Abstract
Vibrio cholerae is a facultative pathogen that thrives in two nutritionally disparate environments, aquatic and human small intestine. Phosphate (Pi) is an essential nutrient that is limited in aquatic ecosystems and of unknown availability in the small intestine. Here we show that the Pi (Pho) regulon, which is controlled by the Pi-specific transporter (Pst) and two-component system PhoBR, is required for V. cholerae survival in both environments, though for differing reasons. While induction of Pi acquisition systems including Pst is critical for survival in the aquatic environment, regulation of virulence genes by PhoB and not Pi transport per se is required for colonization of the small intestine. We show that PhoB regulates virulence genes by directly controlling expression of a key upstream transcriptional regulator, tcpPH. Thus, the Pho regulon includes virulence genes and represents a diverse gene set essential to pathogenic V. cholerae throughout its life cycle.
Introduction
Phosphate is an essential nutrient for all life. Both aquatic and terrestrial environments are generally thought to be limiting for phosphate. Therefore, bacteria and other microorganisms must actively pursue phosphate to ensure survival. One method bacteria have developed to acquire phosphate is the phosphate-specific transport (Pst) system. The Pst system is a high-affinity inorganic phosphate (Pi) transporter and has been well studied in Escherichia coli (Rao and Torriani, 1990; Wanner, 1996). The Pst system is composed of five components encoded within the pstSCAB-phoU operon. PstSCAB have been shown to mediate Pi transport, while the function of PhoU remains unclear (Steed and Wanner, 1993). In addition to the Pi transport function, the Pst system has also been shown to be a regulator of the two-component system, PhoBR. PhoR is a histidine kinase known to phosphorylate the response regulator PhoB in conditions of low environmental Pi (< 4 µM), in turn phospho-PhoB regulates transcription of a large gene set, known as the Pho regulon, generally involved in phosphate homeostasis. By some unknown mechanism, the activation of PhoB is blocked by the Pst system when environmental Pi is in excess. However, when Pi is limiting this repression is relieved, thus allowing induction of the Pho regulon. Null mutations in the Pst genes disrupt regulation of PhoB activation, which leads to constitutive expression of the Pho regulon, regardless of environmental phosphate availability (Rao and Torriani, 1990; Wanner, 1996).
Recent work has highlighted the association of the Pho regulon with bacterial virulence (reviewed in Lamarche et al., 2008). Both constitutive activation and constitutive repression of the Pho regulon can have deleterious effects on the virulence of several species. For example, transposon mutations in the pst operon attenuate the virulence of Yersinia enterocolitica, Streptococcus pneumoniae and uropathogenic E. coli in various models of infection (Darwin and Miller, 1999; Hava and Camilli, 2002; Bahrani-Mougeot et al., 2002). Additionally, mutation of chvI, a phoB ortholog, in Agrobacterium tumefaciens attenuates virulence (Mantis and Winans, 1993). Finally, microarray and in vivo expression experiments have revealed that Pho regulon genes are induced during infection in Yersinia pestis, Erwinia chrysanthemi, Listeria monocytogenes and Mycobacterium tuberculosis in diverse models (Grabenstein et al., 2006; Yang et al., 2004; Chatterjee et al., 2006; Dubail et al., 2000; Talaat et al., 2004;). However, despite the solid connection of Pst and PhoB with bacterial virulence the mechanisms by which Pst and PhoB control virulence have not been elucidated.
Vibrio cholerae is a natural inhabitant of temperate aquatic ecosystems around the world, including salt, brackish and some fresh waters. Upon entry into a human host by ingestion of contaminated food or water, the bacteria pass through the gastric acid barrier of the stomach and colonize the small intestine. As the bacterium transitions from its natural environment to that of the host small intestine, it undergoes a shift from environmental to virulence gene expression (Herrington et al., 1988; Lee et al., 1999; Lee et al., 2001; Miller and Mekalanos 1985; Taylor et al., 1987). As aquatic environments are generally limited for Pi, the Pho regulon is likely to be required for survival in these conditions. However, a function for the Pho regulon during colonization of the small intestine remains unclear, despite the observation that phoB is required for V. cholerae colonization in the rabbit ligated ileal loop model of infection and that pstC-1∷mTn5 and phoU∷mTn5 mutants were shown to be attenuated for colonization in a large-scale signature-tagged mutagenesis (STM) screen using the infant mouse model of infection (Merrell et al., 2002; von Kruger et al., 1999).
Here we show that both constitutive activation and loss of expression of the Pho regulon in V. cholerae leads to severe attenuation of colonization in the infant mouse model of cholera. We show that the attenuation is due to dysregulation of virulence gene expression, and that PhoB is a direct negative regulator of tcpPH expression. Additionally, we show that PhoB is required for survival in pond water, indicating that the Pho regulon is essential throughout the life cycle of pathogenic V. cholerae.
Results
Constitutive activation of PhoB in V. cholerae leads to elevated fitness in low Pi conditions
In E. coli the phosphate transport (pst) genes are encoded within a single operon, pstSCAB-phoU, but the genetic organization in V. cholerae is slightly different. The majority of the operon remains intact, pstCAB-phoU, with pstS encoded just upstream. We deleted the pstCAB-phoU operon in V. cholerae and refer to this strain as Δpst.
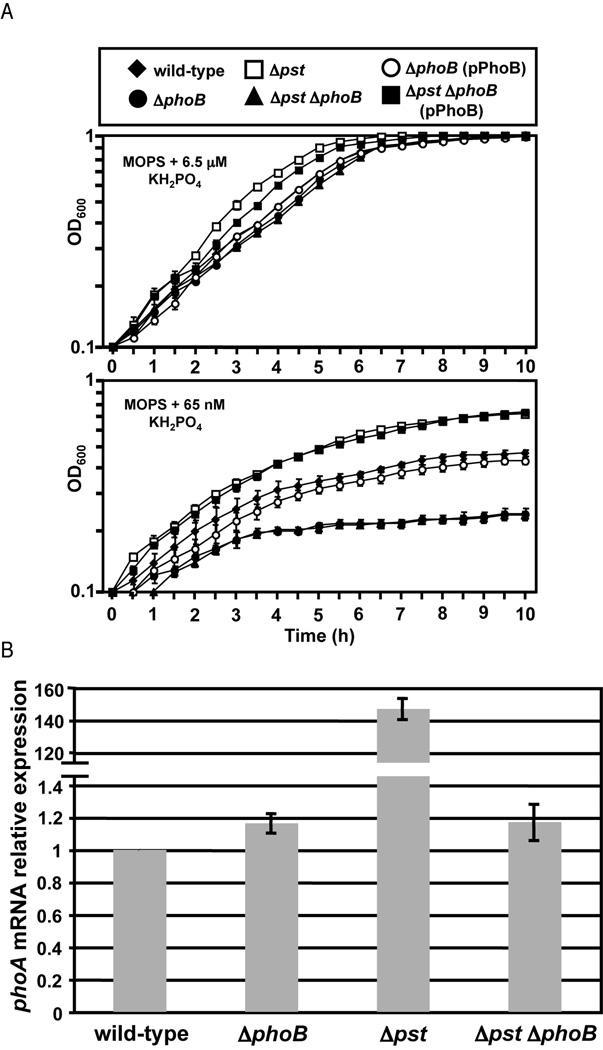
To confirm that the pst mutation leads to induction of the Pho regulon in V. cholerae, as has been described in other bacteria, wild-type and mutant bacteria were grown in media with varying Pi concentrations and the optical density was measured over time. All strains grew similarly in conditions in which Pi is in excess (LB and MOPS minimal media plus 6.5 µM KH2PO4), however, when the concentration of Pi is growth limiting (MOPS plus 65 nM KH2PO4) there is a clear difference in growth between the strains (Fig. 1A and data not shown). As has been shown previously, ΔphoB has a growth defect in low Pi media compared to wild-type (von Kruger et al., 1999). Alternatively, Δpst, which has constitutive activation of the Pho regulon, begins growth much faster than wild-type and reaches a higher cell density. The growth observed is presumably due to the induction of Pi transporter systems that fully compensate for loss of Pst. Mutation of phoB in the Δpst background eliminated this phenotype and the double mutant showed a growth defect similar to ΔphoB, suggesting that constitutive expression of the Pho regulon has primed Δpst for growth in Pi-limiting conditions and that it is due to the activity of PhoB. The growth defects of ΔphoB and Δpst ΔphoB were complemented by expression of phoB in trans.
Figure 1.
Mutation of pst operon leads to activation of PhoB in V. cholerae. V. cholerae strains were grown in MOPS minimal medium supplemented with 6.5 µM or 65 nM KH2PO4 for 10 hours at 37°C. Mean and standard deviation are shown for each time point. All samples were analyzed in triplicate. B) Quantitative RT-PCR (qRT-PCR) analysis of phoA expression. V. cholerae strains were grown in LB at 37°C and RNA collected at OD600=0.3. Expression was normalized to rpoB expression and shown relative to wild-type. The mean and standard deviation for three independent replicates are shown.
To further confirm the PhoB-activating effect of the pst mutation we measured expression of phoA, a known Pho regulon gene, by qRT-PCR during growth in LB, a high Pi condition in which phoA is not normally induced (von Kruger et al., 2006; Wanner 1996). Expression of phoA was measured in wild-type, Δpst, ΔphoB and Δpst ΔphoB, normalized to the expression of rpoB and shown relative to wild-type. Both wild-type and ΔphoB showed approximately equal levels of phoA expression, whereas Δpst expressed phoA approximately 140-fold higher (Fig. 1B). Increased expression of phoA was eliminated by mutation of phoB in the Δpst background. This serves as further confirmation that Δpst leads to activation of PhoB and the Pho regulon in V. cholerae.
PhoB regulates virulence gene expression in V. cholerae
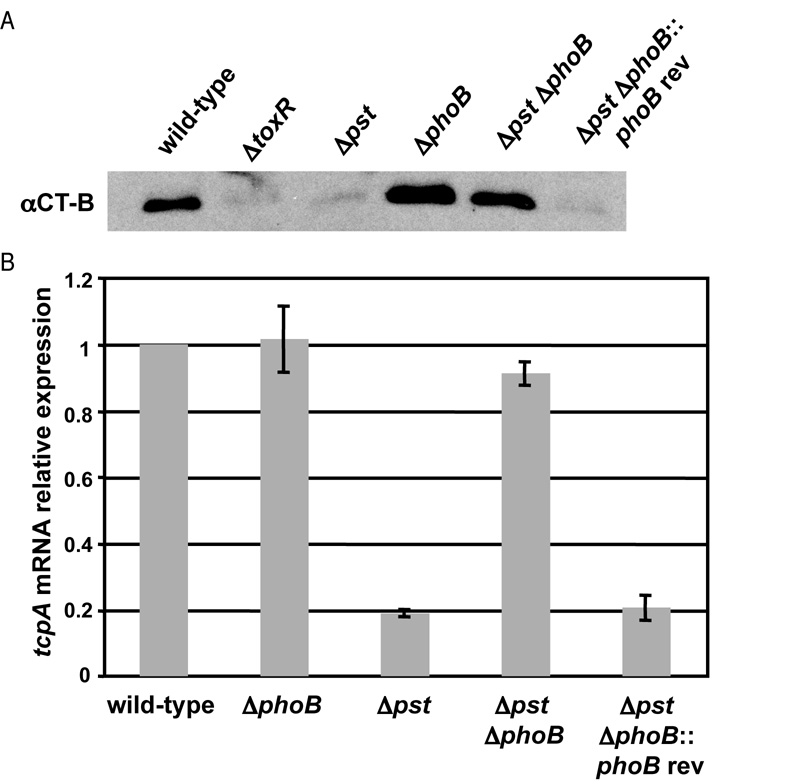
Given prior reports that mutation of pst and phoB attenuate virulence of V. cholerae, we examined whether PhoB regulates virulence gene expression (Merrell et al., 2002; von Kruger et al., 1999). We measured expression of the core virulence determinants CT and TCP in Δpst, ΔphoB and Δpst ΔphoB during growth in virulence gene inducing conditions (M9 minimal medium supplemented with amino acids N, R, E and S at 30°C). Expression of CT was measured by western blot against the CT-B subunit and was shown to be defective in Δpst, while ΔphoB showed no changes in expression compared to wild-type (Fig. 2A). Mutation of ΔphoB in the pst background led to restoration of CT-B expression to near wild-type levels, suggesting that PhoB regulates expression of CT.
Figure 2.
PhoB regulates virulence gene expression in V. cholerae. V. cholerae strains were grown in M9 + NRES at 30°C. A) Western blot analysis for CT-B subunit. Secreted proteins were isolated after overnight incubation. Western blot was performed as outlined in Experimental Procedures. B) qRT-PCR analysis of tcpA expression. RNA was collected at OD600=0.3. Expression was normalized to rpoB expression and shown relative to wild-type. The mean and standard deviation for three independent replicates are shown.
We examined the expression of TCP in each strain by measuring transcription of tcpA, the major subunit of TCP, using qRT-PCR. We observed a similar trend as with expression of CT-B, ΔphoB had no effect, but Δpst showed approximately 5-fold reduction in tcpA expression. Mutation of phoB in the Δpst background restored tcpA expression to wild-type levels (Fig. 2B). For expression of both CT and TCP, the Δpst ΔphoB phenotypes could not be fully complemented by expression of phoB in trans, presumably due to incorrect phoB expression level. However, the phenotypes were complemented by reversion of the phoB mutation, restoring the original defective virulence gene expression of Δpst. This showed that the mutant phenotypes of the Δpst and ΔphoB strains were not due to secondary mutations.
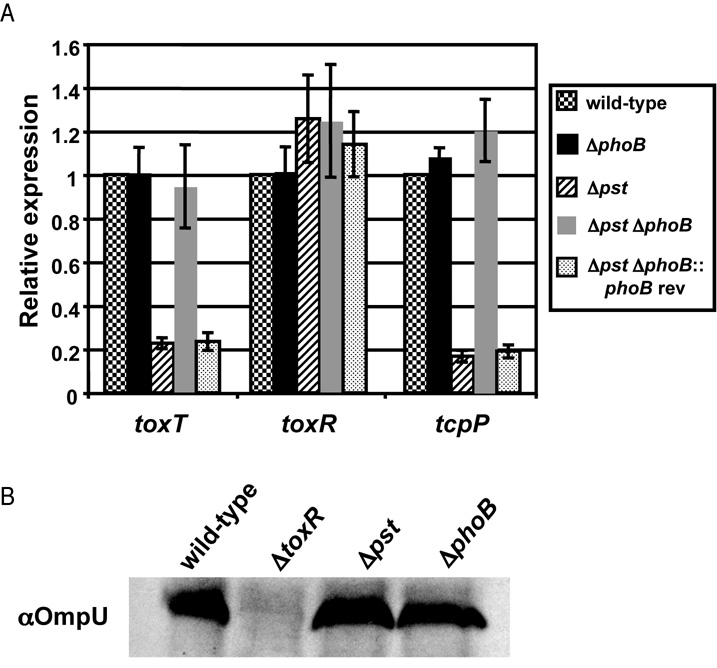
CT and TCP are regulated by a complex cascade of virulence activators, known as the ToxR regulon (reviewed in Childers and Klose, 2007). To determine if PhoB intersects with components of this regulatory cascade we measured expression of each component by qRT-PCR as above (Fig. 3A). We observed that expression of the direct regulator of TCP and CT expression, ToxT, was reduced in Δpst approximately 5-fold compared to wild-type, and that the defect was eliminated in Δpst ΔphoB. We next measured expression of direct regulators of toxT, TcpP and ToxR, by qRT-PCR. We observed no change in toxR transcription in any mutant strain tested. To confirm that the activity of ToxR was not altered by activation of PhoB, we measured expression of OmpU, another ToxR-regulated protein, by western blot. We did not observe a change in OmpU expression in any strain tested, suggesting that ToxR activity is not affected by PhoB (Fig. 3B).
Figure 3.
PhoB regulates the expression of toxT and tcpP. V. cholerae strains were grown in M9 + NRES at 30°C to OD600=0.3. A) qRT-PCR analysis of toxT, tcpP and toxR expression. Expression was normalized to rpoB expression and shown relative to wild-type. The mean and standard deviation for three biological replicates are shown. B) Western blot analysis for OmpU. Western blot was performed as outlined in Experimental Procedures.
However, we did observe an alteration in tcpP expression. We observed an approximately 5-fold decrease in tcpP transcript in Δpst compared to wild-type and mutation of phoB in Δpst background restored tcpP expression back to wild-type levels (Fig. 3A). We next measured the expression of known direct regulators of tcpP transcription, aphA, aphB and crp, but we did not observe any changes in transcription (data not shown). Therefore, based on these data we hypothesize that PhoB negatively regulates virulence gene expression by repressing the tcpPH promoter.
PhoB binds to the tcpPH promoter region
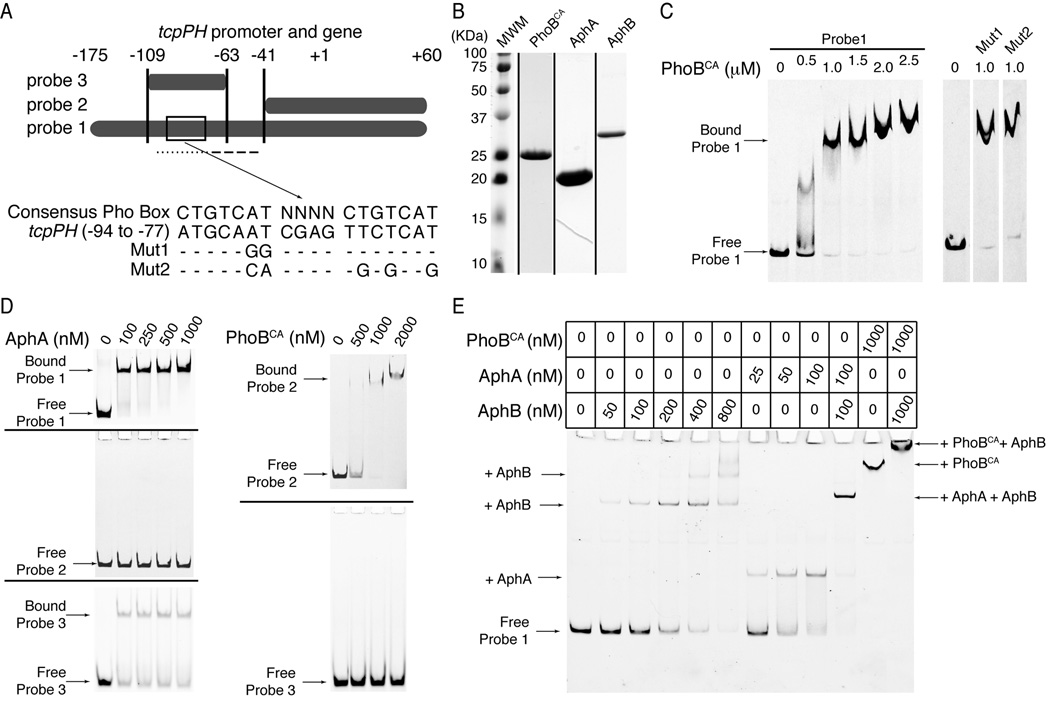
AphA and AphB are two transcriptional activators known to cooperate to regulate the tcpPH promoter (Kovacikova et al., 2004). Analysis of this promoter revealed a potential PhoB-binding site (Pho Box) spanning positions −94 to −77, which overlaps the binding site for AphA (−101 to −75) (Fig. 4A) (Kovacikova and Skorupski, 2001). In order to determine if PhoB acts as a direct or indirect regulator of tcpPH transcription we performed 6FAM fluorescence based gel mobility shift experiments using the tcpPH promoter region. Using purified constitutively active V. cholerae PhoB mutant protein (PhoBCA; PhoBD10A/D53E; Fig. 4B) (Arribas-Bosacoma et al., 2007) we observed binding of PhoBCA to a tcpPH promoter fragment (probe 1; Fig. 4C).
Figure 4.
PhoB binds to the tcpPH promoter. A) Illustration of the DNA sequences used as probes for protein binding in the gel mobility shift assays in C-E. The boxed area in probe 1 is region −94 to −77 of the tcpPH promoter. Its sequence is shown below it, aligned to the consensus Pho Box sequence. Mut1 and Mut2 show mutations made within this putative tcpPH promoter Pho Box. The dotted line below probe 1 represents the binding site for AphA, whereas the dashed line represents the binding site for AphB.. B) Coomassie stained SDS-PAGE gels for PhoBCA, AphA and AphB. Each lane is from a different gel, representing the peak fraction of the gel filtration run of each protein. C-E) Gel mobility shift assays for binding of PhoBCA, AphA and AphB to the tcpPH promoter region. Zero denotes that no protein was added to the reaction mix. C) On the left: electro-mobility of 6FAM-labeled probe 1 in the presence of increasing concentrations (0.5 to 2.5 µM) of PhoBCA. On the right: electro-mobility of 6FAM-labeled Mut1 and Mut2 of probe 1 in the presence of 1 µM PhoBCA. The free wild type, Mut1 and Mut2 of probe 1 have the same mobility (not shown). The lanes are non-contiguous on the same gel. D) On the left: electro-mobility of 6FAM-labeled probe 1 (upper gel), probe 2 (middle gel) or probe 3 (lower gel) in the presence of increasing concentrations (100 to 1000 nM) of AphA. On the right: electro-mobility of 6FAM-labeled probe 2 (upper gel) or probe 3 (lower gel) in the presence of increasing concentrations (500 to 2000 nM) of PhoBCA. E) Electro-mobility of 6FAM-labeled probe 1 in the presence of PhoBCA, and/or AphA and/or AphB at the concentrations indicated in the table above the gel. The arrows in C-E) indicate the migration level of the complexes formed by the DNA probes and the proteins they are bound to.
To determine if the putative binding site is a bona fide Pho Box we introduced point mutations within the region targeting the nucleotides that match the consensus Pho Box sequence and had previously been shown to be inconsequential for AphA binding (Mut1 and Mut2, Fig. 4A) (Kovacikova et al., 2003). We tested the ability of PhoBCA to bind these mutant tcpPH promoter regions and observed no alteration in PhoBCA binding compared to probe 1, suggesting that the predicted Pho Box is not a true PhoB binding site (Fig. 4C).
To further delineate the location of the PhoB binding site, we designed probe 2, which exludes the known AphA and AphB (−75 to −48) (Kovacikova and Skorupski, 2001) binding sites and probe 3, which contains the known AphA binding site flanked by 8 to 13 nucleotides on each side (Fig. 4A). We examined the binding of PhoBCA to each probe and found that while PhoBCA binds to probe 1 and probe 2 with similar titration profiles, it was unable to bind probe 3 (Fig. 4C, D). Additionally, we examined the ability of purified AphA (Fig. 4B) to bind probes 1, 2 and 3 and found that, as expected, AphA binds probes 1 and 3, which contain the known AphA binding site, but not probe 2 (Fig. 4D). These data suggest that PhoB and AphA bind to distinct regions of the tcpPH promoter and do not directly compete for binding sites.
An alternative hypothesis is that PhoB interferes with the binding of AphB to the tcpPH promoter, thus affecting transcription of tcpPH, which requires the binding of both AphA and AphB. To investigate this possibility, we examined the ability of purified PhoBCA and AphB (Fig. 4B) to bind probe 1 simultaneously. Contrary to our hypothesis, we observed that addition of both AphB and PhoBCA to probe 1 led to the appearance of a unique high molecular weight shifted species, which runs higher than a species corresponding to PhoBCA bound to probe 1 (Fig. 4E). Additionally, we confirmed that our purified preparations of AphA and AphB bind cooperatively to the tcpPH promoter as was previously shown (Fig. 4E) (Kovacikova et al., 2004). These observations suggest that PhoB and AphB can bind the tcpPH promoter simultaneously, therefore, they do not compete for the same binding site. Thus, activated PhoB does bind the tcpPH promoter at a site distinct from both AphA and AphB binding sites.
Proper regulation of the Pho regulon is required for efficient colonization
In order to confirm that regulation of virulence genes by PhoB was not an in vitro artifact, we investigated the ability of Δpst, ΔphoB, Δpst ΔphoB and ΔphoR strains to colonize the infant mouse small intestine in competition assays versus the wild-type. We found that all four mutants were severely attenuated (approximately 500-fold) suggesting that the Pho regulon is required for efficient colonization, but also correct regulation is required, as constitutive activation also leads to attenuation (Fig. 5). Again, we were unable to complement the colonization defect of ΔphoB in trans, however, chromosomal reversion of the phoB deletion restored colonization back to the level of wild-type (von Kruger et al., 1999).
Figure 5.
Proper regulation of the Pho regulon is required during V. cholerae infection. Competition assays were performed using the infant mouse model of infection. All strains were competed against wild-type O395. The competitive index is the ratio of mutant to wild-type bacteria recovered from the small intestine corrected for the input ratio. Each data point represents the competitive index from an individual mouse; the gray bar represents the geometric mean. The ΔphoB, Δpst, ΔphoB Δpst and ΔphoR strains are significantly attenuated (P<0.01) by Student’s two-tailed t-test.
To determine if the phosphate uptake function of the Pst system is required for V. cholerae colonization, we introduced the point mutation R454Q into PstA. This point mutation is equivalent to PstAR220Q, which has previously been shown to allow wild-type expression of genes in the Pho regulon but prevent Pst-mediated phosphate transport in E. coli (Cox et al., 1988). To confirm that pstAR454Q does not lead to induction of the Pho regulon in high Pi conditions, expression of phoA was measured by qRT-PCR and found to be equal to wild-type in LB, suggesting that the mutation does not alter regulation of the Pho regulon as expected (data not shown). When tested for colonization in the infant mouse model of infection, pstAR454Q competed 1:1 against wild-type, suggesting that the attenuation observed in Δpst is due to induction of the Pho regulon, not loss of Pst-mediated phosphate transport (Fig. 5).
PhoB regulates TCP expression in vivo
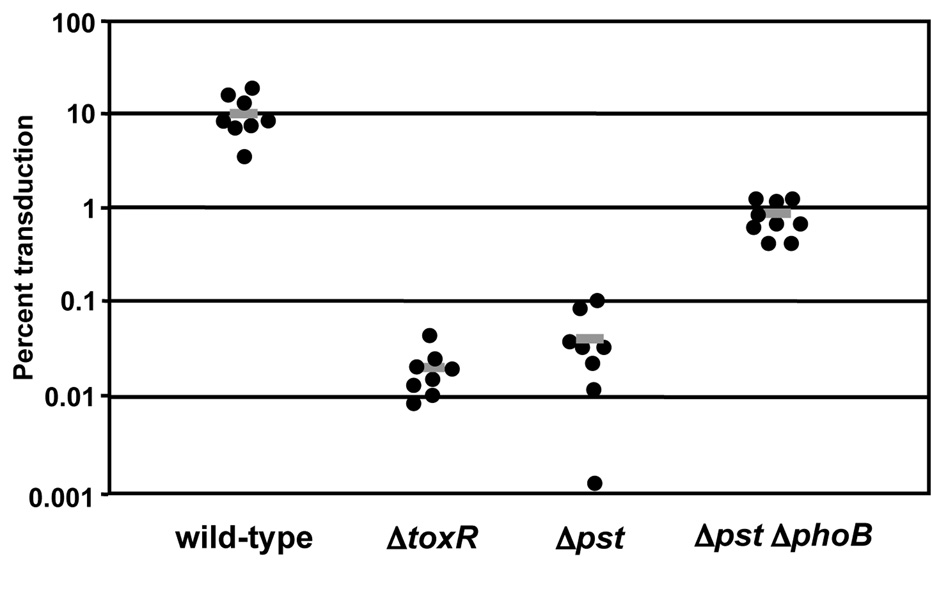
In order to confirm that PhoB regulates TCP expression during colonization, we performed an intraintestinal phage transduction assay. TCP serves as the receptor for the lysogenic bacteriophage, CTXΦ, and it has previously been shown that CTXΦ transduction during infection could be used to monitor TCP expression (Lee et al., 1999). In this assay, infant mice were co-infected with a V. cholerae donor strain carrying a Kn-marked derivative of CTXcalcΦ ( CTXcalc-KnΦ), and one of the following recipient strains: wild-type, toxR∷pGP704, Δpst or Δpst ΔphoB. We observed that approximately 10% of wild-type bacteria became CTXcalc-KnΦ positive following infection, whereas, Δpst showed about 200-fold less transductants, similar to the ΔtoxR mutant that is incapable of synthesizing TCP. Mutation of phoB in the Δpst background led to a partial restoration of phage transduction, an increase of approximately 20-fold (Fig. 6). Perhaps complete complementation was not observed due to the severe colonization defect of Δpst ΔphoB. These data suggest that PhoB negatively regulates TCP expression in Δpst in vivo. The loss of TCP expression in vivo at least partially explains the attenuation for colonization of Δpst, as TCP is an essential colonization factor.
Figure 6.
PhoB regulates the expression of TCP in vivo. TCP expression was measured by in vivo CTXΦ transduction. An O395 CTXcalc-KnΦ donor strain was co-inoculated intra-gastrically into infant mice with wild-type, toxR∷pGP704, Δpst or Δpst ΔphoB. At 21 hrs post-infection V. cholerae were recovered and the frequency of CTXcalc-KnΦ transduction was determined. Each data point represents the transduction frequency from an individual mouse; the gray bar represents the mean. The Δpst strain is significantly attenuated compared to wild-type and Δpst ΔphoB (P<0.01) by the Mann-Whitney U test.
PhoB is required for survival in pond water
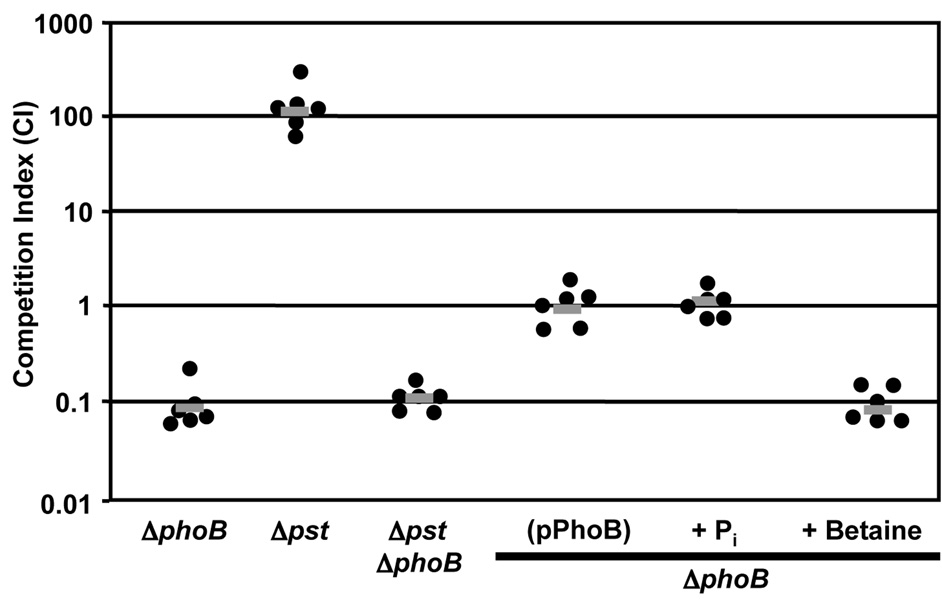
Because pond water, a natural habitat of V. cholerae in cholera endemic areas around the world, is a low Pi environment we hypothesized that V. cholerae phoB mutants would be attenuated for survival in the pond environment (Schild et al., 2007). To test this hypothesis we investigated the ability of Δpst, ΔphoB and Δpst ΔphoB to survive in pond water in competition assays versus wild-type (Fig. 7). We found that ΔphoB was attenuated for survival by approximately 10-fold compared to wild-type. Interestingly, Δpst was substantially more fit than wild-type (approximately 100-fold) in the pond environment, perhaps due to the fact that pst mutant bacteria are constitutively expressing the Pho regulon and can initially acquire more Pi. Indeed, mutation of phoB in the Δpst background reduced the fitness of this strain to the level of ΔphoB alone (approximately 10-fold compared to wild-type). The fitness defect of phoB mutants could be complemented by expression of phoB in trans. Additionally, the fitness defect of ΔphoB could be complemented by addition of 6.5 µM KH2PO4 to the pond water. An additional stress in pond water is hypo-osmolarity; to confirm that the complementation with exogenous Pi was not due to an increase in osmolarity, an equal concentration of an osmolyte, betaine, was added to pond water and had no effect on survival of ΔphoB (Fig. 7). These data confirm that Pi limitation in pond water is responsible for the survival defect of ΔphoB.
Figure 7.
PhoB is required for survival of V. cholerae in pond water. Competition assays were performed in pond water. All strains were competed against wild-type O395. The competitive index is the ratio of mutant to wild-type bacteria recovered from the pond water following 4 h incubation at 37°C with aeration and corrected for the input ratio. Each data point represents the competitive index from an individual competition; the gray bar represents the geometric mean. Pond water was supplemented with 6.5 µM KH2PO4 (Pi) or 6.5 µM betaine as noted. The ΔphoB and ΔphoB Δpst strains are significantly attenuated (P<0.01) and Δpst is significantly more fit (P<0.01) by Student’s two-tailed t-test.
Discussion
In this report we show that the Pho regulon is required for V. cholerae survival in both a fresh water environment and the host small intestine, and that maintenance of proper regulation is critical, since both under- and over-expression was deleterious to fitness in vivo. Additionally, we show that PhoB regulates V. cholerae virulence gene expression by negatively regulating the expression of the important virulence activator tcpP. Thus, we identified a novel role of PhoB as a transcriptional regulator essential for V. cholerae survival throughout its life cycle.
Previous studies have shown that PhoB is required for V. cholerae colonization in the rabbit ligated ileal loop model of infection, here we report data that extend this finding to a more natural, open intestinal tract model of infection by showing that PhoB is required for colonization in the infant mouse model of infection (von Kruger et al., 1999). Moreover, we show that constitutive activation of the Pho regulon through mutation of the pst operon leads to severe attenuation for colonization, suggesting that while PhoB is required for colonization, maintaining proper regulation of the Pho regulon is essential for colonization as well. This suggests that there may be a temporal requirement for PhoB and that the Pho regulon may be activated at some points and deactivated at other points during V. cholerae infection.
Additionally, we show that PhoB negatively regulates expression of the two major virulence determinants of V. cholerae, TCP and CT. This is in contrast to a previous report, which concluded that PhoB does not regulate CT expression (von Kruger et al., 1999). Our experimental design was different than the previous study as we used the pst mutation as a proxy to study activated PhoB. The pst mutant allowed us to study the role of PhoB in virulence gene regulation using standard in vitro virulence gene inducing conditions, rather than altering the phosphate concentration in these conditions in order to activate PhoB as was done in the previous study. Modifying the phosphate concentration changes the growth conditions, the physiology of V. cholerae and alters virulence gene induction, thus making such experiments difficult to interpret.
Consistent with our in vitro data, we show that Δpst has a defect in TCP expression during colonization of the infant mouse small intestine using an intraintestinal phage transduction assay and that Δpst ΔphoB shows partial complementation of TCP expression. While mutation of phoB in the Δpst background did not lead to complete complementation, CTXΦ transduction did increase by 20-fold compared to Δpst. The fact that Δpst ΔphoB is severely attenuated for survival may play a role in this observation, suggesting that more cells may have obtained the phage, but did not survive in the infant mouse small intestine.
Targeted expression profiling revealed that the most upstream member of the ToxR regulon regulated by PhoB was tcpPH. Further study showed that this regulation may be direct as PhoB bound specifically to the tcpPH promoter. A potential PhoB binding site was identified within the known binding region for the positive regulator AphA, however, mutational analysis of the tcpPH promoter region revealed that PhoB does not bind to the AphA binding site and does not compete for binding sites with AphA. Additionally, we found that PhoB and AphB can bind the tcpPH promoter region simultaneously, thus PhoB does not compete for binding sites with AphB either. These data suggest that PhoB binds to the tcpPH promoter region at a distinct site downstream of the AphA/AphB binding sites and that PhoB is not in competition with AphA or AphB. This leaves us with the hypothesis that PhoB interferes with the function of the RNA polymerase at the tcpPH promoter, perhaps by disrupting its interaction with AphB, preventing RNA polymerase binding to the promoter or blocking initiation of transcription. This finding makes a novel connection between phosphate homeostasis and virulence gene regulation in V. cholerae. While a role for PhoB in the pathogenesis of other bacteria has recently become apparent, this represents the first observation suggesting that PhoB directly regulates known virulence genes essential for pathogenesis.
All these data taken together suggest that PhoB acts a virulence gene regulator, a role previously unknown in V. cholerae. By regulating tcpPH expression, the bacterium is able to turn off expression of all major virulence genes, rather than binding each promoter individually. The appearance of activated PhoB may serve as a timing mechanism for the bacterium, leading to repression of virulence gene expression at a time point after colonization has been established in preparation for dissemination in secretory diarrhea. Signals from the host may arise within the small intestine, such as changes in metabolite concentration, which allow the bacteria to monitor the infection and alter their behavior accordingly using PhoB. The signal that leads to induction of the Pho regulon is likely to be Pi limitation. However, we cannot be certain as PhoB can be regulated by a number of signals in addition to phosphate concentration (Fisher et al., 1995; Suziedeliene et al., 1999; Wanner, 1996; Wanner and Wilmes-Riesenberg, 1992). There are a wide variety of stresses that V. cholerae endures during colonization of a host; further study would be required to determine the exact inducer(s). It should be noted that deletion of phoR, encoding the cognate histidine kinase of PhoB is attenuated to a similar extent as ΔphoB, suggesting that the relevant signaling is occurring through PhoR during infection and therefore Pi concentration likely plays a role.
We also show that PhoB is required for V. cholerae survival in pond water, a natural habitat of the bacterium. This was expected given that pond water is a phosphate-limiting environment. We show that the attenuation of ΔphoB is due to Pi limitation because addition of excess Pi to pond water complements the attenuation of ΔphoB, but addition of a non-Pi osmoprotectant does not.
In addition to promoting survival in pond water through activation of Pi acquisition genes, we show that PhoB also serves to maintain repression of virulence genes in this condition, thus ensuring that expression does not occur in inappropriate environments. This also sets up a potentially complex regulatory circuit, whereby the Pho regulon would be expressed during V. cholerae life in the aquatic ecosystem, but entrance into a host would potentially require PhoB to be inactivated in order to allow expression of colonization and virulence factors, including TCP and CT. However, based on our data showing that ΔphoB is severely attenuated, PhoB would need to be activated at some point, potentially after colonization has been initiated, in order to allow maximal colonization/survival. Thus, PhoB and the Pho regulon are essential factors required throughout the entire life cycle of pathogenic V. cholerae.
Experimental Procedures
Growth Conditions
Bacteria were grown in Luria-Bertani (LB) broth at 37°C with aeration unless otherwise noted. M9 minimal medium supplemented with 0.5% glycerol, trace metals (1ml/l of 5% MgSO4, 0.5% MnCl2•4H20, 0.5% FeCl3, 0.4% trinitriloacetic acid) (Callahan et al., 1971) and 25 mM each of L-Asn, L-Arg, L-Glu, and L-Ser (M9 + NRES), was prepared as previously described (Miller and Mekalanos, 1988). MOPS minimal medium supplemented with KH2PO4 (MOPS) was prepared as previously described (Tischler and Camilli 2004). Antibiotics were added when appropriate at the following concentrations: streptomycin (Sm) 100 µg/ml, ampicillin (Amp) 50 µg/ml, kanamycin (Kn) 50 µg/ml and tetracycline (Tc) 2 µg/ml.
Plasmid and strain construction
All strains and plasmids used in this study are listed in supplementary table 1. All primers used in this study are listed in supplementary table 2. Plasmids with oriR6K were propagated in E. coli DH5αλpir; all other plasmids were propagated in E. coli DH5α. Plasmids for generating in-frame deletions and point mutations in V. cholerae were constructed in the allelic exchange vector pCVD442, which encodes the sacB counter-selectable marker (Donnenberg and Kaper, 1991). Splicing by overlapping extension (SOE) PCR was used to generate all deletions (Senanayake and Brian, 1995). DNA fragments of approximately 800 bp upstream and downstream of each deletion were amplified by PCR from V. cholerae O395 genomic DNA, annealed together by complementary sequences in the R1 and F2 primers, and PCR-amplified with the F1 and R2 primers. The final PCR product was blunt-ligated into pCVD442. The respective F1/R1 and F2/R2 primer pairs used for generating deletion alleles of phoB, pstCABphoU and phoR were phoBF1/phoBR1and phoBF2/phoBR2; pstF1/pstR1 and pstF2/pstR2; and phoRF1/phoRR1 and phoRF2 and phoRR2, respectively. Plasmids were conjugated into AC61and Δpst from E. coli SM10λpir as previously described (Lee et al., 1998). After one passage in LB broth in the absence of antibiotics, sucrose-resistant colonies were selected and were subsequently screened for the desired deletion by PCR.
PhoBCA was cloned in a modified pGEX vector that contains a TEV protease recognition site between GST tag and a modified multiple cloning site. PhoBCA template was made in two steps using SOE. The initial phoBD10A was amplified from O395 genomic DNA using primer pairs: phoBF1/D10AR1 and D10AF2/phoBR2. In the second step phoBD10A/D53E was amplified from phoBD10A template using primer pairs: phoBF1/D53ER1 and D53EF2/phoBR2. Another round of modification of the PhoBCA template was done, using SOE, to eliminate the NdeI restriction enzyme site in the template using the primer pairs: F NdeI ntPhoB/R PhoB T201C and R ctPhoB st BamHI/F PhoB T201C. The resulting product and the modified pGEX vector were digested with NdeI/BamHI restriction enzymes pair and ligated together to give plasmid pAIV71.
The aphB gene was cloned into a modified pET15b vector that contains a TEV protease recognition site between 6xHis tag and a modified multiple cloning site. The insert was generated using the primer pair: F NdeI ntAphB/R ct AphB st HindIII, then digested with NdeI/HindIII restriction enzymes pair along with the vector and ligated together to give plasmid pAIV86. AphA expression vector (pWEL18) was previously described (Kovacikova et al., 2004).
Growth curves
V. cholerae strains were grown O/N at 37°C on LB plates supplemented with antibiotics then inoculated to OD600= 0.1 into MOPS minimal medium containing 6.5 µM KH2PO4 and grown O/N at 37°C with aeration. Cultures were then washed three times in MOPS medium without KH2PO4 and then diluted to OD600= 0.1 into LB or MOPS medium plus 6.5 µM or 65 nM KH2PO4. Cultures were grown at 37°C with aeration in 96-well polystyrene plates (Costar) in a Synergy HT plate reader (BioTek)
Western Blot Analysis
V. cholerae strains were grown overnight in M9 + NRES at 30°C. Whole cell lysates were used to measure OmpU expression, while TCA-precipitated culture supernatant was used to measure CT-B expression. Samples were normalized to OD600, resuspended in SDS sample buffer, boiled for 5 minutes, run on SDS-PAGE gels and transferred to nitrocellulose membranes (Invitrogen). Blots were probed with rabbit polyclonal antisera against OmpU or CT-B and donkey anti-rabbit HRP-linked secondary antibody (Amersham). Proteins were detected with the ECL-Plus horseradish peroxidase Western blotting detection kit (Amersham).
RNA Purification and qRT-PCR
RNA was isolated from 0.5 ml of OD600=∼0.3 V. cholerae cultures grown in LB or M9 + NRES at 30°C and purified following resuspension in 1 ml of RNAprotect (Qiagen) using the RNeasy Mini Kit (QIAGEN). DNA was removed using a DNA-free kit (Ambion). cDNA was synthesized from 1 µg RNA using iScript Select SYBR Green RT-PCR Kit (Bio-Rad). Controls lacking reverse transcriptase were included.
qRT-PCR experiments were performed using IQ SYBR Green Supermix (Bio-Rad) and MxP3005P Real-Time PCR System with MxPro qPCR software (Stratagene). Primers used in these studies are listed in supplementary table 2. For each sample, the mean cycle threshold of the test transcript was normalized to that of rpoB and presented relative to wild-type. Values less than one indicate that the transcript is present in lower numbers than wild-type. Three independent samples were tested in each condition.
Protein purification
Vectors carrying AphA, AphB or PhoBCA were transformed into E. coli BL21(DE3) and grown overnight on LB agar/ampicillin plates. Individual colonies were inoculated into starter cultures (10 mL) and grown to OD600 = 0.5 and then transferred into 1 L cultures. The cultures were grown at 37°C until OD600 = 0.6 to 0.8. They were then induced with 1 mM IPTG and grown at 20°C for another 16 hours. The cultures were harvested by centrifugation and the pellets were resuspended into 25 mL of the corresponding lysis buffer (for AphA: LysB1 [20 mM Tris pH8.0, 500 mM NaCl, 1 mM EDTA], for AphB: LysB2 [20 mM Tris pH8, 25 mM Imidazole, 150 mM NaCl, 5 mM βME] and for PhoBCA: LysB3 [20 mM Tris pH8.0, 150 mM NaCl, 1 mM DTT]). Protease inhibitors cocktail tablets were added. Resuspended pellets were lyzed by sonication and the lysate was cleared by centrifugation at 18,000 rpm in a SS34 rotor.
For AphA, supernatant was incubated with chitin beads for 30 min. The beads were washed with LysB1 then with CWB (20 mM Tris pH8.0, 1 M NaCl, 1 mM EDTA), before an overnight incubate with CCB (100 mM Tris pH8.0, 500 mM NaCl, 1 mM EDTA, 50 mM DTT) to induce intein cleavage of the intein chitin-binding protein tag. The following day, cleaved AphA was eluted from the beads, diluted 10 fold with QB1A (20 mM Tris pH8.0, 1 mM DTT) and applied to a 2 mL Source15Q anion exchange column equilibrated in QB1A. The protein was eluted using a 10 to 15% QB1B (20 mM Tris pH8.0, 1 M NaCl, 1 mM DTT) gradient developed over 30 CV. The peak fraction was applied to a 24 mL Superdex75 gel filtration column in EMSA buffer (10 mM Tris pH8.0, 100 mM KCl, 5% glycerol, 1 mM DTT).
For AphB, supernatant was incubated with NiNTA beads for 30 min. The beads were washed with LysB2 then with HWB (20 mM Tris pH8.0, 50 mM Imidazole pH8.0, 150 mM NaCl, 5 mM βME). AphB was eluted in HEB (20 mM Tris pH8.0, 250 mM Imidazole pH8.0, 150 mM NaCl, 5 mM βME) and incubated overnight with TEV protease. The following day, the cleavage reaction was diluted 10 fold with QB1A and applied to a 2 mL Source15Q anion exchange column equilibrated in QB1A. The protein was eluted using a 0 to 70% QB1B gradient developed over 35 CV. The peak fraction was applied to a 24 mL Superose12 gel filtration column in EMSA buffer.
For PhoBCA, supernatant was incubated with Glutathione Sepharose 4B beads for 30 min. The beads were washed with LysB3 and the protein was eluted in GEB (100 nM Tris pH8.0, 20 mM reduced glutathione, 150 mM NaCl, 1 mM DTT), diluted 10 fold with QB1A and applied to a 2 mL Source15Q anion exchange column equilibrated in QB1A. The protein was eluted using a 0 to 30% QB1B gradient developed over 30 CV. The peak fraction was incubated overnight with TEV protease. The following day, the cleavage reaction was incubated with Glutathione Sepharose 4B beads for 15 min and the flowthrough containing cleaved PhoBCA was collected. It was then applied to a 24 mL Superose12 gel filtration column in EMSA buffer.
Gel mobility shift experiments
Probe 1 and probe 2 were amplified from O395 genomic DNA using primer pairs F 6FAM tcpPH −175/tcpPR and F 6FAM tcpPH −41/tcpPR respectively. Two DNA oligomers corresponding to the two strands of probe 3 were purchased and annealing together to form the probe. Mut1 and Mut2 promoter fragments were made using SOE PCR with primer pairs F 6FAM tcpPH −175/Mut1R1 and Mut1F2/tcpPR and F 6FAM tcpPH −175/Mut2R1 and Mut2F2/tcpPR, respectively. All the forward primers (except Mut1F2 and Mut2 F2) used to generate all the probes are 5’ labeled with the fluorophore 6-FAM to enable in gel detection using a fluorescence scanner.
For the mobility shift assays, each reaction mix consisted of one or more of the desired proteins (at the desired concentration), 5 nM final probe concentration, 0.1 mg/mL calf thymus DNA and 0.1 mg/mL BSA in EMSA buffer. Reactions were incubated for 30 min at room temperature, then loaded onto a 8 % native polyacrylamide gel running at 150V in 1X TBE buffer. 6-FAM fluorescent bands were visualized using a Fujifilm Starion FLA-9000 imaging scanner
Intraintestinal CTX phage transduction assay
V. cholerae CTX phage donor strain, MKW107 (O395 [pCTXcalcΦ − Kn]) (Davis et al., 1998) was grown O/N at 37°C on LB plates plus Sm and Kn. V. cholerae recipient strains AC61, toxR∷pGP704, Δpst, and Δpst ΔphoB were grown O/N at 37°C on LB plates plus Sm and Tc. Approximately 107 CFU of the phage donor strain was mixed with 107 CFU (AC61) or 108 CFU (toxR pGP704, Δpst or Δpst ΔphoB) in 50 µl LB, then each mixture was intragastrically inoculated into 5-day-old CD-1 mice. At seven hours post-inoculation bacteria were recovered from the small intestines and serial dilutions were plated on LB plates plus Sm and Tc and LB plates plus Sm, Tc, and Kn. The % transduction was determined by the ratio of Sm/Tc/Kn resistant cells to the total cells (Sm/Tc resistant)
In vivo competition assays
Competition assays using the infant mouse model of infection were performed essentially as described (Tischler and Camilli, 2005). The wild-type and mutant strains were grown overnight on LB agar plus antibiotics at 37°C. For each strain approximately 10 colonies were resuspended in 200 µl LB and the OD600 was determined. The strains were mixed 1:1 and adjusted to a final OD600 of 0.001 (approximately 106 CFU/ml). Five-day-old CD-1 infant mice were anesthetized by isoflurane (2.5%) inhalation and intragastrically inoculated with 50 µl of this mixture. In vitro competitions were performed in parallel by inoculating 2 µl of the mix into 1 ml LB and incubating overnight at 37°C with aeration.
Pond competition assay
Pond competition assays were performed as described previously (Bourassa and Camilli, 2009). Wild-type and mutant bacteria were scraped from LB plates incubated O/N at 37°C and resuspended in 1ml LB to OD600 = 0.2. Samples were washed three times in 1 ml pond water. Pond water collected from Boston, MA was used in all experiments. Samples were mixed 1:1 and incubated at 37°C with aeration for 4 hrs. Serial dilutions were plated to calculate the ratio of wild-type to mutant bacteria. Pond water was supplemented with 6.5 µM KH2PO4 or 6.5 µM betaine monohydrate as noted.
Supplementary Material
Acknowledgements
We are grateful to A. Stock and K. Skorupski for the generous gifts of PhoB antisera and pTXB1 vector carrying aphA, respectively. A. Camilli is a Howard Hughes Medical Institute investigator. The research was supported by NIH grant AI045746.
References
- Arribas-Bosacoma R, Kim SK, Ferrer-Orta C, Blanco AG, Pereira PJ, Gomis-Rüth FX, Wanner BL, Coll M, Solà M. The X-ray crystal structures of two constitutively active mutants of the Escherichia coli PhoB receiver domain give insights into activation. J Mol Biol. 2007;366:626–641. doi: 10.1016/j.jmb.2006.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrani-Mougeot FK, Buckles EL, Lockatell CV, Hebel JR, Johnson DE, Tang CM, Donnenberg MS. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol Microbiol. 2002;45:1079–1093. doi: 10.1046/j.1365-2958.2002.03078.x. [DOI] [PubMed] [Google Scholar]
- Bourassa L, Camilli A. Glycogen contributes to the environmental persistence and transmission of Vibrio cholerae. Mol Microbiol. 2009;72:124–138. doi: 10.1111/j.1365-2958.2009.06629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan LT, Ryder RC, Richardson SH. Biochemistry of Vibrio cholerae virulence. II. Skin permeability factor-cholera enterotoxin production in a chemically defined medium. Infect Immun. 1971;4:611–618. doi: 10.1128/iai.4.5.611-618.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee SS, Hossain H, Otten S, Kuenne C, Kuchmina K, Machata S, Domann E, Chakraborty T, Hain T. Intracellular gene expression profile of Listeria monocytogenes. Infect Immun. 2006;74:1323–1338. doi: 10.1128/IAI.74.2.1323-1338.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers BM, Klose KE. Regulation of virulence in Vibrio cholerae: the ToxR regulon. Future Microbiol. 2007;2:335–344. doi: 10.2217/17460913.2.3.335. [DOI] [PubMed] [Google Scholar]
- Cox GB, Webb D, Godovac-Zimmermann J, Rosenberg H. Arg-220 of the PstA protein is required for phosphate transport through the phosphate-specific transport system in Escherichia coli but not for alkaline phosphatase repression. J Bacteriol. 1988;170:2283–2286. doi: 10.1128/jb.170.5.2283-2286.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin AJ, Miller VL. Identification of Yersinia enterocolitica genes affecting survival in an animal host using signature-tagged transposon mutagenesis. Mol Microbiol. 1999;32:51–62. doi: 10.1046/j.1365-2958.1999.01324.x. [DOI] [PubMed] [Google Scholar]
- Donnenberg MS, Kaper JB. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubail I, Berche P, Charbit A. Listeriolysin O as a reporter to identify constitutive and in vivo-inducible promoters in the pathogen Listeria monocytogenes. Infect Immun. 2000;68:3242–3250. doi: 10.1128/iai.68.6.3242-3250.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SL, Jiang W, Wanner BL, Walsh CT. Cross-talk between the histidine protein kinase VanS and the response regulator PhoB. Characterization and identification of a VanS domain that inhibits activation of PhoB. J Biol Chem. 1995;270:23143–23149. doi: 10.1074/jbc.270.39.23143. [DOI] [PubMed] [Google Scholar]
- Grabenstein JP, Fukuto HS, Palmer LE, Bliska JB. Characterization of phagosome trafficking and identification of PhoP-regulated genes important for survival of Yersinia pestis in macrophages. Infect Immun. 2006;74:3727–3741. doi: 10.1128/IAI.00255-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hava DL, Camilli A. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol Microbiol. 2002;45:1389–1406. [PMC free article] [PubMed] [Google Scholar]
- Herrington DA, Hall RH, Losonsky G, Mekalanos JJ, Taylor RK, Levine MM. Toxin, toxin co-regulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988;168:1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacikova G, Skorupski K. Overlapping binding sites for the virulence gene regulators AphA, AphB and cAMP-CRP at the Vibrio cholerae tcpPH promoter. Mol Microbiol. 2001;41:393–407. doi: 10.1046/j.1365-2958.2001.02518.x. [DOI] [PubMed] [Google Scholar]
- Kovacikova G, Lin W, Skorupski K. The virulence activator AphA links quorum sensing to pathogenesis and physiology in Vibrio cholerae by repressing the expression of a penicillin amidase gene on the small chromosome. J Bacteriol. 2003;185:4825–4836. doi: 10.1128/JB.185.16.4825-4836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacikova G, Lin W, Skorupski K. Vibrio cholerae AphA uses a novel mechanism for virulence gene activation that involves interaction with the LysR-type regulator AphB at the tcpPH promoter. Mol Microbiol. 2004;53:129–142. doi: 10.1111/j.1365-2958.2004.04121.x. [DOI] [PubMed] [Google Scholar]
- Lamarche MG, Wanner BL, Crépin S, Harel J. The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol Rev. 2008;32:461–473. doi: 10.1111/j.1574-6976.2008.00101.x. [DOI] [PubMed] [Google Scholar]
- Lee SH, Butler SM, Camilli A. Selection for in vivo regulators of bacterial virulence. Proc Natl Acad Sci USA. 2001;98:6889–6894. doi: 10.1073/pnas.111581598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Angelichio MJ, Mekalanos JJ, Camilli A. Nucleotide sequence and spatiotemporal expression of the Vibrio cholerae vieSAB genes during infection. J Bacteriol. 1998;180:2298–2305. doi: 10.1128/jb.180.9.2298-2305.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Hava DL, Waldor MK, Camilli A. Regulation and temporal expression patterns of Vibrio cholerae virulence genes during infection. Cell. 1999;99:625–634. doi: 10.1016/s0092-8674(00)81551-2. [DOI] [PubMed] [Google Scholar]
- Mantis NJ, Winans SC. The chromosomal response regulatory gene chvI of Agrobacterium tumefaciens complements an Escherichia coli phoB mutation and is required for virulence. J Bacteriol. 1993;175:6626–6636. doi: 10.1128/jb.175.20.6626-6636.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell DS, Hava DL, Camilli A. Identification of novel factors involved in colonization and acid tolerance of Vibrio cholerae. Mol Microbiol. 2002;43:1471–1491. doi: 10.1046/j.1365-2958.2002.02857.x. [DOI] [PubMed] [Google Scholar]
- Miller VL, Mekalanos JJ. Genetic analysis of the cholera toxin-positive regulatory gene toxR. J Bacteriol. 1985;163:580–585. doi: 10.1128/jb.163.2.580-585.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VL, Mekalanos JJ. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao NN, Torriani A. Molecular aspects of phosphate transport in Escherichia coli. Mol Microbiol. 1990;4:1083–1090. doi: 10.1111/j.1365-2958.1990.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Schild S, Tamayo R, Nelson EJ, Qadri F, Calderwood SB, Camilli A. Genes induced late in infection increase fitness of Vibrio cholerae after release into the environment. Cell Host Microbe. 2007;2:264–277. doi: 10.1016/j.chom.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senanayake SD, Brian DA. Precise large deletions by the PCR-based overlap extension method. Mol Biotechnol. 1995;4:13–15. doi: 10.1007/BF02907467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steed PM, Wanner BL. Use of the rep technique for allele replacement to construct mutants with deletions of the pstSCAB-phoU operon: evidence of a new role for the PhoU protein in the phosphate regulon. J Bacteriol. 1993;175:6797–6809. doi: 10.1128/jb.175.21.6797-6809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suziedeliene E, Suziedelis K, Garbenciute V, Normark S. The acid-inducible asr gene in Escherichia coli: transcriptional control by the phoBR operon. J Bacteriol. 1999;181:2084–2093. doi: 10.1128/jb.181.7.2084-2093.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaat AM, Lyons R, Howard ST, Johnston SA. The temporal expression profile of Mycobacterium tuberculosis infection in mice. Proc Natl Acad Sci USA. 2004;101:4602–4607. doi: 10.1073/pnas.0306023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RK, Miller VL, Furlong DB, Mekalanos JJ. Use of phoA fusions to identify a pilus colonization factor co-coordinately regulated with cholera toxin. Proc Natl Acad Sci USA. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler AD, Camilli A. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol Microbiol. 2004;53:857–869. doi: 10.1111/j.1365-2958.2004.04155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler AD, Camilli A. Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect Immun. 2005;73:5873–5882. doi: 10.1128/IAI.73.9.5873-5882.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Krüger WM, Humphreys S, Ketley JM. A role for the PhoBR regulatory system homologue in the Vibrio cholerae phosphate-limitation response and intestinal colonization. Microbiology. 1999;145:2463–2475. doi: 10.1099/00221287-145-9-2463. [DOI] [PubMed] [Google Scholar]
- von Krüger WM, Lery LM, Soares MR, de Neves-Manta FS, Batista e Silva CM, Neves-Ferreira AG, Perales J, Bisch PM. The phosphate-starvation response in Vibrio cholerae O1 and phoB mutant under proteomic analysis: disclosing functions involved in adaptation, survival and virulence. Proteomics. 2006;6:1495–1511. doi: 10.1002/pmic.200500238. [DOI] [PubMed] [Google Scholar]
- Wanner BL. Escherichia coli and Salmonella: Cellular and Molecular Biology. Washington DC: American Society for Microbiology Press; 1996. Phosphorus assimilation and control of the phosphate regulon; pp. 1357–1381. [Google Scholar]
- Wanner BL, Wilmes-Riesenberg MR. Involvement of phosphotransacetylase, acetate kinase, and acetyl phosphate synthesis in control of the phosphate regulon in Escherichia coli. J Bacteriol. 1992;174:2124–2130. doi: 10.1128/jb.174.7.2124-2130.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Perna NT, Cooksey DA, Okinaka Y, Lindow SE, Ibekwe AM, Keen NT, Yang CH. Genome-wide identification of plant-upregulated genes of Erwinia chrysanthemi 3937 using a GFP-based IVET leaf array. Mol Plant Microbe Interact. 2004;17:999–1008. doi: 10.1094/MPMI.2004.17.9.999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.