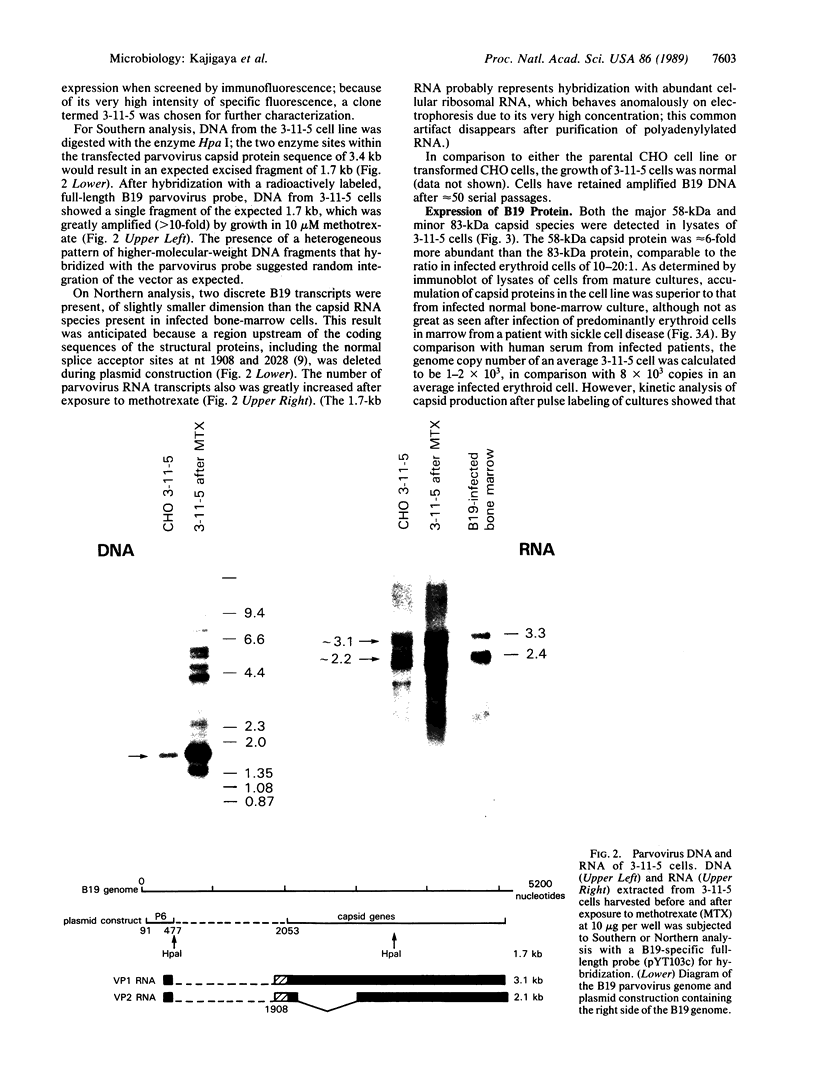
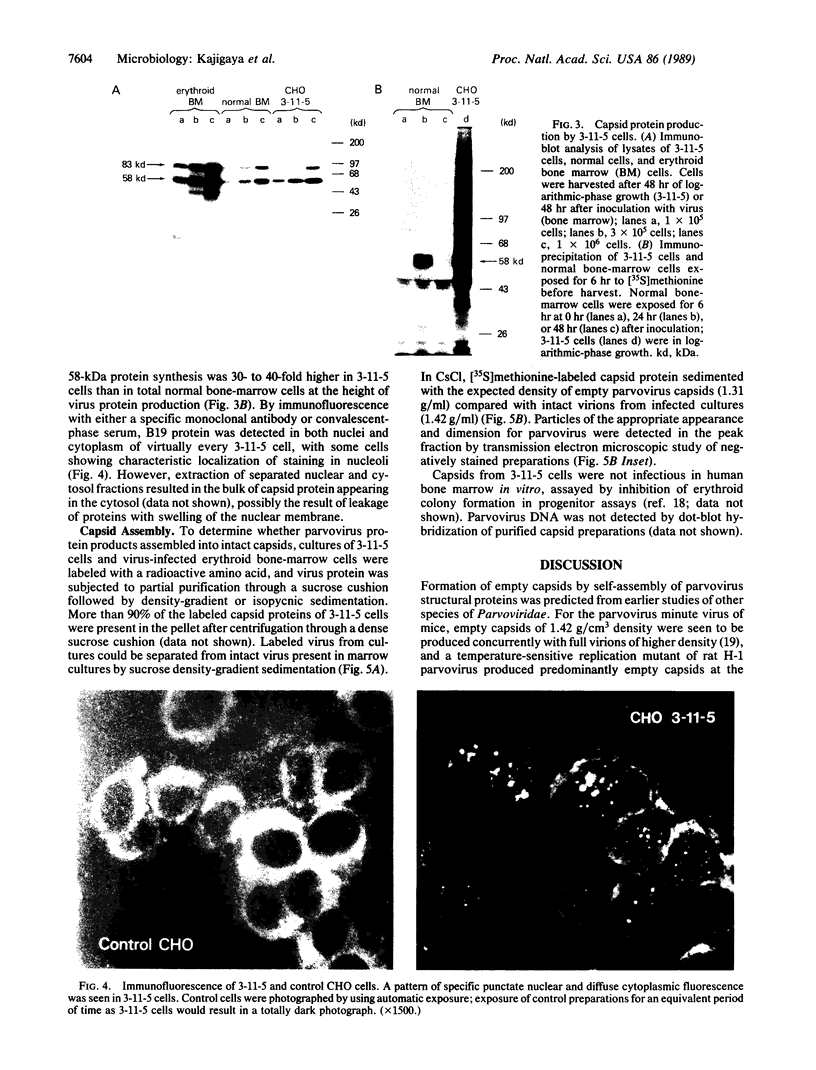
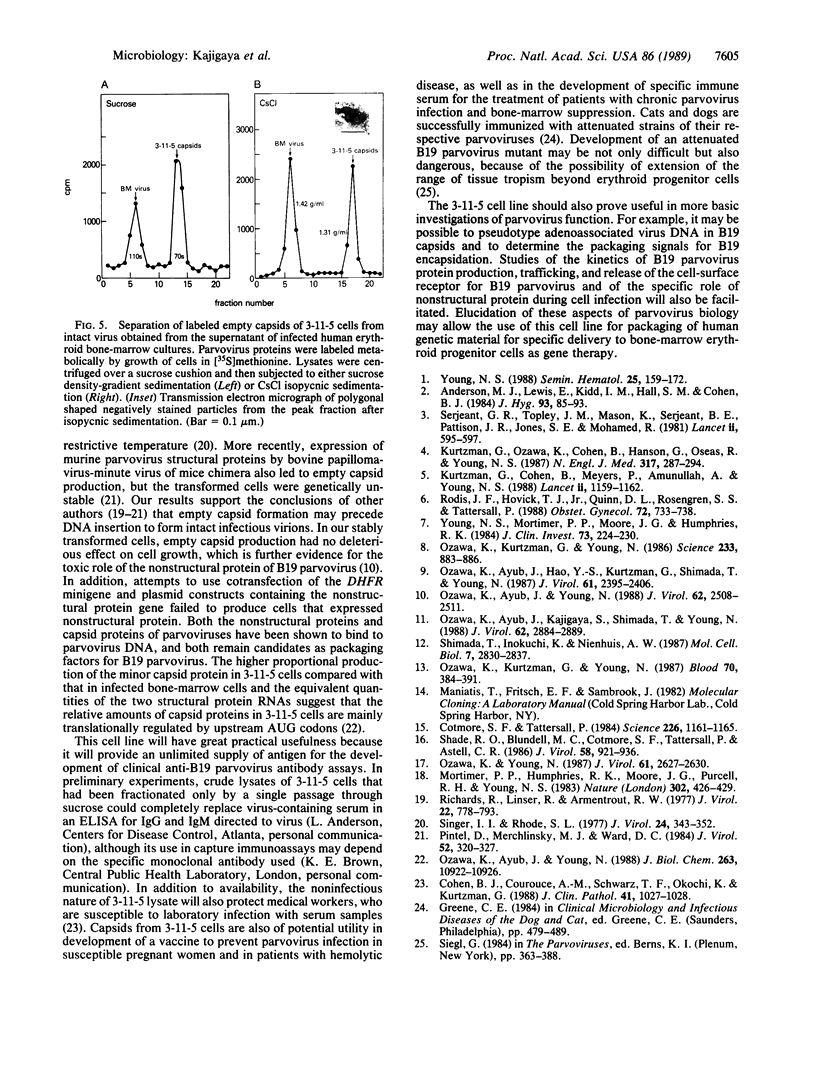
Abstract
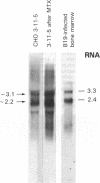
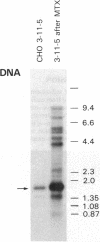
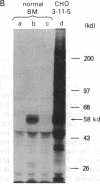
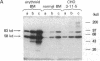
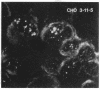
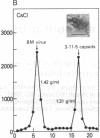
B19 parvovirus is pathogenic in humans, causing the common childhood exanthem fifth disease and bone-marrow failure, both acute (transient aplastic crisis of hemolysis) and chronic (pure erythrocyte aplasia in immunodeficiency). The virus is tropic for a human red cell progenitor cell, and failure to culture B19 in a cell line has limited its clinical study. We cotransfected the right half of the cloned B19 genome and a minigene derived from the human dihydrofolate reductase gene (DHFR) into dhfr--Chinese hamster ovary cells and screened selected clones by RNA analysis; after amplification in methotrexate, clones were tested for capsid protein expression. A cell line, designated 3-11-5, stably expressed nearly full-length transcripts for the two capsid proteins. These cells produced the major and minor structural protein species in natural proportions that self-assembled into virion capsids. Capsids from 3-11-5 cells could be separated from virions by sucrose gradient sedimentation and had the density on cesium chloride isopycnic sedimentation of empty parvovirus capsids. Capsid protein was present in both nuclei and cytoplasm on immunofluorescence study but fractionated with the cytosol on purification. Empty capsid production was equal to or greater than virion production by infected bone-marrow cells, 1000-2000 capsids per cell, but cell growth was not diminished by capsid production. This cell line will be useful in developing practical assays for B19 parvovirus antibody and a vaccine for the virus, as well as potentially serving as a packaging cell line for gene therapy.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Lewis E., Kidd I. M., Hall S. M., Cohen B. J. An outbreak of erythema infectiosum associated with human parvovirus infection. J Hyg (Lond) 1984 Aug;93(1):85–93. doi: 10.1017/s0022172400060964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B. J., Couroucé A. M., Schwarz T. F., Okochi K., Kurtzman G. J. Laboratory infection with parvovirus B19. J Clin Pathol. 1988 Sep;41(9):1027–1028. doi: 10.1136/jcp.41.9.1027-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. Characterization and molecular cloning of a human parvovirus genome. Science. 1984 Dec 7;226(4679):1161–1165. doi: 10.1126/science.6095448. [DOI] [PubMed] [Google Scholar]
- Kurtzman G. J., Cohen B., Meyers P., Amunullah A., Young N. S. Persistent B19 parvovirus infection as a cause of severe chronic anaemia in children with acute lymphocytic leukaemia. Lancet. 1988 Nov 19;2(8621):1159–1162. doi: 10.1016/s0140-6736(88)90233-4. [DOI] [PubMed] [Google Scholar]
- Kurtzman G. J., Ozawa K., Cohen B., Hanson G., Oseas R., Young N. S. Chronic bone marrow failure due to persistent B19 parvovirus infection. N Engl J Med. 1987 Jul 30;317(5):287–294. doi: 10.1056/NEJM198707303170506. [DOI] [PubMed] [Google Scholar]
- Mortimer P. P., Humphries R. K., Moore J. G., Purcell R. H., Young N. S. A human parvovirus-like virus inhibits haematopoietic colony formation in vitro. 1983 Mar 31-Apr 6Nature. 302(5907):426–429. doi: 10.1038/302426a0. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Ayub J., Hao Y. S., Kurtzman G., Shimada T., Young N. Novel transcription map for the B19 (human) pathogenic parvovirus. J Virol. 1987 Aug;61(8):2395–2406. doi: 10.1128/jvi.61.8.2395-2406.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K., Ayub J., Kajigaya S., Shimada T., Young N. The gene encoding the nonstructural protein of B19 (human) parvovirus may be lethal in transfected cells. J Virol. 1988 Aug;62(8):2884–2889. doi: 10.1128/jvi.62.8.2884-2889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K., Ayub J., Young N. Functional mapping of the genome of the B19 (human) parvovirus by in vitro translation after negative hybrid selection. J Virol. 1988 Jul;62(7):2508–2511. doi: 10.1128/jvi.62.7.2508-2511.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K., Ayub J., Young N. Translational regulation of B19 parvovirus capsid protein production by multiple upstream AUG triplets. J Biol Chem. 1988 Aug 5;263(22):10922–10926. [PubMed] [Google Scholar]
- Ozawa K., Kurtzman G., Young N. Productive infection by B19 parvovirus of human erythroid bone marrow cells in vitro. Blood. 1987 Aug;70(2):384–391. [PubMed] [Google Scholar]
- Ozawa K., Kurtzman G., Young N. Replication of the B19 parvovirus in human bone marrow cell cultures. Science. 1986 Aug 22;233(4766):883–886. doi: 10.1126/science.3738514. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Young N. Characterization of capsid and noncapsid proteins of B19 parvovirus propagated in human erythroid bone marrow cell cultures. J Virol. 1987 Aug;61(8):2627–2630. doi: 10.1128/jvi.61.8.2627-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintel D., Merchlinsky M. J., Ward D. C. Expression of minute virus of mice structural proteins in murine cell lines transformed by bovine papillomavirus-minute virus of mice plasmid chimera. J Virol. 1984 Nov;52(2):320–327. doi: 10.1128/jvi.52.2.320-327.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards R., Linser P., Armentrout R. W. Kinetics of assembly of a parvovirus, minute virus of mice, in synchronized rat brain cells. J Virol. 1977 Jun;22(3):778–793. doi: 10.1128/jvi.22.3.778-793.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodis J. F., Hovick T. J., Jr, Quinn D. L., Rosengren S. S., Tattersall P. Human parvovirus infection in pregnancy. Obstet Gynecol. 1988 Nov;72(5):733–738. [PubMed] [Google Scholar]
- Serjeant G. R., Topley J. M., Mason K., Serjeant B. E., Pattison J. R., Jones S. E., Mohamed R. Outbreak of aplastic crises in sickle cell anaemia associated with parvovirus-like agent. Lancet. 1981 Sep 19;2(8247):595–597. doi: 10.1016/s0140-6736(81)92739-2. [DOI] [PubMed] [Google Scholar]
- Shade R. O., Blundell M. C., Cotmore S. F., Tattersall P., Astell C. R. Nucleotide sequence and genome organization of human parvovirus B19 isolated from the serum of a child during aplastic crisis. J Virol. 1986 Jun;58(3):921–936. doi: 10.1128/jvi.58.3.921-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T., Inokuchi K., Nienhuis A. W. Site-specific demethylation and normal chromatin structure of the human dihydrofolate reductase gene promoter after transfection into CHO cells. Mol Cell Biol. 1987 Aug;7(8):2830–2837. doi: 10.1128/mcb.7.8.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Rhode S. L., 3rd Ultrastructural studies of H-1 parvovirus replication. IV. Crystal development and structure with the temperature-sensitive mutant ts1. J Virol. 1977 Oct;24(1):343–352. doi: 10.1128/jvi.24.1.343-352.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N. S., Mortimer P. P., Moore J. G., Humphries R. K. Characterization of a virus that causes transient aplastic crisis. J Clin Invest. 1984 Jan;73(1):224–230. doi: 10.1172/JCI111195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N. Hematologic and hematopoietic consequences of B19 parvovirus infection. Semin Hematol. 1988 Apr;25(2):159–172. [PubMed] [Google Scholar]