Abstract
Background
Ischemia-related neurologic injury is a primary cause of stroke disability. Studies have demonstrated that xenon (Xe) may have potential as an effective and nontoxic neuroprotectant. Xe delivery is, however, hampered by lack of suitable administration methods. We have developed a pressurization-freeze method to encapsulate Xe into echogenic liposomes (Xe-ELIP) and have modulated local gas release with transvascular ultrasound exposure.
Methods and Results
Fifteen microliters of Xe were encapsulated into each 1 mg of liposomes (70% Xe and 30% argon). Xe delivery from Xe-ELIP into cells and consequent neuroprotective effects were evaluated with oxygen-glucose deprived and control neuronal cells in vitro. Xe-ELIP were administered into Sprague-Dawley rats intravenously or intraarterially following right middle cerebral artery occlusion. 1-MHz low-amplitude (0.18 MPa) continuous wave ultrasound directed onto the internal carotid artery triggered Xe release from circulating Xe-ELIP. Effects of Xe delivery on ischemia-induced neurologic injury and disability were evaluated. Xe-ELIP delivery to oxygen-glucose deprived neuronal cells improved cell viability in vitro and 48% infarct volume decrease in vivo. Intravenous Xe-ELIP administration in combination with the ultrasound directed onto the carotid artery enhanced local Xe release from circulating Xe-ELIP and demonstrated 75% infarct volume reduction. This was comparable to the effect following intraarterial administration. Behavioral tests on limb placement and grid and beam walking correlated with infarct reduction.
Conclusions
This novel methodology may provide a noninvasive strategy for ultrasound-enhanced local therapeutic gas delivery for cerebral ischemia-related injury while minimizing systemic side effects.
Keywords: Cerebral ischemia, Contrast media, Stroke, Xenon, Liposomes
Background
Stroke is the third leading cause of death in the United States and the most common cause of adult disabilities. Ischemic stroke follows occlusion of a cerebral artery, resulting in obstructed blood flow to a portion of the brain.1 Emergent treatments of acute ischemic stroke have two objectives: rapid restoration of cerebral blood flow (reperfusion) and restriction of neuronal injury (neuroprotection). Currently, the only FDA-approved thrombolytic agent for the treatment of ischemic stroke is tissue plasminogen activator (tPA) in selected patients who present early with no evidence of intracerebral hemorrhage.2,3 Other approaches using neuroprotective agents have generated great interest as an adjunct to thrombolytic therapy4 but no agent has been approved for clinical application.
Several neuroprotective agents have been developed to interrupt ischemic injury by targeting the NMDA (N-methyl D-aspartate) receptor.5–8 Many of the NMDA receptor antagonists have not been approved for clinical use due to neurotoxicity9–11 or failure to cross the blood-brain barrier to the site of injury.12 Bioactive gases such as Xe, isoflurane, sevoflurane or nitrous oxide are promising neuroprotective agents with minimal adverse effects due to their low blood/gas solubility, with resultant rapid inflow and washout.13 Inhalation of 70% Xe provides a pharmacologic profile similar to that of low-affinity NMDA receptor antagonists with few neurotoxic side effects.14–20 This neuroprotective effect is dose-dependent requiring a high concentration of inhaled Xe (50–70%) for a noticeable effect. Such a concentration would not provide sufficient inspired oxygen essential for cell survival, and thus this would be difficult to translate clinically.
Liposomes are artificial sub-micron vesicles consisting of a phospholipid bilayer and a hydrophilic core. The phospholipid bilayer is ideal for incorporating a variety of hydrophobic drugs, including gases, whilst maintaining the physiological inertness of its contents. We have developed echogenic liposomes (ELIP) that can encapsulate a variety of bioactive gases such as nitric oxide allowing both echogenicity as well as gas delivery.21 Xe may be encapsulated into ELIP using the same methodology, allowing efficient Xe delivery with therapeutic benefit.
In this study, we have: (1) developed Xe-containing ELIP (Xe-ELIP) using a pressurization-freeze method and evaluated their physical and sonographic characteristics; (2) investigated the effects of ultrasound exposure for triggering Xe release from Xe-ELIP; (3) determined the therapeutic effects of Xe-ELIP on cultured hypoxic PC12 cells in vitro; and (4) evaluated the pathologic and behavioral changes in a rodent model of cerebral ischemia with ultrasound-enhanced Xe delivery using Xe-ELIP.
Methods
Preparation of Xe-Containing Liposomes
Liposomes were composed of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC, Avanti Polar Lipids, Alabaster, AL): 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC, Avanti Polar Lipids, Alabaster, AL): and cholesterol (CH, Sigma, St Louis) at a molar ratio of 60:30:10. Five milligrams of lipids were mixed in chloroform and the solvent was evaporated with argon in a 50°C water bath to form a thin film on the glass vial. The lipid film was placed under vacuum (<100 mTorr) for 4–6 hours for complete solvent removal. The dried lipid film was hydrated with 0.32 M mannitol to a concentration of 10 mg lipid/ml, followed by sonication for 5 minutes. The sonicated liposomes were transferred to a 2 ml glass vial with a cap sealed with a Teflon-rubber septum. Ten milliliters of a Xe (70%) (Concorde Specialty Gas Inc., Eatontown, NJ) and argon (30%) mixture was injected into the glass vial through the Teflon-rubber septum using a 12 ml syringe attached to a 27G × ½” needle. A ratio of 70% Xe and 30% argon was chosen as preliminary data showed 70% Xe produced the maximal neuroprotective effect.22 The pressurized liposomal dispersion was frozen at −70°C with dry ice for at least half an hour. The liposomal dispersion was allowed to thaw after the vial was unpressurized by removing the cap.
Xe Release Profile
The spontaneous Xe release profiles from Xe-ELIP were determined by a syringe method as previously described.23 A dispersion of 200 µl of Xe-ELIP was added into 1.8 ml of phosphate-buffered saline (GIBCO™ PBS, Invitrogen Co., Carlsbad, CA) or human serum plasma (HSP, Innovative Research Inc, Novi, Michigan) and incubated at 37°C. The release of Xe was measured at different time points (0, 5, 10, 30, 60 minutes and 18 hours) after incubation. PBS and HSP solutions devoid of Xe-ELIP were used as controls to correct Xe release from Xe-ELIP with respect to the residual Xe in the solution. Each Xe-ELIP sample was prepared separately at each time point and Xe release was compared between the PBS and HSP groups, i.e. comparison of Xe release was performed independently at each time point.
Ultrasound-Triggered Xe Release from Xe-ELIP
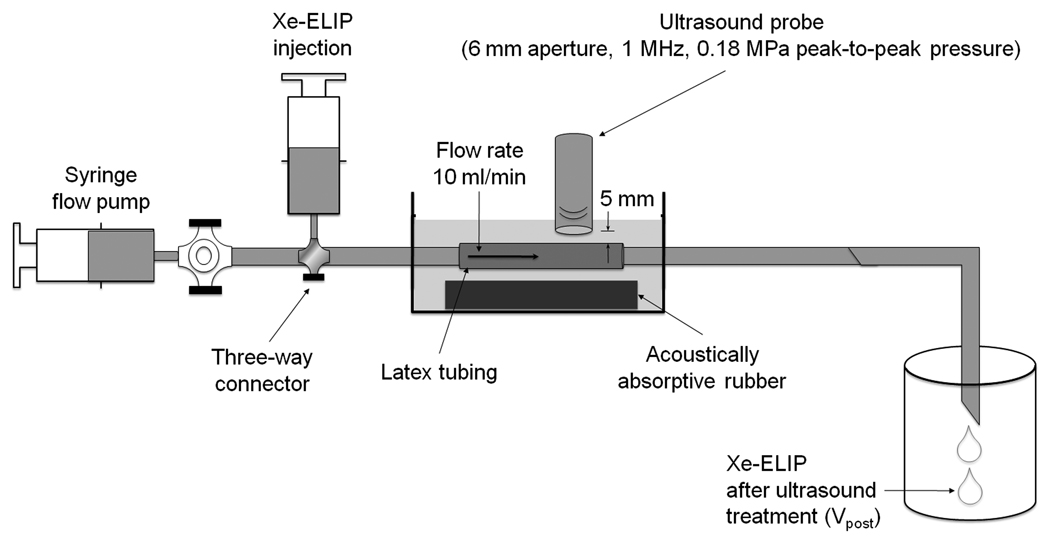
Ultrasound-triggered Xe release from Xe-ELIP was evaluated using a flow system that mimics physiological flow conditions (Fig. 1). Xe-ELIP (250 µl, 10 mg lipid/ml) were diluted in 1.75 ml PBS, and 2 ml of the diluted Xe-ELIP were injected into 2 cm of latex tubing (1/8” inner diameter; 1/32” wall thickness, McMaster-Carr, Atlanta, GA), submerged in degassed water at 22°C, and placed directly on acoustically absorptive rubber (Precision Acoustics LTD, Dorchester, Dorset, UK) to minimize acoustic reflections. A calibrated 1-MHz continuous wave ultrasound transducer which has a 6 mm aperture (Sonitron 2000, Rich-Mar Corp, Inola, OK) was mounted 5 mm above the latex tubing, and the flow was exposed to 0.18 MPa peak-to-peak pressure amplitude. A constant flow rate of 10 ml/min was used with a syringe pump. The ultrasound-exposed Xe-ELIP solution was collected to measure residual Xe.
Figure 1.
In vitro setup for ultrasound-triggered Xe release from Xe-ELIP under physiologic flow conditions.
The amount of Xe released (Vreleased) from Xe-ELIP with ultrasound exposure was determined by subtracting the residual Xe (Vpost) from the initial Xe amount (Vpre) in the Xe-ELIP using the same syringe method. The percentage of Xe release, Vreleased%, was calculated as:
Neuroprotective Effects of Xe on Cultured PC12 Cells
PC12 cells (ATCC, Manassas, VA) were placed in 24-multiwell plates (Costar, Cambridge, MA) and incubated in medium consisting of Eagle's minimum essential medium (ATCC, Manassas, VA) supplemented with 20 mM glucose, 26 mM NaHCO3, 10% fetal bovine serum (GIBCO), 10% heat-inactivated horse serum, penicillin-streptomycin solution (GIBCO), 2 mM glutamine (Sigma, Poole, United Kingdom), and 10 ng/ml murine epidermal growth factor (GIBCO). After reaching confluence, deprivation of oxygen and glucose (OGD) was induced by incubating PC12 cells with deoxygenated PBS solution in the absence of glucose in an anoxic chamber for 3 hours at 37°C. After hypoxic treatment, deoxygenated PBS was removed. Standard PBS was placed in the control wells, no treatment wells, air-containing ELIP (air-ELIP) treatment wells (80 µl of 1 mg/ml), and Xe-containing ELIP (Xe-ELIP) treatment wells (80 µl of 1 mg of lipid/ml). All cells were incubated for 1 hour at 37°C, and neuronal injury was evaluated by the ability of viable cells to reduce the tetrazolium derivative, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) into a blue formazan salt using protocol previously validated in our labs.24 Cells were incubated with MTT (100 µg/mL) at 37°C in culture medium for 3 hours, washed and incubated in 0.08 M HCl/isoproponal to dissolve the blue formazan product. The concentration of blue formazan salt, a surrogate of cell viability, was quantified by measuring absorbance at 570 nm using a TECAN Safire2 plate reader (Tecan Group Ltd., Männedorf, Switzerland). Data were reported as percentage of the released blue formazan compared to controls.
Rat Model of MCA Ischemia-Reperfusion
All animal experiments were approved by the Animal Welfare Committee at The University of Texas Health Science Center at Houston. A total of 40 Sprague-Dawley male rats (260–280 g, Harlan Laboratories Inc., Indianapolis, IN) were fasted for 24 hours with free access to water prior to surgery. Before surgery, anesthesia was induced by intraperitoneal injection of a cocktail solution of ketamine (25 mg/ml), diazepam (2 mg/ml), and atropine (0.1 mg/ml) at a dose of 2.5 ml/kg. Marcaine (2 mg/kg) was injected subcutaneously at the surgical site to provide topical analgesia. Cerebral ischemia was induced by occluding the right middle cerebral artery (MCA) for 2 hours using the intraluminal suture method.25–27
Using an operating microscope, the right common carotid artery (CCA) was exposed through a midline neck incision and carefully dissected free from surrounding nerves and fascia. The external carotid artery (ECA) was isolated and dissected distally to the bifurcation of the lingual and maxillary artery branches. The ECA was ligated close to its distal end. The internal carotid artery (ICA) was isolated and separated from adjacent tissues. A 25 cm 4-0 monofilament nylon suture (Ethicon, Somerville, NJ) was blunted by heating the tip using a low temperature cauterizer (World Precision Instruments, Sarasota, FL). The suture was coated with poly-L-lysine (0.1% [wt/vol]) and heparin solution (1,000 units/ml) in deionized water, and dried with the cauterized tip downward at room temperature for 12 hours. The suture was inserted into the MCA lumen located 18 to 20 mm from the ECA/CCA bifurcation for 2 hours to provoke ischemia. As soon as the suture was removed, Xe-saturated saline (200 µl) or Xe-ELIP (200 µl, 10 mg/ml) were administered via the tail vein or through a catheter into the ICA in the antegrade direction. In all experiments, body temperature was monitored and maintained at 37°C during ischemia and for the first hour of reperfusion using a feed-forward temperature controller that has a heating lamp and heating pad (Harvard Apparatus, Holliston, MA). A polyethylene catheter was introduced into the right femoral artery for pressure recording.
Cerebral blood flow was measured before and after MCA occlusion and after reperfusion, just before Xe delivery and after Xe delivery using a PR407-1 straight needle LDF-probe (Perimed, Järfälla, Stockholm, Sweden) connected to a standard laser Doppler monitor (PF5010 LDPM Unit and PF5001 main unit, Perimed, Järfälla, Stockholm, Sweden). Interruption of blood flow was recorded in the region of ischemic penumbra (2 mm lateral and 2 mm posterior to the bregma).24
Animals were randomly divided into seven groups (n=8 in each group): (1) normal group – skin incision without MCA occlusion; (2) no treatment group – MCA occlusion only; (3) treatment group “a” – intraarterial Xe-ELIP administration into CCA after MCA occlusion; (4) treatment group “b” – intravenous Xe-saturated saline administration after MCA occlusion; (5) treatment group “c” – intravenous Xe-saturated saline administration with ultrasound activation on ICA after MCA occlusion; (6) treatment group “d” – intravenous Xe-ELIP injection after MCA occlusion; (7) treatment group “e” – intravenous Xe-ELIP injection with ultrasound activation on ICA after MCA occlusion.
Transvascular Ultrasound Application for Enhanced Xe Release
The Sonitron ultrasound probe was placed 5 mm above the ICA to trigger Xe release from Xe-ELIP during the 4-minute period of liposomal injection. A PBS solution was filled between the artery and probe to ensure adequate acoustic coupling. The ICA was exposed to 1-MHz continuous wave ultrasound at a peak-to-peak pressure amplitude of 0.18 MPa (1 W/cm2 dial setting) during tail vein injection of the Xe-ELIP.
Neurologic Assessment
All behavioral tests in mouse were conducted in a quiet and low-lit room by an observer blinded with respect to the treatment groups. At days 1, 2 and 3 after surgery, each animal was tested for motor function and neurologic outcomes by recording limb placing, beam walking and grid walking abilities.24 Limb placement was assessed by observing the animal’s ability to lift its head and extend its forelimbs toward a table while the animal was suspended over the table by its tail (zero score – no response; score of 1 – when response was sluggish or delayed; score of 2 when response was rapid and fully executed). The ability to walk across a beam (2.5×2.5×80 cm) was assessed by observing the ability to maintain balance while navigating across the beam. The response scores were assigned as follows: score 0 – traversed the beam with no foot slip; score 1 – traversed with grasping of the lateral side of the beam; score 2 – showed difficulty crawling across the beam but able to traverse; score 3 – required a more than 10 seconds to traverse the beam due to difficulty in walking; score 4 – unable to traverse the beam; score 5 – unable to move the body or any limb on the beam; score 6 – unable to stay on the beam for more than 10 seconds. Grid walking ability was assessed by placing the animal on a stainless steel grid floor (20 cm × 40 cm with a mesh size of 2 cm × 2 cm). The total number of steps was counted up to a maximum of 50 steps. The number of foot fault errors as defined by the misplacement of a forelimb or hindlimb that fell through the grid was recorded.
Infarct Volume Measurement
Animals were sacrificed on the third day following neurological assessment. Brains were harvested. Using a Jacobowitz brain slicer, 2 mm thick coronal sections were cut prior to staining with 2% 2,3,5-triphenyltetrazolium chloride (TTC) in PBS for 20 minutes at 37°C for infarct volume determination. Stained sections were transferred to 10% phosphate buffered formalin for storage. Sections were photographed with a Canon G7 10.0 megapixel camera fitted on a Polaroid land-tripod at an object distance of 8.5 cm. Images were transferred and analyzed with Image Pro-Plus to calculate infarct volumes. Infarct volume was calculated by measuring infarct areas on evenly-sliced (1 mm) brain sections and adding them together (Simpson’s rule). Normalized infarct volume with respect to whole brain volume was calculated by dividing the volume of TTC unstained (infarcted) tissue by that of the whole brain.
Statistical Analysis
In this preclinical animal study, due to the small sample sizes within the experimental groups, nonparametric statistical methods were used to assess differences between the groups. Pairwise comparisons of spontaneous Xe release from Xe-ELIP in PBS or HSP and release with or without ultrasound were performed by the Wilcoxon rank sum test for two groups. For comparisons of multiple groups, the Kruskal-Wallis analysis of variance of ranks and median test was used to assess if there were global differences between the groups. This was followed by post hoc multiple comparisons of mean ranks for all groups by computation of normal z-values for each comparison followed by probabilities adjusted for the number of comparisons for two-sided tests of significance.28 Data were plotted as means and standard deviations for most experiments. Neurologic outcomes between the treatment groups were also reported as medians and quartiles. Statistica (Version 9, StatSoft Inc, Tulsa, OK) software was utilized for the statistical analyses. A p<0.05 was considered significant.
Results
Encapsulation and Release of Xe
Using the pressurization-freeze method at 6 atm, a total of 15±2.3 µl of Xe gas was encapsulated into 1 mg of liposomes. The spontaneous Xe release profiles from Xe-ELIP demonstrated a biphasic pattern with the rapid phase (initial 10 minutes) characterized by 30% release of the encapsulated gas (Fig. 2). Although Xe release from Xe-ELIP in HSP was somewhat slower than in PBS, there was no difference between the two groups for the first hour.
Figure 2.
Spontaneous Xe release profiles from Xe-ELIP in PBS and HSP over the first hour and up to 18 hours. Data are demonstrated using normalized Xe release as a percentage with respect to the initial amount of Xe in the Xe-ELIP.
Ultrasound-Triggered Xe Release from Xe-ELIP
The average of initial Xe volume encapsulated in 2 mg Xe-ELIP (10 mg/ml) was 33±5 µl. Under physiologic steady flow conditions (10 ml/min, Fig. 1) mimicking blood flow in the internal carotid artery, it took 12 seconds to have 2 ml of Xe-ELIP pass through the latex tubing. Ultrasound exposure triggered enhanced Xe release (44±4%) compared to 5±1% Xe release without ultrasound exposure (Fig. 3).
Figure 3.
Effects of ultrasound on Xe release from Xe-ELIP under physiological flow conditions. Low-amplitude continuous ultrasound (0.18 MPa) increased Xe release (44%) from Xe-ELIP compared to no ultrasound (5%).
Effects of Xe-ELIP on Cell Viability during Oxygen-Glucose Deprivation
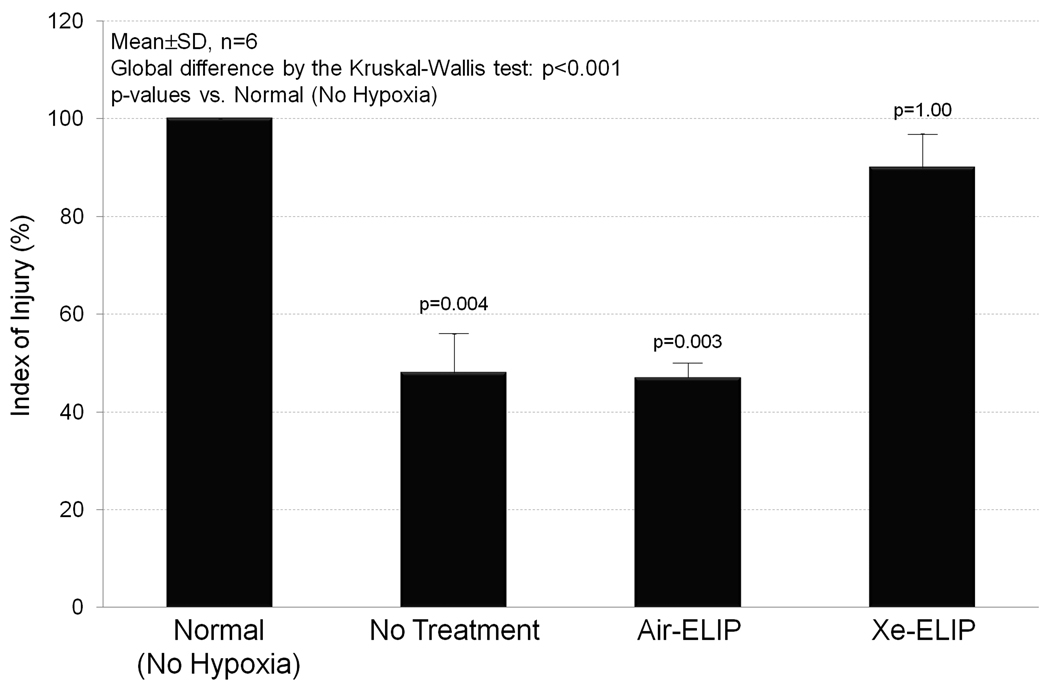
The effects of Xe-ELIP on cell viability during oxygenglucose deprivation (OGD) were evaluated by measuring release of blue formazan salt. Live cells maintain their metabolic capability to convert MTT to blue formazan salt. Fig. 4 demonstrates 54% decrease in viability of the cultured PC12 cells at 3 hours due to OGD in the no treatment group (p=0.004 vs. No Hypoxia). Air-ELIP did not protect the cells from OGD damage. However, with exposure to 40 µg/ml of Xe-ELIP, 90% of the cells remained viable (p=1.00 vs. No Hypoxia).
Figure 4.
Effects of Xe-ELIP on cell viability during hypoxia. Using the MTT cell viability assay, this demonstrates the capability of Xe-ELIP to protect cells from hypoxic damage with 90% cells remaining viable.
Neurologic Assessment
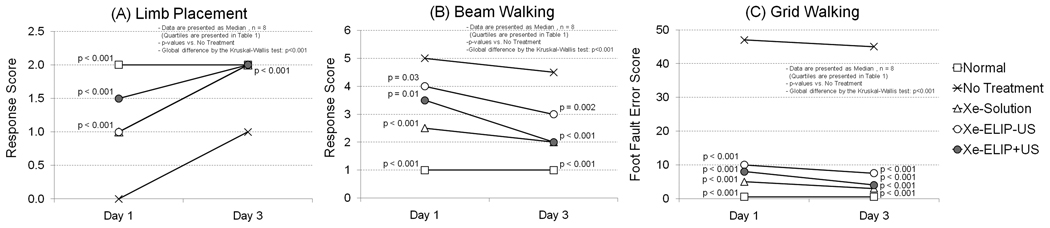
The effects of Xe-ELIP treatment on the neurologic disability of animals with ischemia after transient MCA occlusion were investigated (Table 1 and Fig. 5). Animals without treatment showed major deficits in locomotor performance on day 1 with little improvement on days 2 and 3. Both Xe-saturated saline and Xe-ELIP treatment groups demonstrated improved performance in all behavioral tests from day 1, and almost close to normal in the limb placement test on day 3 (p<0.001 vs. no treatment group). Animals with Xe-ELIP treatment combined with ultrasound exposure demonstrated an enhanced neurologic improvement on all tests compared to those without ultrasound exposure. Animals treated with Xe-ELIP showed earlier recovery from anesthesia and earlier restoration of daily activities including grooming, exploratory behavior and feeding compared to no treatment.
Table 1.
Changes in neurologic disability of animals with ischemia on day 1 and day 3 after transient MCA occlusion.
| Treatment Group | Limb Placement | Beam Walking | Grid Walking | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 1 | Day 3 | Day 1 | Day 3 | |||||||||||||
| Med | 25% | 75% | Med | 25% | 75% | Med | 25% | 75% | Med | 25% | 75% | Med | 25% | 75% | Med | 25% | 75% | |
| Normal (No Hypoxia) | 2.0* | 2.0 | 2.0 | 2.0* | 2.0 | 2.0 | 1.0* | 1.0 | 1.0 | 1.0* | 1.0 | 1.3 | 0.5* | 0.0 | 1.3 | 0.5* | 0.0 | 1.3 |
| No Treatment | 0.0 | 0.0 | 0.0 | 1.0 | 0.8 | 1.0 | 5.0 | 4.8 | 5.0 | 4.5 | 4.0 | 5.0 | 47 | 46 | 48 | 45 | 42 | 47 |
| Xe-Solution Treatment | 1.0* | 1.0 | 1.0 | 2.0* | 2.0 | 2.0 | 2.5* | 2.0 | 3.0 | 2.0* | 2.0 | 2.0 | 5.0* | 4.8 | 6.3 | 3.0* | 2.8 | 3.3 |
| Xe-ELIP Treatment | 1.0* | 1.0 | 1.0 | 2.0* | 2.0 | 2.0 | 4.0++ | 4.0 | 4.0 | 3.0** | 3.0 | 4.0 | 10* | 10 | 12 | 7.5* | 6.0 | 8.3 |
| Xe-ELIP Treatment + US Exposure | 1.5* | 1.0 | 2.0 | 2.0* | 2.0 | 2.0 | 3.5+ | 3.0 | 4.0 | 2.0* | 2.0 | 3.0 | 8.0* | 7.8 | 8.8 | 4.0* | 3.0 | 5.3 |
Data are presented as median and quartiles, US – ultrasound.
p<0.001,
p=0.002,
p=0.01,
p=0.03 vs. No treatment group, n=8.
Scores in the limb placement: 0 – no response; 1 – response was sluggish or delayed; 2 response was rapid and fully executed.
Scores in the beam walking: 0 – traversed the beam with no foot slip; 1 – traversed with grasping of the lateral side of the beam; 2 – showed difficulty crawling across the beam but able to traverse; 3 – required a more than 10 seconds to traverse the beam due to difficulty in walking; 4 – unable to traverse the beam; 5 – unable to move the body or any limb on the beam; 6 – unable to stay on the beam for more than 10 seconds.
Scores in the grid walking: Total number of foot fault errors.
Figure 5.
Effect of intravenous Xe-ELIP treatment in combination with ultrasound application on neurologic disability in animal model of ischemia. (A) Limb placement, (B) Beam walking, (C) Grid walking.
Effects of Xe-ELIP on Cerebral Infarct Volume After Middle Cerebral Artery Occlusion
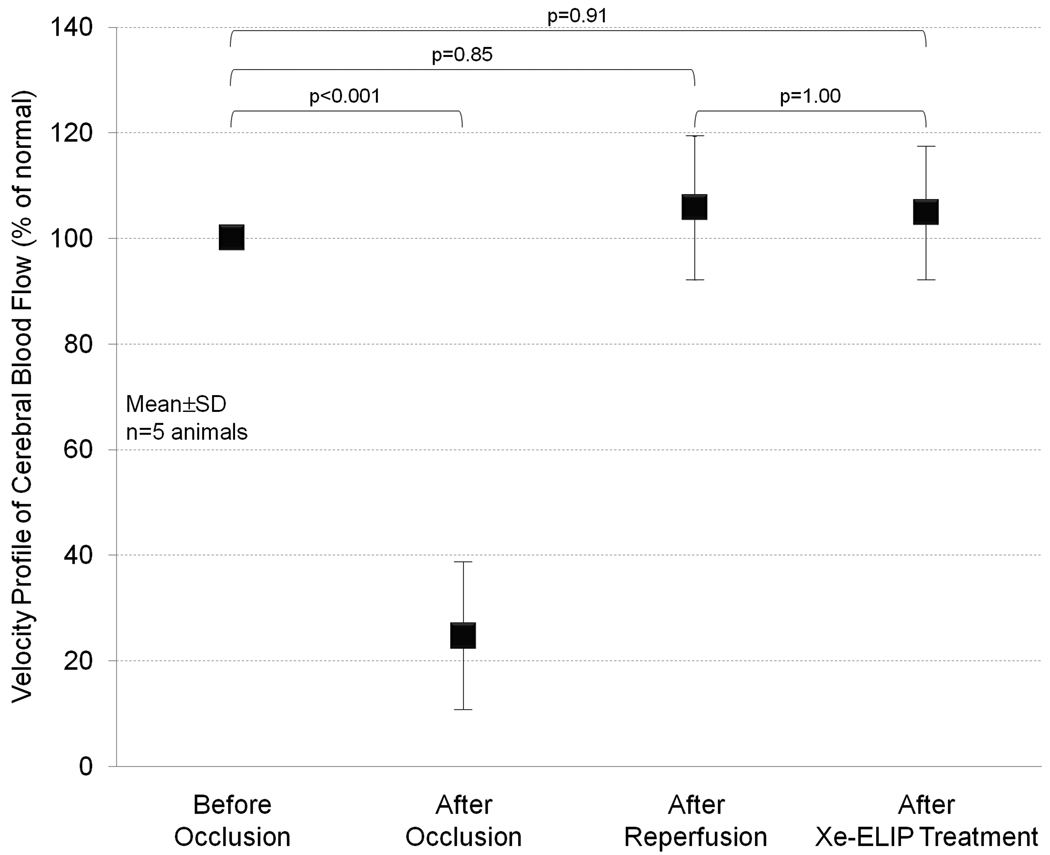
Local delivery of Xe-ELIP to moderate cerebral ischemia was achieved by tail vein injection in combination with or without ultrasound application over the ICA. Representative images of the TTC-stained brain section of rat subjected to MCA occlusion are shown in Fig. 6A. In the no treatment group, a large infarction developed and predominately involved the cerebral cortex and striatum. Fig. 6B demonstrates the effects of Xe delivery by Xe-saturated saline solution or Xe-ELIP. The normalized infarct volume in the no treatment group was 16±5.2% (228±74 mm3). The normalized infarct volume with intravenous Xe-saturated saline treatment with and without ultrasound activation were 7.5±1.4% (p=0.23 vs. no treatment) and 7.8±2.0% (p=0.40 vs. no treatment), respectively. The normalized infarct volume with intravenous delivery of Xe-ELIP without ultrasound treatment was 8.3±1.4% (108±18 mm3, p=1.00 vs no treatment). However, intravenous administration of Xe-ELIP combined with ultrasound treatment over the carotid artery further decreased the normalized infarct size to 4.0±1.4% (56±19 mm3), a 75% reduction compared to the no treatment group (p<0.001). The effect of intraarterial delivery of Xe-ELIP was compared with the treatment groups with intravenous injection (Table 2). Intraarterial injection of Xe-ELIP decreased the normalized infarct volume to 4.9±1.3 (82±18 mm3, 64% reduction, p<0.001), which was comparable to the treatment group with intravenous Xe-ELIP injection with ultrasound activation (p=1.00). No deaths or seizures were observed in all groups. There was no difference in core body temperature between the groups during MCA occlusion and initial hours of reperfusion. Fig.7 demonstrates an estimate of blood velocity profile which supports blood restoration after removal of MCA occlusion. We-ELIP treatment showed no additional vasodilatory effect.
Figure 6.
Effects of intravenous Xe-ELIP delivery in combination with ultrasound application on cerebral infarct volume in an animal model. (A) Representative TTC-stained coronal brain sections demonstrating brain infarction in rats at 3 days after MCA occlusion with no treatment, Xe-saturated saline treatment, and Xe-ELIP treatment, (B) Infarct size comparison between treatment groups demonstrating a 48% infarct volume reduction with Xe-ELIP alone and 75% reduction with Xe-ELIP combined with ultrasound activation. IA – intraarterial, IV – intravenous, US – ultrasound.
Table 2.
Changes in normalized infarct size with intraarterial or intravenous Xe delivery.
| No Treatment |
Xe-ELIP w/o US (IA) |
Xe-Saturated Saline w/o US (IV) |
Xe-Saturated Saline w/ US (IV) |
Xe-ELIP w/o US (IV) |
Xe-ELIP w/ US (IV) |
|
|---|---|---|---|---|---|---|
| Normalized Infarct Volume (%) | 15.9±5.2 | 4.9±1.3* | 7.5±1.4 | 7.8±2.0†,*** | 8.3±1.4 | 4.0±1.4*,**,†† |
Data are presented as mean and standard deviation, n=8.
IA – intraarterial, IV – intravenous, US – ultrasound.
p<0.001 vs. No treatment group,
p=0.004 vs. Xe-ELIP w/o US (IV) group,
p=0.03 vs. Xe-ELIP w/ US (IV) group,
p=1.00 vs. Xe-Saturated Saline w/o (IV) group,
p=1.00 vs. Xe-ELIP w/o US (IA) group.
Figure 7.
Velocity profile of cerebral blood flow after the MCA occlusion, reperfusion, and Xe-ELIP delivery estimated by using a laser Doppler flowmeter.
Discussion
Over the last two decades, neuroprotective agents designed to block the NMDA receptor have demonstrated promising results in animal models of cerebral ischemia but have failed to produce clinical benefits.29 This lack of efficacy may be related to factors such as delay in administration of the neuroprotective agents beyond 6 hours after stroke onset,29 use of a single agent for complicated neuroprotective therapy,30 inability to achieve sufficiently high local doses in the ischemic area.26 The present study is the first to demonstrate the therapeutic effect of Xe delivery intravenously with ultrasound-enhanced local Xe release after cerebral ischemic injury. This novel strategy with Xe-ELIP may represent a major step in overcoming these obstacles in the management of ischemic and reperfusion brain injury.
ELIP as Theranostic Agents
The encapsulation of therapeutic and diagnostic gases into ELIP provides enhanced echogenicity as an echo contrast agent, as well as a vehicle to deliver a bioactive gas for therapeutic effect. We have previously demonstrated a novel methodology to encapsulate nitric oxide into ELIP with directed local delivery to balloon-injured carotid arteries to attenuate neointimal hyperplasia.21 In the present study, we employed the same gas encapsulation technique and investigated the Xe release profiles. Spontaneous Xe release from Xe-ELIP was observed in two phases; an initial rapid release in the first 10 minutes and a slow release over 18 hours (Fig. 2).
ELIP are effective vehicles for delivering bioactive gases to target tissues with the potential to overcome obstacles in delivering therapeutic gases by inhalation, such as the reduction of partial pressure of inspired oxygen, and toxicity associated with inhaling high concentrations of bioactive gases. Xe delivery efficiency to infarct area by Xe-saturated saline is limited by the solubility of Xe in solution, while Xe delivery by Xe-containing ELIP can be conducted via both soluble and insoluble formats. Xe solubility in solution is 89 µl/ml, while in lipid is 1,853 µl/ml.31, 32 The total amount of Xe loaded in ELIP consists of three components; Xe dissolved in solution (89 µl), Xe in vapor phase (150 µl) and Xe dissolved in lipid bilayer (19 µl) per 1 ml of lipid dispersion (10 mg lipid/ml). Xe-containing ELIP thus provide a more effective strategy for Xe delivery. Gas-containing liposomes, therefore, represent a new class of “theranostic” agents with unique characteristics that allow their visualization in the circulation and delivery of the payload in a site-specific fashion.
In addition, liposomes can be conjugated to ligands for targeted delivery of payload to specific tissues. We have incorporated tissue plasminogen activator (tPA) into our ELIP and demonstrated a formulation that is suitable for fibrin targeting and clot lysis.33–35 We have also demonstrated that the combination of an ultrasound contrast agent and ultrasound treatment can facilitate thrombolysis.34–36 Co-encapsulation of both Xe and tPA into ELIP may provide another approach to targeted treatment of ischemic stroke when both reperfusion and neuroprotection are vital to reduce neuronal damage.
Ultrasound for Therapeutic Gas Release
Drug delivery by intravenous administration has low efficiency due to the large dilation in the blood volume. An alternative delivery method to increase local drug concentration would be intraarterial administration. Intraarterial delivery of tissue plasminogen activator has been used clinically for selected cases of embolic cerebral infarction37 and intraarterial delivery of papaverine for relief of vasospasm after subarachnoid hemorrhage38. Although intraarterial drug administration is efficient, it involves invasive arterial catheterization procedures with potential complication and risk to patients. Intravenous administration of ELIP carrying a neuroprotective gas in combination with ultrasound application over the carotid artery can overcome this problem. In this study, we have demonstrated intravenous Xe-ELIP injection with good effects.
Xe release from circulating Xe-ELIP can be triggered by low power ultrasound exposure. There are two types of ultrasound induced gas release from ELIP; ultrasound-driven diffusion (MI<0.4) or rapid fragmentation (MI>0.4). We have previously demonstrated these ELIP destruction thresholds.39 Rapid fragmentation can be accompanied by cavitation, which has the potential to cause harmful target tissue bioeffects. In this study, low power ultrasound (ultrasound-driven diffusion) was utilized with the ultrasound focused over the ICA, but not directly over the brain tissue. This strategy avoids possible ultrasound bioeffects on the ischemia-compromised microvasculature and neurons.
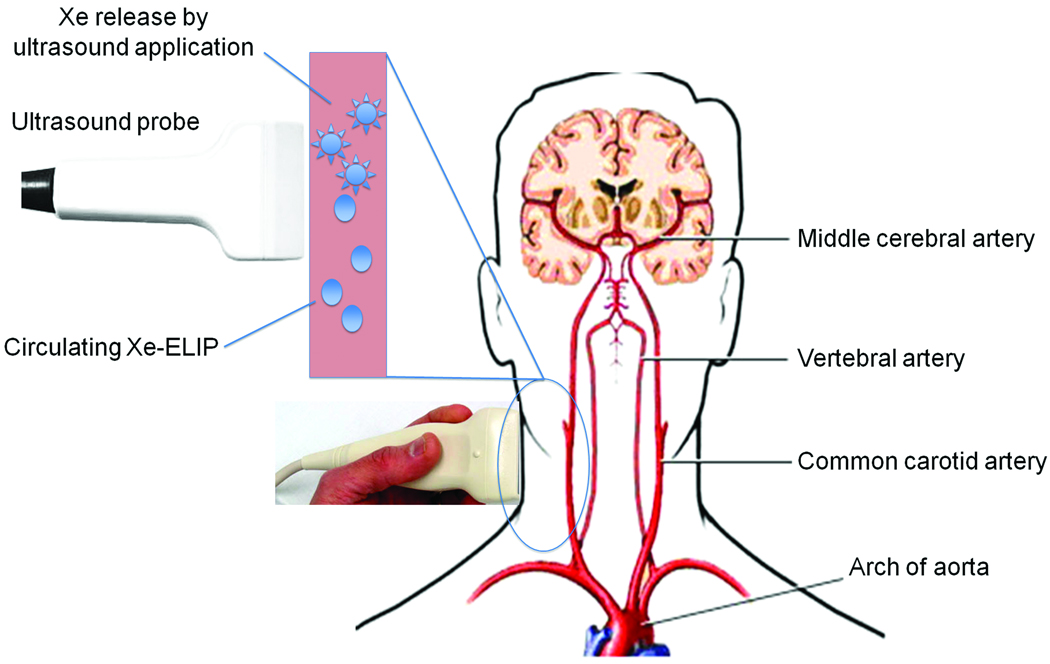
Xe-ELIP are stable under physiological conditions allowing Xe release for hours. This ensures that Xe-ELIP administered into the tail vein can circulate systemically and release Xe locally to the cerebral infarction sites upon ultrasound activation. 1-MHz continuous wave ultrasound with 0.18 MPa peak-to-peak pressure amplitude was able to trigger 44% of Xe release from Xe-ELIP under a flow rate of 10 ml/min (Fig. 3). Xe release from intravenously injected Xe-ELIP resulted in 48% reduction of infarction size, and further increased to 75% reduction following ultrasound enhanced Xe release. This was comparable to the therapeutic effect by intraarterially injected Xe-ELIP (Fig. 6, Table 2). Ultrasound treatment demonstrated further infarct volume reduction only in Xe-ELIP group while there was no ultrasound effect in Xe-saturated solution group. This indicates, with strategic ultrasound activation over the ICA, Xe can be continuously released from circulating Xe-ELIP into the cerebral circulation allowing a therapeutic amount of Xe delivery to ischemic areas (Fig. 8).
Figure 8.
Diagram of a strategy to apply ultrasound treatment over the common carotid artery, a site that is distant from the cerebral infarct site, for both imaging of Xe-ELIP and ultrasound-enhanced therapeutic gas delivery.
Limitations
There are a limited number of methods available to determine Xe concentration in vivo. Although CT or MRI have potential to investigate Xe distribution in vivo, no modality can accurately determine Xe concentration. We injected 200 µl (10 mg/ml) of Xe-ELIP via the tail vein which contained a total of 20 µl Xe for our desired neuroprotective effect in vivo. Our in-vitro testing demonstrated 40% of Xe release from Xe-ELIP upon ultrasound exposure (Fig. 1). We thus speculate that, with ultrasound application, 40% of Xe release from the circulating Xe-ELIP which reached to the ICA via intravenous injection was achieved in our in-vivo studies.
The mechanisms of increased infarct size reduction following Xe release from our Xe-ELIP have not been fully investigated. Previous studies suggest that Xe may share similar characteristics to some NMDA antagonists.20,40,41 The activation of NMDA receptors is a very early event during cascade of ischemic injury and viable neurons in the ischemic penumbra may only be salvageable with early administration of an NMDA antagonist or other neuroprotective agent.29 However in a rat myocardial infarct model, reduction of infarct size following Xe administration was greater than that produced by an NMDA antagonist (MK801), suggesting other mechanisms play a role for Xe protections.42
Conclusions
This study demonstrates a methodology to encapsulate therapeutic gases such as Xe into ELIP using a pressurization-freeze method. Xe-ELIP protected PC12 cells from hypoxic cell death. In an animal model of cerebral ischemia-reperfusion injury, intravenous administration of Xe-ELIP reduced infarct size. Upon ultrasound activation over the carotid artery, Xe release from circulating Xe-ELIP was enhanced with further reduction of infarct size and restoration of sensorimotor function. Intravenous administration of ELIP carrying a neuroprotective gas in combination with ultrasound application over the carotid artery is a novel non-invasive strategy for local therapeutic delivery to modulate cerebral ischemia while minimizing systemic side effects.
Acknowledgments
The authors thank Dianna B. Roberts, Ph.D., at The University of Texas M. D. Anderson Cancer Center, for assisting with the statistical analyses, Roger Strong, M.S., for providing expertise with animal ischemic model, and Jonathan Kopechek, B.S., at The University of Cincinnati for providing expertise with ultrasound characterization.
Funding Sources
This work was in part supported by the American Heart Association (0535512Z, Huang), the University of Texas Health Science Center at Houston (Biotechnology Seed Grant Phase 2, Huang), and the National Institutes of Health (NS067454, Huang; HL074002, HL059586, McPherson; NS047603, Holland).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Stroke is the third leading cause of death and the most common cause of adult disabilities in the United States. The emergent treatment of acute ischemic stroke has two primary goals: rapid restoration of cerebral blood flow (reperfusion) and limitation of neural injury (neuroprotection). Although many therapeutic strategies have demonstrated pharmacologic effect for neuroprotection including their ability to decrease stroke size, these strategies often fail when translated to the clinical setting. Inability to deliver high local doses sufficiently to the ischemic area and inadequate penetration of therapeutic agents across the blood-brain barrier are major contributory factors.
The present study suggests that intravenous delivery of a noble gas, xenon, which readily diffuses across the blood-brain barrier, using a delivery vehicle (liposomes) and a release mechanism (ultrasound) enhances local xenon delivery and decreases stroke size. Site-specific payload release from liposomes can be controlled by low power ultrasound over the common carotid artery with enhanced neuroprotective delivery to the ischemic circulation. This study demonstrated that both intraarterial and intravenous carrier delivery with low power ultrasound activation over the common carotid artery had the same therapeutic effect for neuroprotectant payload delivery to the cerebral ischemic area.
Together this novel delivery vehicle and delivery methodology has the potential to provide a clinically effective and efficient means for therapeutic gas/pharmaceutic delivery for improved stroke treatment.
Disclosures
There are no conflicts to disclose.
References
- 1.Suwanwela NC, Eusattasak N, Phanthumchinda K, Piravej K, Locharoenkul C. Combination of acute stroke unit and short-term stroke ward with early supported discharge decreases mortality and complications after acute ischemic stroke. J Med Assoc Thai. 2007;90:1089–1096. [PubMed] [Google Scholar]
- 2.Adibhatla RM, Hatcher JF. Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets. 2008;7:243–253. doi: 10.2174/187152708784936608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein LB, Rothwell PM. Primary prevention and health services delivery. Stroke. 2007;38:222–224. doi: 10.1161/01.STR.0000254717.89942.67. [DOI] [PubMed] [Google Scholar]
- 4.Wagner KR, Jauch EC. Extending the window for acute stroke treatment: thrombolytics plus CNS protective therapies. Exp Neurol. 2004;188:195–199. doi: 10.1016/j.expneurol.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Catarzi D, Colotta V, Varano F. Competitive Gly/NMDA receptor antagonists. Curr Top Med Chem. 2006;6:809–821. doi: 10.2174/156802606777057544. [DOI] [PubMed] [Google Scholar]
- 6.Kalia LV, Kalia SK, Salter MW. NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol. 2008;7:742–755. doi: 10.1016/S1474-4422(08)70165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipton SA. Pathologically-activated therapeutics for neuroprotection: mechanism of NMDA receptor block by memantine and S-nitrosylation. Curr Drug Targets. 2007;8:621–632. doi: 10.2174/138945007780618472. [DOI] [PubMed] [Google Scholar]
- 8.Parsons CG, Danysz W, Quack G. Glutamate in CNS disorders as a target for drug development: an update. Drug News Perspect. 1998;11:523–569. doi: 10.1358/dnp.1998.11.9.863689. [DOI] [PubMed] [Google Scholar]
- 9.Davis SM, Lees KR, Albers GW, Diener HC, Markabi S, Karlsson G, Norris J. Selfotel in acute ischemic stroke : possible neurotoxic effects of an NMDA antagonist. Stroke. 2000;31:347–354. doi: 10.1161/01.str.31.2.347. [DOI] [PubMed] [Google Scholar]
- 10.Olney JW, Ikonomidou C, Mosinger JL, Frierdich G. MK-801 prevents hypobaric-ischemic neuronal degeneration in infant rat brain. J Neurosci. 1989;9:1701–1704. doi: 10.1523/JNEUROSCI.09-05-01701.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olney JW, Labruyere J, Wang G, Wozniak DF, Price MT, Sesma MA. NMDA antagonist neurotoxicity: mechanism and prevention. Science. 1991;254:1515–1518. doi: 10.1126/science.1835799. [DOI] [PubMed] [Google Scholar]
- 12.Arrowsmith JE, Harrison MJ, Newman SP, Stygall J, Timberlake N, Pugsley WB. Neuroprotection of the brain during cardiopulmonary bypass: a randomized trial of remacemide during coronary artery bypass in 171 patients. Stroke. 1998;29:2357–2362. doi: 10.1161/01.str.29.11.2357. [DOI] [PubMed] [Google Scholar]
- 13.Derwall M, Coburn M, Rex S, Hein M, Rossaint R, Fries M. Xenon: recent developments and future perspectives. Minerva Anestesiol. 2009;75:37–45. [PubMed] [Google Scholar]
- 14.David HN, Ansseau M, Lemaire M, Abraini JH. Nitrous oxide and xenon prevent amphetamine-induced carrier-mediated dopamine release in a memantine-like fashion and protect against behavioral sensitization. Biol Psychiatry. 2006;60:49–57. doi: 10.1016/j.biopsych.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Dworschak M. Pharmacologic neuroprotection--is xenon the light at the end of the tunnel? Crit Care Med. 2008;36:2477–2479. doi: 10.1097/CCM.0b013e31818113d2. [DOI] [PubMed] [Google Scholar]
- 16.Fries M, Nolte KW, Coburn M, Rex S, Timper A, Kottmann K, Siepmann K, Hausler M, Weis J, Rossaint R. Xenon reduces neurohistopathological damage and improves the early neurological deficit after cardiac arrest in pigs. Crit Care Med. 2008;36:2420–2426. doi: 10.1097/CCM.0b013e3181802874. [DOI] [PubMed] [Google Scholar]
- 17.Ma J, Zhang GY. Lithium reduced N-methyl-D-aspartate receptor subunit 2A tyrosine phosphorylation and its interactions with Src and Fyn mediated by PSD-95 in rat hippocampus following cerebral ischemia. Neurosci Lett. 2003;348:185–189. doi: 10.1016/s0304-3940(03)00784-5. [DOI] [PubMed] [Google Scholar]
- 18.Palmer GC, Widzowski D. Low affinity use-dependent NMDA receptor antagonists show promise for clinical development. Amino Acids. 2000;19:151–155. doi: 10.1007/s007260070043. [DOI] [PubMed] [Google Scholar]
- 19.Petzelt C, Blom P, Schmehl W, Muller J, Kox WJ. Prevention of neurotoxicity in hypoxic cortical neurons by the noble gas xenon. Life Sci. 2003;72:1909–1918. doi: 10.1016/s0024-3205(02)02439-6. [DOI] [PubMed] [Google Scholar]
- 20.Yamakura T, Harris RA. Effects of gaseous anesthetics nitrous oxide and xenon on ligand-gated ion channels. Comparison with isoflurane and ethanol. Anesthesiology. 2000;93:1095–1101. doi: 10.1097/00000542-200010000-00034. [DOI] [PubMed] [Google Scholar]
- 21.Huang SL, Kee PH, Kim H, Moody MR, Chrzanowski SM, Macdonald RC, McPherson DD. Nitric oxide-loaded echogenic liposomes for nitric oxide delivery and inhibition of intimal hyperplasia. J Am Coll Cardiol. 2009;54:652–659. doi: 10.1016/j.jacc.2009.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Britton GL, Kee PH, McPherson DD, Huang SL. Novel Drug Delivery for Neuroprotection Against Ischemic Insults. Ann Neurol. 2008;64:S13. [Google Scholar]
- 23.Huang SL, Hamilton AJ, Pozharski E, Nagaraj A, Klegerman ME, McPherson DD, MacDonald RC. Physical correlates of the ultrasonic reflectivity of lipid dispersions suitable as diagnostic contrast agents. Ultrasound Med Biol. 2002;28:339–348. doi: 10.1016/s0301-5629(01)00512-9. [DOI] [PubMed] [Google Scholar]
- 24.Zhao X, Sun G, Zhang J, Strong R, Song W, Gonzales N, Grotta JC, Aronowski J. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol. 2007;61:352–362. doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
- 25.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 26.Wahlgren NG, Diez-Tejedor E, Teitelbaum J, Arboix A, Leys D, Ashwood T, Grossman E. Results in 95 hemorrhagic stroke patients included in CLASS, a controlled trial of clomethiazole versus placebo in acute stroke patients. Stroke. 2000;31:82–85. doi: 10.1161/01.str.31.1.82. [DOI] [PubMed] [Google Scholar]
- 27.Zhao X, Liu SJ, Zhang J, Strong R, Aronowski J, Grotta JC. Combining insulin-like growth factor derivatives plus caffeinol produces robust neuroprotection after stroke in rats. Stroke. 2005;36:129–134. doi: 10.1161/01.STR.0000149624.87661.18. [DOI] [PubMed] [Google Scholar]
- 28.Siegel S, Castellan NJ. Nonparametric statistics for the behavioral sciences. 2nd ed. New York: McGraw-Hill; 1988. pp. 213–215. [Google Scholar]
- 29.Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher M, Ratan R. New perspectives on developing acute stroke therapy. Ann Neurol. 2003;53:10–20. doi: 10.1002/ana.10407. [DOI] [PubMed] [Google Scholar]
- 31.Ladefoged J, Andersen AM. Solubility of Xenon-133 at 37°C in Water, Saline, Olive Oil, Liquid Paraffin, Solutions of Albumin, and Blood. Physics in Medicine and Biology. 1967;12:353. [Google Scholar]
- 32.Yeh SY, Peterson RE. Solubility of carbon dioxide, krypton, and xenon in lipids. J Pharm Sci. 1963;52:453–458. doi: 10.1002/jps.2600520511. [DOI] [PubMed] [Google Scholar]
- 33.Klegerman ME, Zou Y, McPherson DD. Fibrin targeting of echogenic liposomes with inactivated tissue plasminogen activator. J Liposome Res. 2008;18:95–112. doi: 10.1080/08982100802118482. [DOI] [PubMed] [Google Scholar]
- 34.Shaw GJ, Meunier JM, Huang SL, Lindsell CJ, McPherson DD, Holland CK. Ultrasound-enhanced thrombolysis with tPA-loaded echogenic liposomes. Thrombosis research. 2009;124:306–310. doi: 10.1016/j.thromres.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tiukinhoy-Laing SD, Huang S, Klegerman M, Holland CK, McPherson DD. Ultrasound-facilitated thrombolysis using tissue-plasminogen activator-loaded echogenic liposomes. Thrombosis research. 2007;119:777–784. doi: 10.1016/j.thromres.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Datta S, Coussios CC, Ammi AY, Mast TD, de Courten-Myers GM, Holland CK. Ultrasound-enhanced thrombolysis using Definity as a cavitation nucleation agent. Ultrasound Med Biol. 2008;34:1421–1433. doi: 10.1016/j.ultrasmedbio.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juttler E, Kohrmann M, Schellinger PD. Therapy for early reperfusion after stroke. Nat Clin Pract Cardiovasc Med. 2006;3:656–663. doi: 10.1038/ncpcardio0721. [DOI] [PubMed] [Google Scholar]
- 38.Brisman JL, Eskridge JM, Newell DW. Neurointerventional treatment of vasospasm. Neurol Res. 2006;28:769–776. doi: 10.1179/016164106X152043. [DOI] [PubMed] [Google Scholar]
- 39.Smith DA, Porter TM, Martinez J, Huang S, MacDonald RC, McPherson DD, Holland CK. Destruction thresholds of echogenic liposomes with clinical diagnostic ultrasound. Ultrasound Med Biol. 2007;33:797–809. doi: 10.1016/j.ultrasmedbio.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 40.Franks NP, Dickinson R, de Sousa SL, Hall AC, Lieb WR. How does xenon produce anaesthesia? Nature. 1998;396:324. doi: 10.1038/24525. [DOI] [PubMed] [Google Scholar]
- 41.Jevtovic-Todorovic V, Todorovic SM, Mennerick S, Powell S, Dikranian K, Benshoff N, Zorumski CF, Olney JW. Nitrous oxide (laughing gas) is an NMDA antagonist, neuroprotectant and neurotoxin. Nat Med. 1998;4:460–463. doi: 10.1038/nm0498-460. [DOI] [PubMed] [Google Scholar]
- 42.Ma D, Yang H, Lynch J, Franks NP, Maze M, Grocott HP. Xenon attenuates cardiopulmonary bypass-induced neurologic and neurocognitive dysfunction in the rat. Anesthesiology. 2003;98:690–698. doi: 10.1097/00000542-200303000-00017. [DOI] [PubMed] [Google Scholar]