1358. doi: 10.1080/10715760600921153. [DOI] [PubMed] [Google Scholar]
- 47.Hansberg W, de Groot H, Sies H. Reactive oxygen species associated with cell differentiation in Neurospora crassa. Free radical biology & medicine. 1993;14:287–293. doi: 10.1016/0891-5849(93)90025-p. [DOI] [PubMed] [Google Scholar]
- 48.Hansberg W, Aguirre J. Hyperoxidant states cause microbial cell differentiation by cell isolation from dioxygen. J Theor Biol. 1990;142:201–221. doi: 10.1016/s0022-5193(05)80222-x. [DOI] [PubMed] [Google Scholar]
- 49.Malagnac F, Lalucque H, Lepere G, Silar P. Two NADPH oxidase isoforms are required for sexual reproduction and ascospore germination in the filamentous fungus Podospora anserina. Fungal Genet Biol. 2004;41:982–997. doi: 10.1016/j.fgb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 50.Egan MJ, Wang ZY, Jones MA, Smirnoff N, Talbot NJ. Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0700574104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giesbert S, Schürg T, Scheele S, Tudzynski P. The NADPH oxidase Cpnox1 is required for full pathogenicity of the ergot fungus Claviceps purpurea. Molec Plant Pathol. 2008;9:317–327. doi: 10.1111/j.1364-3703.2008.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edens WA, Sharling L, Cheng G, Shapira R, Kinkade JM, Edens HA, Tang X, Flaherty DB, Benian G, Lambeth JD. Tyrosine cross-linking of extracellullar matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. Journal of Cell Biology. 2001;154:879–891. doi: 10.1083/jcb.200103132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hill AA, Hunter CP, Tsung BT, Tucker-Kellogg G, Brown EL. Genomic analysis of gene expression in C. elegans. Science (New York, N.Y. 2000;290:809–812. doi: 10.1126/science.290.5492.809. [DOI] [PubMed] [Google Scholar]
- 54.Chavez V, Mohri-Shiomi A, Garsin DA. Ce-Duox1/BLI-3 generates reactive oxygen species as a protective innate immune mechanism in Caenorhabditis elegans. Infection and immunity. 2009;77:4983–4989. doi: 10.1128/IAI.00627-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meitzler JL, Ortiz de Montellano PR. Caenorhabditis elegans and human dual oxidase 1 (DUOX1) "peroxidase" domains: insights into heme binding and catalytic activity. The Journal of biological chemistry. 2009;284:18634–18643. doi: 10.1074/jbc.M109.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thein MC, Winter AD, Stepek G, McCormack G, Stapleton G, Johnstone IL, Page AP. Combined extracellular matrix cross-linking activity of the peroxidase MLT-7 and the dual oxidase BLI-3 is critical for post-embryonic viability in Caenorhabditis elegans. The Journal of biological chemistry. 2009;284:17549–17563. doi: 10.1074/jbc.M900831200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- 58.Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. Faseb J. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 59.De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. Journal of Biological Chemistry. 2000;275:23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- 60.Dupuy C, Ohayon R, Valent A, Noe-Hudson M, Dee D, Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Journal of Biological Chemistry. 1999;274:37265–37269. doi: 10.1074/jbc.274.52.37265. [DOI] [PubMed] [Google Scholar]
- 61.Ritsick DR, Edens WA, Finnerty V, Lambeth JD. Nox regulation of smooth muscle contraction. Free radical biology & medicine. 2007;43:31–38. doi: 10.1016/j.freeradbiomed.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ha EM, Oh CT, Bae YS, Lee WJ. Science (New York, N.Y. Vol. 310. 2005. A direct role for dual oxidase in Drosophila gut immunity; pp. 847–850. [DOI] [PubMed] [Google Scholar]
- 63.Kumar S, Molina-Cruz A, Gupta L, Rodrigues J, Barillas-Mury C. A peroxidase/dual oxidase system modulates midgut epithelial immunity in Anopheles gambiae. Science. 2010;327:1644–1648. doi: 10.1126/science.1184008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ha EM, Lee KA, Park SH, Kim SH, Nam HJ, Lee HY, Kang D, Lee WJ. Regulation of DUOX by the Galphaq-phospholipase Cbeta-Ca2+ pathway in Drosophila gut immunity. Developmental cell. 2009;16:386–397. doi: 10.1016/j.devcel.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 65.Leto TL, Geiszt M. Role of Nox family NADPH oxidases in host defense. Antioxidants & redox signaling. 2006;8:1549–1561. doi: 10.1089/ars.2006.8.1549. [DOI] [PubMed] [Google Scholar]
- 66.Maru Y, Nishino T, Kakinuma K. Expression of Nox genes in rat organs, mouse oocytes, and sea urchin eggs. DNA Seq. 2005;16:83–88. doi: 10.1080/10425170500069734. [DOI] [PubMed] [Google Scholar]
- 67.Eddy EM, Shapiro BM. Membrane events of fertilization in the sea urchin. Scanning electron microscopy. 1979:287–297. [PubMed] [Google Scholar]
- 68.Foerder CA, Shapiro BM. Release of ovoperoxidase from sea urchin eggs hardens the fertilization membrane with tyrosine crosslinks. Proceedings of the National Academy of Sciences of the United States of America. 1977;74:4214–4218. doi: 10.1073/pnas.74.10.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heinecke JW, Shapiro BM. Respiratory burst oxidase of fertilization. Proceedings of the National Academy of Sciences. 1989;86:1259–1263. doi: 10.1073/pnas.86.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heinecke JW, Meier KE, Lorenzen JA, Shapiro BM. A specific requirement for protein kinase C in activation of the respiratory burst oxidase of fertilization. The Journal of biological chemistry. 1990;265:7717–7720. [PubMed] [Google Scholar]
- 71.Wong JL, Creton R, Wessel GM. The oxidative burst at fertilization is dependent upon activation of the dual oxidase Udx1. Developmental cell. 2004;7:801–814. doi: 10.1016/j.devcel.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 72.Wong JL, Wessel GM. Reactive oxygen species and Udx1 during early sea urchin development. Developmental biology. 2005;288:317–333. doi: 10.1016/j.ydbio.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 73.Inoue Y, Ogasawara M, Moroi T, Satake M, Azumi K, Moritomo T, Nakanishi T. Characteristics of NADPH oxidase genes (Nox2, p22, p47, and p67) and Nox4 gene expressed in blood cells of juvenile Ciona intestinalis. Immunogenetics. 2005;57:520–534. doi: 10.1007/s00251-005-0010-4. [DOI] [PubMed] [Google Scholar]
- 74.Hiruta J, Mazet F, Ogasawara M. Restricted expression of NADPH oxidase/peroxidase gene (Duox) in zone VII of the ascidian endostyle. Cell and tissue research. 2006;326:835–841. doi: 10.1007/s00441-006-0220-6. [DOI] [PubMed] [Google Scholar]
- 75.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 77.Lambeth JD, Krause KH, Clark RA. NOX enzymes as novel targets for drug development. Seminars in immunopathology. 2008;30:339–363. doi: 10.1007/s00281-008-0123-6. [DOI] [PubMed] [Google Scholar]
- 78.Jaquet V, Scapozza L, Clark R, Krause KH, Lambeth JD. Small Molecule NOX Inhibitors: ROS-generating NADPH Oxidases as Therapeutic Targets. Antioxidants & redox signaling. 2009 doi: 10.1089/ars.2009.2585. [DOI] [PubMed] [Google Scholar]
- 79.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li J, Stouffs M, Serrander L, Banfi B, Bettiol E, Charnay Y, Steger K, Krause KH, Jaconi ME. The NADPH oxidase NOX4 drives cardiac differentiation: Role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell. 2006;17:3978–3988. doi: 10.1091/mbc.E05-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free radical biology & medicine. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 82.Finkel T. Reactive oxygen species and signal transduction. IUBMB Life. 2001;52:3–6. doi: 10.1080/15216540252774694. [DOI] [PubMed] [Google Scholar]
- 83.Lee S-R, Kwon K-S, Kim S-R, Rhee SG. Reversible inactivation of protein tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. Journal of Biological Chemistry. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 84.Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 85.El Jamali A, Valente AJ, Lechleiter JD, Gamez MJ, Pearson DW, Nauseef WM, Clark RA. Novel redox-dependent regulation of Nox5 NADPH oxidase by the non-receptor tyrosine kinase c-Abl. Free Radical Biology and Medicine. 2008:yyy–zzz. doi: 10.1016/j.freeradbiomed.2007.11.020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.El Jamali A, Valente AJ, Clark RA. Regulation of phagocyte NADPH oxidase by hydrogen peroxide through a Ca(2+)/c-Abl signaling pathway. Free radical biology & medicine. 2010;48:798–810. doi: 10.1016/j.freeradbiomed.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Perrone LA, Plowden JK, Garcia-Sastre A, Katz JM, Tumpey TM. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 2008;4:e1000115. doi: 10.1371/journal.ppat.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hazen SL, Gaut JP, Hsu FF, Crowley JR, d’Avignon A, Heinecke JW. p-Hydroxyphenylacetaldehyde, the major product of L-tyrosine oxidation by the myeloperoxidase-H2O2-chloride system of phagocytes, covalently modifies epsilon-amino groups of protein lysine residues. The Journal of biological chemistry. 1997;272:16990–16998. doi: 10.1074/jbc.272.27.16990. [DOI] [PubMed] [Google Scholar]
- 89.Heinecke J, Li W, Daehnke H, Goldstein J. Dityrosine, a specific marker of oxidation, is synthesized by the myeloperoxidase-hydrogen peroxide system of human neutrophils and macrophages. Journal of Biological Chemistry. 1993;268:4069–4077. [PubMed] [Google Scholar]
- 90.Salazar G, Falcon-Perez JM, Harrison R, Faundez V. SLC30A3 (ZnT3) oligomerization by dityrosine bonds regulates its subcellular localization and metal transport capacity. PLoS One. 2009;4:e5896. doi: 10.1371/journal.pone.0005896. [DOI] [PMC free article] [PubMed] [Google Scholar]