Abstract
Human studies show that the number of teenagers abusing anabolic androgenic steroids (AAS) is increasing. During adolescence, brain development is altered by androgen exposure, which suggests that AAS may potentially alter central nervous system development. The goal of the present study was to determine whether pubertal AAS exposure increased dendritic spine densities on neurons within the medial amygdala and the dorsal hippocampus. Pubertal gonadally intact male rats received the AAS testosterone propionate (5mg/kg) or vehicle for 5 days/week for four weeks. To determine the long-term implications of pubertal AAS use, another set of males received the same AAS treatment and were then withdrawn from AAS exposure for 4 weeks. Results showed that pubertal AAS exposure significantly increased spine densities on neurons in the anterior medial amygdala, posterodorsal medial amygdala, and the CA1 region of the hippocampus compared to gonadally intact control males. Spine densities returned to control levels within the anterior medial amygdala and the posterodorsal medial amygdala 4 weeks after withdrawal. However, spine densities remained significantly elevated after AAS withdrawal in the CA1 region of the hippocampus, suggesting that pubertal AAS exposure may have a long-lasting impact on CA1 hippocampal neuroanatomy. Since pubertal AAS exposure increased spine densities and most excitatory synapses in the central nervous system occur on dendritic spines, AAS may increase neuronal excitation. It is proposed that this increase in excitation may underlie the behavioral responses seen in pubertal AAS-treated male rats.
Keywords: Testosterone, Medial Amygdala, Hippocampus, Withdrawal, DiI
Anabolic androgenic steroid (AAS) use by human adolescent males is on the rise (Burnett and Kleiman, 1994, DuRant et al., 1995, Melia et al., 1996). This is of particular concern because puberty is a time when increasing endogenous hormone levels influence brain maturation (Primus and Kellogg, 1990, Sisk et al., 2003), which may be adversely affected by AAS exposure.
Two brain regions that have been found to be sensitive to pubertal hormones in both human and animal studies are the hippocampus and amygdala (Meyer et al., 1978, Giedd et al., 1996a, Giedd et al., 1996b, Romeo and Sisk, 2001, Giedd et al., 2006, Zehr et al., 2006). The volume of the amygdala and hippocampus has been shown to increase with age in human adolescents (Giedd et al., 1996a, Giedd et al., 1996b). Additionally, after puberty rodent males have an increased posterodorsal medial amygdala volume (Romeo and Sisk, 2001, Cooke et al., 2002, Zehr et al., 2006). Furthermore, hippocampal CA1 spine density levels increase throughout puberty, and castration before puberty can abolish this increase in male rodents (Meyer et al., 1978). Therefore, these two brain regions are clearly sensitive to AAS exposure during puberty.
Both androgens and estrogens have been shown to affect the density of dendritic spines in the amygdala and the hippocampal formation (Woolley et al., 1990, Johansen et al., 2004, Cooke and Woolley, 2005, Garza-Meilandt et al., 2006). For example, estradiol treatment has been shown to increase neuronal spine density within the posterodorsal medial amygdala (Gomez and Newman, 1991, Cooke and Woolley, 2005) and pyramidal neuronal spine density in the CA1 region of the dorsal hippocampus of adult ovariectomized female rats (Gould et al., 1990, Woolley et al., 1990, Cooke and Woolley, 2005, Garza-Meilandt et al., 2006). Recently, it has been found that exogenous testosterone treatment increased the number of spines on neurons in the medial amygdala and the number of spine synapse densities, which can reflect an increase in spine density, in the hippocampus of adult castrated male rats (Fowler et al., 2003, Leranth et al., 2003).
It has been previously shown that many of the effects of testosterone are due to its conversion to estrogen (Gorski, 1985). However, several studies suggest that testosterone is more salient in male brain morphology than estrogen. For example, male rats typically have a higher density of dendritic spines on neurons in the medial amygdala than female rats (McDonald, 1992). Furthermore, castration of adult male rats or monkeys resulted in decreased CA1 pyramidal spine synapse density, and testosterone or dihydrotestosterone (DHT) exposure restored spine synapse density levels to control levels (Leranth et al., 2003), whereas estrogen administration had no effect on restoring spine synapse density levels in male rats (Leranth et al., 2003).
The goal of this study was to determine the effects of pubertal exposure to chronic high doses of the widely abused AAS testosterone (Pope and Katz, 1994, Mottram and George, 2000) on dendritic spines on neurons in the medial amygdala and the CA1 region of the dorsal hippocampus. Since, both human and animal studies have shown that pubertal AAS exposure has varied effects on mood, such as increased irritability and aggression (Kopera, 1985, Kindlundh et al., 2001, Thiblin and Parlklo, 2002, Farrell and McGinnis, 2003a, Wesson and McGinnis, 2006), pubertal exposure to AAS may alter the central nervous system. Therefore, we hypothesized that gonadally intact males exposed to chronic high doses of AAS during puberty would show increased spine densities on neurons within the anterior medial amygdala, posterodorsal medial amygdala, and the CA1 region of the dorsal hippocampus compared to gonadally intact controls treated with vehicle. Additionally, we predicted that the proposed increased in spine densities would remain following withdrawal from pubertal AAS exposure.
Experimental Procedures
Animals
Long Evans male rats were purchased from Charles River Laboratory (Wilmington, MA) and received on postnatal day 38. Animals were housed in a temperature-controlled room (23°C) in standard Plexiglas cages (25 × 20 × 18 cm) with ad libitum access to food and water. Lights were maintained on a 12:12 hour reversed light/dark cycle, with lights off at 1200h. Body weights were recorded weekly and found not to be significantly different from controls (AAS males, 348.9± 13.8g Control males, 368.5± 7.9g, AAS withdrawal males, 451.4± 26.5g, Control withdrawal males, 484.8± 10.3g). Experimental procedures were performed in accordance with National Institutes of Health’s guidelines for animal care and use.
Anabolic Androgenic Steroid Treatment
Males were randomly assigned to one of two treatment groups: 4 week pubertal exposure to the AAS testosterone (n=9) or vehicle control (n=8) (polyethylene glycol 200). In addition, 3 rats from each treatment group were withdrawn from the experimental treatment for 4 weeks to examine the long-term effects of pubertal AAS exposure. A four-week withdrawal period from pubertal AAS exposure has previously been shown to be sufficient to allow the endocrine system to return to a physiological state, as evidenced by similar wet testes and prostate weights between AAS withdrawal males and vehicle control males (Feinberg et al., 1997).
AAS (Sigma, St. Louis, MO) exposure was initiated on postnatal day 40, which is the time of preputial separation (Korenbrot et al., 1977). Testosterone propionate or vehicle control was subcutaneously injected at a dosage of 5mg/kg body weight five days a week for four weeks (Farrell and McGinnis, 2003a, Cunningham and McGinnis, 2006, Wesson and McGinnis, 2006), which is comparable to the AAS levels (10-100 times physiological testosterone levels) abused by humans (Pope and Katz, 1988, Bahrke et al., 1990, Bahrke et al., 1998, Mottram and George, 2000). In male rats this dosage of testosterone results in several endocrine changes, such as ~30 fold increase in serum testosterone levels, decreased testes weights, and increased seminal vesicles and prostate weights (Feinberg et al., 1997, Farrell and McGinnis, 2003b).
Brain Regions
The anterior medial amygdala, posterodorsal medial amygdala, and the CA1 region of the dorsal hippocampus of the brain were selected for spine density analysis (Figure 1). The CA1 region of the hippocampus has been shown to be sensitive to testosterone, as determined by the decrease in spine synapse density following castration in adult male rats (Leranth et al., 2003). In addition, both sub-regions of the medial amygdala, anterior and posterodorsal, undergo changes in volume throughout puberty, which is a critical period during which testosterone levels increase (Romeo and Sisk, 2001).
Figure 1.
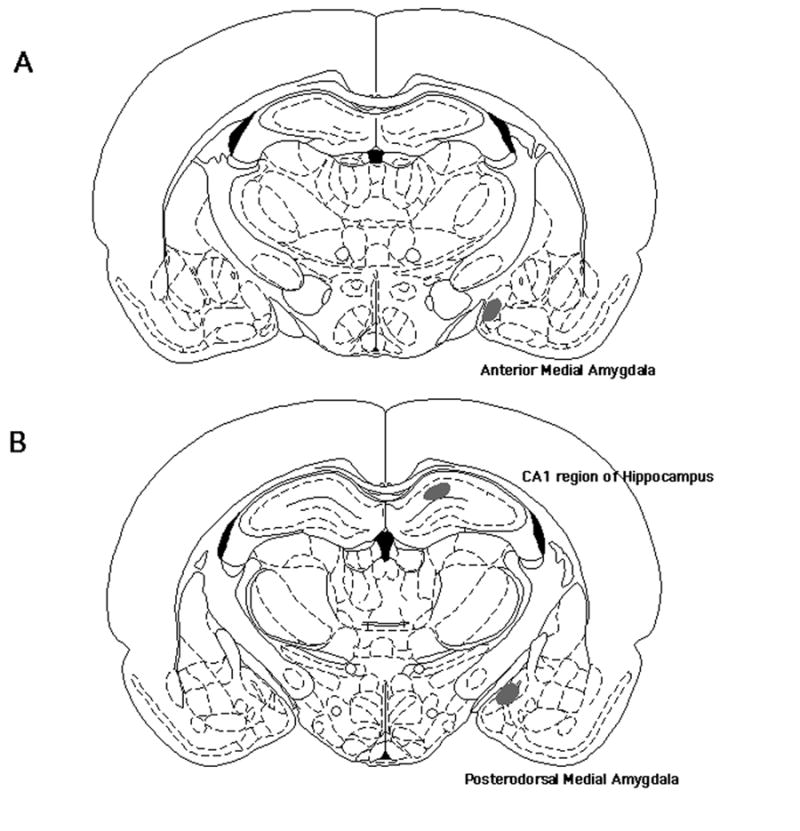
Shaded regions indicate the placement of DiI crystals within the anterior medial amygdala (A), posterodorsal medial amygdala (B), and CA1 region of the dorsal hippocampus (B).
Fixation of Brain Tissue
The animals were sacrificed by a lethal administration of pentobarbital at a dose of 50 mg/kg i.p., flushed and perfused with lactated Ringers (Brawn Medical Inc, Irvine, CA) and 4% paraformaldehyde in 0.1M phosphate buffered saline (PBS). Immediately following decapitation, the brains were removed and immersed in ice-cold 4% paraformaldehyde in 0.1M PBS for 1 hour, followed by 6 washes of 0.1M PBS every 5 minutes. After washing the brain, the tissue was sectioned at 300 μm on a Lancer vibratome (Lancer, St. Louis, MO). The thickness of the tissue was chosen to minimize the number of severed dendrites within the brain slices (Claiborne et al., 1990, Garza-Meilandt et al., 2006).
DiI Neuronal Labeling
The neurons and associated dendritic components in the perfused brains were labeled with 1,1,1’-dioctadecyl-3,3,3,3’ tetramethlindocarbocyanine percholate (DiI, Molecular Probes, Eugene, OR), a lipophilic carbocyanine dye. In perfused tissue the DiI can travel 0.4mm/day and is confined to the membrane that has absorbed it (Balthazart et al., 1994). Crushed crystals of DiI were placed on the tip of a micropipette. The micropipette was placed on a manipulator that was used to advance the micropipette into the tissue. Crystals were placed in three different locations within each brain region. In CA1 region of the dorsal hippocampus, crystals were placed along the transverse axis of the stratum lacunosum-moleculare. Also, in both the anterior and posterodorsal medial amygdala, crystals were place in the dorsal, middle, and ventral regions.
Once the DiI crystals were placed in the appropriate regions, the sections were stored in a vial containing a 2% paraformaldehyde and 0.1M phosphate buffer (7.4 pH) solution. The vial was placed in the dark at room temperature for a three-day incubation period (Garza-Meilandt et al., 2006).
Visualization and Analysis of Dendritic Spine Densities
Both the anterior and posterodorsal medial amygdala segments were sampled from the secondary dendrites of bipolar or multipolar cells (Rasia-Filho et al., 1999). There are many types of neurons in the medial amygdala, but the majority (~ 85%) of these neurons are pyramidal neurons with long branches (Rasia-Filho et al., 1999, Muller et al., 2006). Since these neurons can be easily visualized with DiI, they were selected for study. In the dorsal hippocampus, spine densities were analyzed on secondary apical dendrites of the pyramidal cells within the stratum radiatum of the CA1 region (Galea et al., 1997, Danzer et al., 1998). Segments were sampled bilaterally. Utilizing secondary segments for each brain region allowed for uniformity across all dendritic segments.
The labeled neurons were randomly assigned numeric identifiers for analysis. This procedure allowed the investigator to analyze the segments without bias. The dendrites were imaged by utilizing a scanning confocal laser microscope (Bio-Rad, Hercules, CA) mounted on a Nikon Labophot-2 microscope equipped with a fluorescence emission laser and a 60x oil immersion objective (Zeiss). The fluorescent laser was set at 568-nm emission length, allowing visualization of the DiI-labeled segments. Only intensely and discretely labeled segments of secondary branches of pyramidal cells were selected for analysis. Additionally, another criterion for analysis was that the chosen segment be at least 20 μm in length and 100 μm from the cell body. Each segment was imaged by making scanning sequential scans at 0.3 μm z-step intervals.
Spine density was analyzed by an investigator, blind to the treatment groups, using an Igor-based spine counting program (WaveMetric, Inc, Lake Oswego, OR). Examination of individual segments was performed by the investigator scrolling through the individual confocal scans and placing a mark by each visible spine. Spines were categorized as dendritic protrusions that were at least 0.25 μm in length (Desmond and Levy, 1985) and were observed as separate protrusions in one or more scans. The number of spines was summed and the dendritic length in three dimensions was quantified to determine the number of visible spines per dendritic micrometer (Garza-Meilandt et al., 2006). No correction factor was applied for hidden spines (Woolley and McEwen, 1993).
Spines within the medial amygdala are of three types: small bulb on a thin stalk, mushroom-shaped, and thin stalk with two or more branched heads (Rall, 1974). Dendritic spines within the stratum radiatum layer of the CA1 region of the dorsal hippocampus are thin, stubby, or mushroom-shaped (Desmond and Levy, 1985, Harris et al., 1992). The dendritic spines of neurons in both the medial amygdala and the CA1 region of the dorsal hippocampus observed within this experiment were consistent with these structural characteristics. However, within this study dendritic spines were not categorized based on shape (Rall, 1974, Desmond and Levy, 1985, Harris et al., 1992)
A total of 6 dendritic segments from different neurons were quantified for each brain region per rat. Therefore the dendritic segments for 6 animals totaled 36 segments per brain region. Dendritic segments for 8 control animals totaled 48 segments per brain region. For three animals in the AAS withdrawal group, there were a total of 18 segments in each brain region. Average segment length was approximately 30 μm.
Statistical Analysis
Spine densities from all segments in each brain region were averaged per animal. The spine density was analyzed with either a Kruskal-Wallis or Kolmogorov-Smirnov non-parametric tests to determine main effects, followed by Tukey/Kramer test for post hoc comparisons (Statview 5.0). Data were displayed as mean ± SEM. P values ≤ 0.05 were designated as significant.
Results
Kolmogorov-Smirnov analysis of age on spine density levels between control males and 4 week withdrawal control males showed similar means between groups with no significant differences, regardless of nuclei. Therefore, spine densities from these two control groups were compiled into one overall control group for further analysis.
Anterior Medial Amygdala
There was a significant overall effect of pubertal AAS treatment on spine densities of neurons within the anterior medial amygdala (Kruskal-Wallis, p<0.018). Post hoc analysis of spine densities revealed that pubertal AAS treatment significantly increased spine densities from 1.48 spines/μm to 2.33 spines/μm (Tukey/Kramer, p<0.05) (Figure 2). Following withdrawal from AAS, spine densities were not significantly different from controls. Figure 3 illustrates representative dendritic segments from the anterior medial amygdala showing that spine densities increased in AAS treated males compared to gonadally intact control males (Figure 3, A vs. B), and returned to control levels after four weeks of AAS withdrawal (Figure 3, C vs. A).
Figure 2.
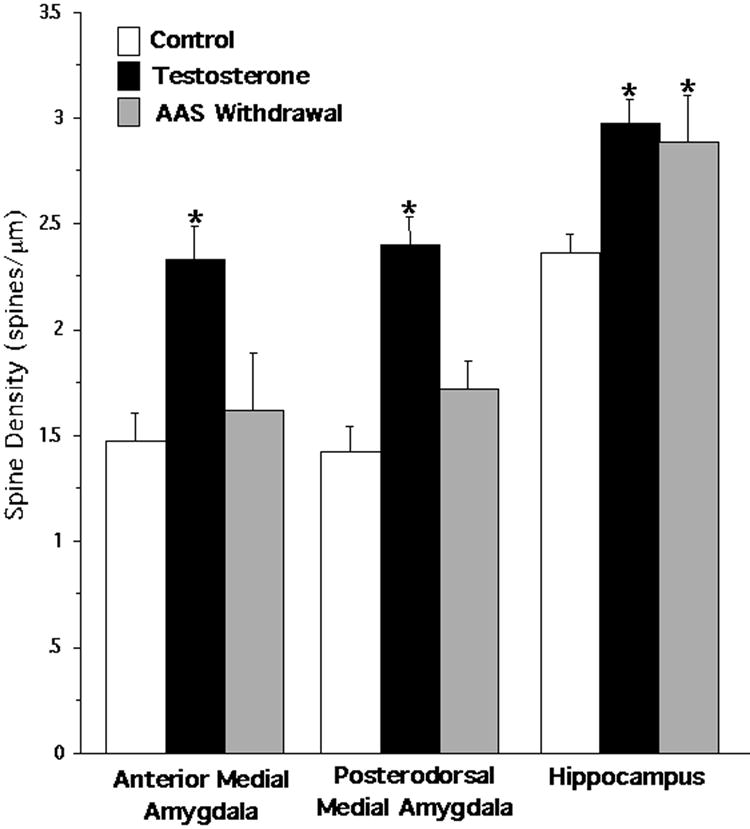
Spine density (mean ± SEM) resulting from three treatment conditions: vehicle control (n = 8), AAS testosterone (n = 6), and AAS withdrawal (n = 3). Secondary dendritic segments originating from three nuclei were analyzed: anterior medial amygdala, posterodorsal medial amygdala, and the CA1 region of the dorsal hippocampus. Spine densities within the anterior medial amygdala and the posterodorsal medial amygdala were significantly higher after AAS testosterone treatment compared to control (Tukey/Kramer, * = p<0.05). Spine densities returned to control levels within both regions of the medial amygdala following AAS withdrawal. AAS testosterone significantly increased spine densities in the CA1 region compared to control, and spine densities remained elevated after 4 weeks of AAS withdrawal (* = p<0.05).
Figure 3.
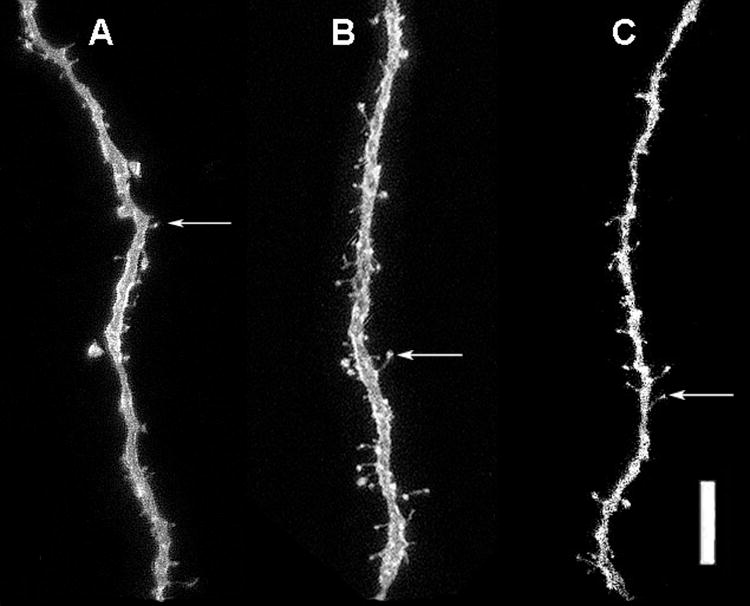
Representative photomicrographs of DiI-labeled dendrite segments of neurons in the anterior medial amygdala in the 3 treatment conditions: vehicle control (A), AAS testosterone (B), and AAS withdrawal (C). Example of a dendritic spine is indicated by arrow. Scale bar = 10 μm.
Posterodorsal Medial Amygdala
The same pattern of AAS-induced spine density alterations was found in the posterodorsal medial amygdala. There was a significant overall effect of AAS treatment on spine densities on neurons in the posterodorsal medial amygdala (Kruskal-Wallis, p<0.005). Figure 2 shows that pubertal AAS exposure significantly increased spine densities from 1.43 spines/μm to 2.40 spines/μm (Tukey/Kramer, p<0.05), but there were no significant differences between AAS withdrawal and control spine densities. Figure 4 depicts representative dendritic segments from the posterodorsal medial amygdala showing that pubertal AAS exposure increased spine densities and that spine density levels returned to control levels after 4 weeks from withdrawal from AAS.
Figure 4.
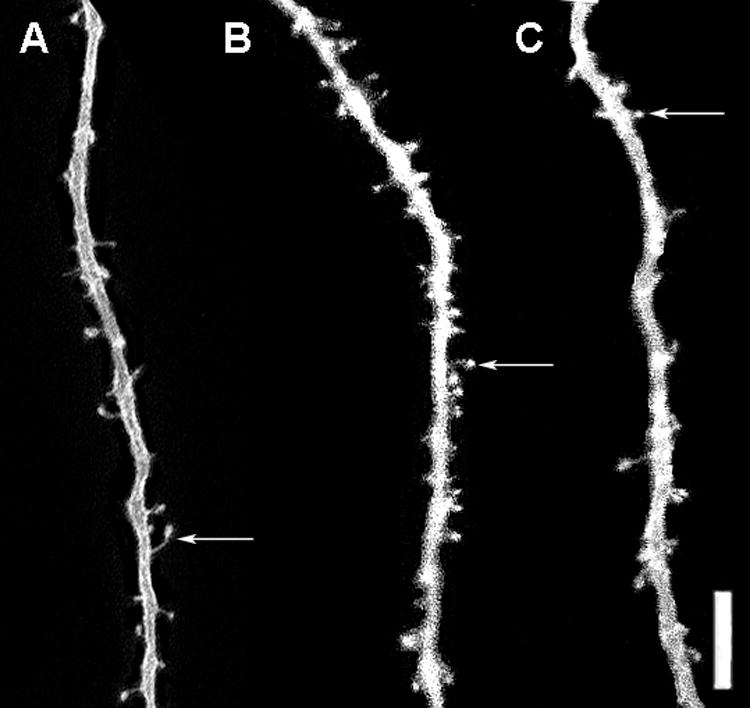
Representative photomicrographs of DiI-labeled dendrite segments of neurons in the posterodorsal medial amygdala in the 3 treatment conditions: vehicle control (A), AAS testosterone (B), and AAS withdrawal (C). Example of a dendritic spine is indicated by arrow. Scale bar = 10 μm
CA1 Region of the Dorsal Hippocampus
There was a significant overall effect of AAS treatment on hippocampal spine densities (Kruskal-Wallis, p<0.006). Post hoc analysis showed that pubertal AAS exposure significantly increased spine density levels in the CA1 region of the dorsal hippocampus from 2.37 spines/μm to 2.98 spines/μm (Tukey/Kramer, p<0.05), and spine densities continued to be significantly elevated at 2.89 spines/μm following four weeks of AAS withdrawal (p<0.05) (Figure 2). Representative dendritic segments from the stratum radiatum of the CA1 region of the dorsal hippocampus are seen in Figure 5. Spine densities were increased as a result of pubertal AAS exposure (Figure 5, B vs. A), and remained elevated four weeks after AAS withdrawal compared to controls (Figure 5, C vs. A).
Figure 5.
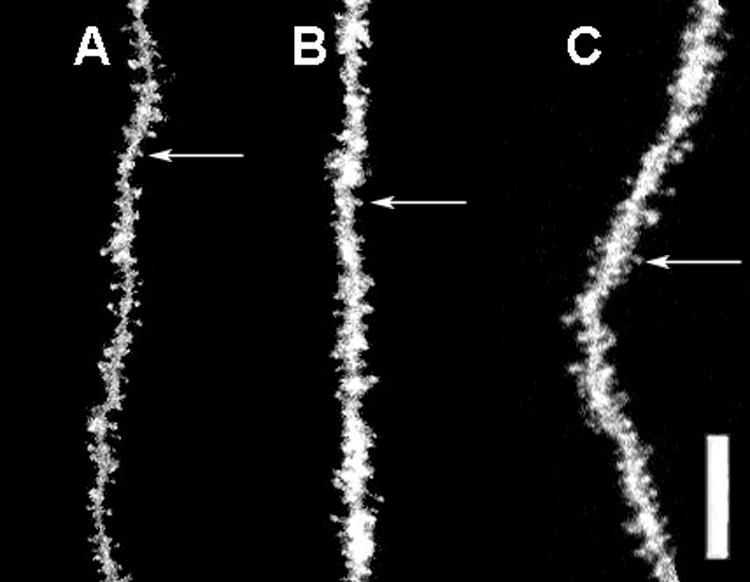
Representative photomicrographs of DiI-labeled dendrite segments of neurons in the CA1 region of the dorsal hippocampus in the 3 treatment conditions: vehicle control (A), AAS testosterone (B), and AAS withdrawal (C). Example of a dendritic spine is indicated by arrow. Scale bar = 10 μm
The micrographs in Figures 3-5 are composite images from a series of confocal scans. Although it may appear at times that the dendrite diameter was altered, it was actually an artifact of superimposing several confocal scans into one composite image. At a confocal microscopic level, no structural differences in dendritic spine or dendritic diameter were observed in any of the treatment groups.
Discussion
This study quantified dendritic spines on neurons to study the effects of pubertal exposure to the AAS testosterone within the medial amygdala and the hippocampus. In the current study, we demonstrated that pubertal AAS exposure increased spine density on neurons within the anterior medial amygdala, posterodorsal medial amygdala, and the CA1 region of the dorsal hippocampus in male rats compared to gonadally intact control males. Additionally, we found that pubertal AAS exposure had long-term effects on spine density levels within the CA1 region of the dorsal hippocampus. Spine densities remained elevated four weeks following pubertal AAS withdrawal.
Spine density levels for gonadally intact control males were consistent with spine density levels reported in previous studies (Trommald et al., 1995, Rasia-Filho et al., 1999, Markham et al., 2005). For example, spine densities on neurons for gonadally intact control males in this study were 1.4 spines/μm in the medial amygdala compared to 1.9 spines/μm reported by Rasia-Filho (Rasia-Filho et al., 1999). Also, a spine density of 2.37 spines/μm in the CA1 region of the dorsal hippocampus was similar to spine densities of 2.0 spines/μm reported by Trommald (Trommald et al., 1995) and 2.8 spines/μm by Markham (Markham et al., 2005).
It has been postulated that spine densities can be a measure of excitatory potential (Nuriya et al., 2006), since excitatory synapses are usually made on spines (Nimchinsky et al., 2002). Moreover, testosterone has been shown to enhance excitation of neuronal parameters. For example, testosterone application has been shown to decrease the absolute refractory period of neurons in the medial amygdala, thus resulting in increased neuronal excitation (Kendrick and Drewett, 1979, Kendrick and Drewett, 1980). Also, in vitro 20 minute bath application of testosterone increased the frequency of excitatory post synaptic potentials (EPSPs) in hippocampal CA1 pyramidal neurons from castrated adult males and ovariectomized adult females (Smith et al., 2002). However, when male rats are castrated during puberty and given replacement testosterone, suppressive effects on hippocampal CA1 long term potentiation (LTP), another measure of excitation, have been noted (Harley et al., 2000, Hebbard et al., 2003). These studies suggest that testosterone can influence neuronal activity by different mechanisms, which may underlie the process whereby testosterone lowers the threshold to respond to stimuli.
Interestingly, a temporal parallel linking spine density and aggressive behaviors can be noted. During puberty, AAS exposure increased spine densities on neurons in the medial amygdala and the hippocampus and also increased aggression displayed by intact male rats (Farrell and McGinnis, 2004). This aggressive behavior has been linked to the medial amygdala (Masco and Carrer, 1990, Wood and Coolen, 1997, Grimes et al., 2003, David et al., 2004, Giammanco et al., 2005).
In further support of this premise, long-term behavioral effects have been found in male rats pubertally exposed to AAS. For instance, aggression remained elevated several weeks after withdrawal from pubertal AAS exposure in adult male rats, which may be a result of learned behavior (Farrell and McGinnis, 2004). This behavior is consistent with the time frame that pubertal AAS exposure increased hippocampal spine density, which has been associated with learning and memory (Moser et al., 1994, Jarrard, 1995, Leuner and Shors, 2004). Interestingly, findings from an adult AAS exposure study showed that aggression declined to gonadally intact control levels within three weeks after withdrawal from adult AAS exposure, in contrast to the permanent effects of pubertal AAS exposure (McGinnis et al., 2002). Consequently, the increased spine density on neurons in the medial amygdala and the hippocampus from AAS exposure during the critical period of puberty may play an important role in AAS-induced behaviors.
It is possible that pubertal AAS exposure may have a more global impact on dendritic structure than just spine density as was examined in the current study. In vitro studies using androgen receptor transfected PC12 cells showed that androgens increased neurite length and branching (Lustig, 1994, Lustig et al., 1994). Furthermore, an in vivo study in adult male rats showed that testosterone increased dendritic surface area and dendritic branching of preoptic/anterior hypothalamic area neurons (Cherry et al., 1992). Interestingly, this study used supraphysiological levels of testosterone propionate (5mg/kg body weight) (Cherry et al., 1992), which is actually the same dosage of testosterone propionate used in the current AAS study. Results from these studies in conjunction with our results demonstrate that testosterone can alter neuronal structure, and thus possibly affect neuronal function by increasing the receptivity of neurons.
Notably, it has also been found that gonadal hormones, such as estrogen, can increase spine synapse density by altering the amount of presynaptic inputs per CA1 hippocampal dendritic spine (Yankova et al., 2001), thus further increasing neuronal receptivity to multiple hippocampal pyramidal neurons. Furthermore, it is known that androgens can increase hippocampal spine synapse density (Leranth et al., 2003), but it is not known if androgens also alter the amount of presynaptic inputs per CA1 dendritic spine. Further studies will need to be conducted to determine if pubertal AAS exposure affects other dendritic parameters, such as dendritic length, dendritic branching, and afferent inputs of neurons within the amygdala and hippocampus. Moreover, increased lengths of withdrawal from pubertal AAS exposure needs to be examined to determine the saliency of pubertal AAS exposure on neuronal parameters.
This study is the first empirical demonstration of neuroanatomical changes resulting from pubertal AAS exposure. Both the medial amygdala and the hippocampus showed increased spine densities on neurons as a result of pubertal AAS exposure. Notably, spine densities within the hippocampus did not return to gonadally intact control spine density levels after four weeks of AAS withdrawal, suggesting that pubertal AAS exposure has long-term effects on hippocampal morphology. Since pubertal AAS exposure increases dendritic spine density during brain maturation, the effects of AAS abuse by human adolescents may result in enduring changes in brain function.
Acknowledgments
We would like to thank Drs. Augustus R. Lumia and J. Thomas Cunningham for their insightful comments on the manuscript. We appreciate the expert technical advice Mr. Michael O’Boyle and Mr. Natividad Ybarra. This research was supported by NIH Grant DA 10886 to M.Y.M.
Comprehensive List of Abbreviations
- AAS
Anabolic Androgenic Steroid
- CA1
Cornu Ammonis Region 1
- DHT
Dihydrotestosterone
- DiI
1,1,1’-dioctadecyl-3,3,3,3’ tetramethlindocarbocyanine percholate
- PBS
Phosphate Buffered Saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bahrke MS, Yesalis CE, Brower KJ. Anabolic-androgenic steroid abuse and performance-enhancing drugs among adolescents. Child Adolesc Psychiatr Clin N Am. 1998;7:821–838. [PubMed] [Google Scholar]
- Bahrke MS, Yesalis CEI, Wright JE. Psychological and behavioral effects of endogenous testosterone levels and anabolic-androgenic steroids among males: a review. Sports Med. 1990;10:303–337. doi: 10.2165/00007256-199010050-00003. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Dupiereux V, Aste N, Viglietti-Panzica C, Barrese M, Panzica GC. Afferent and efferent connections of the sexually dimorphic medial preoptic nucleus of the male quail revealed by in vitro transport of DiI. Cell Tissue Res. 1994;276:455–475. doi: 10.1007/BF00343944. [DOI] [PubMed] [Google Scholar]
- Burnett KF, Kleiman ME. Psychological characteristics of adolescent steroid users. Adolescence. 1994;29:81–89. [PubMed] [Google Scholar]
- Cherry JA, Tobet SA, DeVoogd TJ, Baum MJ. Effects of sex and androgen treatment on dendritic dimensions of neurons in the sexually dimorphic preoptic/anterior hypothalamic area of male and female ferrets. J Comp Neurol. 1992;323:577–585. doi: 10.1002/cne.903230410. [DOI] [PubMed] [Google Scholar]
- Claiborne BJ, Amaral DG, Cowan WM. Quantitative, three-dimensional analysis of granule cell dendrites in the rat dentate gyrus. J Comp Neurol. 1990;302:206–219. doi: 10.1002/cne.903020203. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Hegstrom CD, Breedlove SM. Photoperiod-dependent response to androgen in the medial amygdala of the Siberian hamster, Phodopus sungorus. J Biol Rhythms. 2002;17:147–154. doi: 10.1177/074873002129002438. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64:34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- Cunningham RL, McGinnis MY. Physical provocation of pubertal anabolic androgenic steroid exposed male rats elicits aggression towards females. Horm Behav. 2006;50:410–416. doi: 10.1016/j.yhbeh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Danzer SC, McMullen NT, Rance NE. Dendritic growth of arcuate neuroendocrine neurons following orchidectomy in adult rats. J Comp Neurol. 1998;390:234–246. [PubMed] [Google Scholar]
- David JT, Cervantes MC, Trosky KA, Salinas JA, Delville Y. A neural network underlying individual differences in emotion and aggression in male Golden hamsters. Neurosci. 2004;126:567–578. doi: 10.1016/j.neuroscience.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Desmond NL, Levy WB. Granule cell dendritic spine density in the rat hippocampus varies with spine shape and location. Neurosci Lett. 1985;54:219–224. doi: 10.1016/s0304-3940(85)80082-3. [DOI] [PubMed] [Google Scholar]
- DuRant RH, Escobedo LG, Heath GW. Anabolic-steroid use, strength training, and multiple drug use among adolescents in the United States. Pediatrics. 1995;96:23–28. [PubMed] [Google Scholar]
- Farrell SF, McGinnis MY. Effects of pubertal anabolic-androgenic steroid (AAS) administration on reproductive and aggressive behaviors in male rats. Behav Neurosci. 2003a;117:904–911. doi: 10.1037/0735-7044.117.5.904. [DOI] [PubMed] [Google Scholar]
- Farrell SF, McGinnis MY. Effects of pubertal anabolic-androgenic steroid exposure and withdrawal on reproductive endocrinology and physiology in male rats. Soc Neurosci Abstacts 2003b [Google Scholar]
- Farrell SF, McGinnis MY. Long-term effects of pubertal anabolic-androgenic steroid exposure on reproductive and aggressive behaviors in male rats. Horm Behav. 2004;46:193–203. doi: 10.1016/j.yhbeh.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Feinberg MJ, Lumia AR, McGinnis MY. The effect of anabolic-androgenic steroids on sexual behavior and reproductive tissues in male rats. Physiol Behav. 1997;62:23–30. doi: 10.1016/s0031-9384(97)00105-4. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Freeman ME, Wang Z. Newly proliferated cells in the adult male amygdala are affected by gonadal steroid hormones. J Neurobiol. 2003;57:257–269. doi: 10.1002/neu.10273. [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic stress. Neurosci. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Garza-Meilandt A, Cantu RE, Claiborne BJ. Estradiol’s effects on learning and neuronal morphology vary with route of administration. Behav Neurosci. 2006;120:905–916. doi: 10.1037/0735-7044.120.4.905. [DOI] [PubMed] [Google Scholar]
- Giammanco M, Tabacchi G, Di Majo D, La Guardia M. Testosterone and aggressiveness. Med Sci Monit. 2005:11. [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, Molloy EA, Blumenthal JD, Tossell JW, Stayer C, Samango-Sprouse CA, Shen D, Davatzikos C, Merke D, Chrousos GP. Puberty-related influences on brain development. Mol Cell Endocrinol. 2006:254–255. 154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex. 1996a;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. J Comp Neurol. 1996b;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gomez DM, Newman SW. Medial nucleus of the amygdala in the adult Syrian hamster: a quantitative Golgi analysis of gonadal hormonal regulation of neuronal morphology. Anat Rec. 1991;231:498–509. doi: 10.1002/ar.1092310412. [DOI] [PubMed] [Google Scholar]
- Gorski RA. Sexual dimorphisms of the brain. J Anim Sci. 1985;3:38–61. doi: 10.1093/ansci/61.supplement_3.38. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes JM, Ricci LA, Melloni RH., Jr Glutamic acid decarboxylase (GAD65) immunoreactivity in brains of aggressive, adolescent anabolic steroid-treated hamsters. Horm Behav. 2003;44:271–280. doi: 10.1016/s0018-506x(03)00138-7. [DOI] [PubMed] [Google Scholar]
- Harley CW, Malsbury CW, Squires A, Brown RA. Testosterone decreases CA1 plasticity in vivo in gonadectomized male rats. Hippocampus. 2000;10:693–697. doi: 10.1002/1098-1063(2000)10:6<693::AID-HIPO1007>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbard PC, King RR, Malsbury CW, Harley CW. Two organizational effects of pubertal testosterone in male rats: transient social memory and a shift away from long-term potentiation following a tetanus in hippocampal CA1. Exp Neurol. 2003;182:470–475. doi: 10.1016/s0014-4886(03)00119-5. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. What does the hippocampus really do? Behav Brain Res. 1995;71:1–10. doi: 10.1016/0166-4328(95)00034-8. [DOI] [PubMed] [Google Scholar]
- Johansen JA, Jordan CL, Breedlove SM. steroid hormone masculinization of neural structure in rats: a tale of two nuclei. Physiol Behav. 2004;83:271–277. doi: 10.1016/j.physbeh.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Drewett RF. Testosterone reduces the absolute refractory period of the stria terminalis neurons in the rat brain. Science. 1979;204:877–879. doi: 10.1126/science.220709. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Drewett RF. Testosterone-sensitive neurones respond to oestradiol but not to dihydrotestosterone. Nature. 1980;286:67–68. doi: 10.1038/286067a0. [DOI] [PubMed] [Google Scholar]
- Kindlundh AMS, Hagekull DG, Isacson DGL, Nyberg F. Adolescent use of anabolic-androgenic steroids and relations to self-reports of social, personality and health aspects. Eur J Public Health. 2001;11:322–328. doi: 10.1093/eurpub/11.3.322. [DOI] [PubMed] [Google Scholar]
- Kopera H. The history of anabolic androgenic steroids and a review of clinical experience with anabolic steroids. Acta Endocrinol. 1985;271:11–18. doi: 10.1530/acta.0.109s00011. [DOI] [PubMed] [Google Scholar]
- Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of prepubertal development in the male rat. Biol Reprod. 1977;17:298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. New spines, new memories. Mol Neurobiol. 2004;29:117–130. doi: 10.1385/MN:29:2:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig RH. Sex hormone modulation of neural development in vitro. Horm Behav. 1994;28:383–395. doi: 10.1006/hbeh.1994.1035. [DOI] [PubMed] [Google Scholar]
- Lustig RH, Hua P, Smith LS, Wang C, Chang C. An in vitro model for the effects of androgen on neurons employing androgen receptor-transfected PC12 cells. Mol-Cell-Neurosci. 1994;5:587–596. doi: 10.1006/mcne.1994.1072. [DOI] [PubMed] [Google Scholar]
- Markham JA, McKian KP, Stroup TS, Juraska JM. Sexually dimorphic aging of dendritic morphology in CA1 of hippocampus. Hippocampus. 2005;15:97–103. doi: 10.1002/hipo.20034. [DOI] [PubMed] [Google Scholar]
- Masco DH, Carrer HF. Sexual receptivity in female rats after lesions or stimulation in different amygdaloid nuclei. Physiol Behav. 1990;24:1073–1080. doi: 10.1016/0031-9384(80)90050-5. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cell types and intrinsic connections of the amygdala. In: Aggleton JP, editor. The Amygdala. Wiley-Liss; New York: 1992. pp. 67–96. [Google Scholar]
- McGinnis MY, Lumia AR, Possidente BP. Effects of withdrawal from anabolic androgenic steroids on aggression in adult male rats. Physiol Behav. 2002;75:541–549. doi: 10.1016/s0031-9384(02)00657-1. [DOI] [PubMed] [Google Scholar]
- Melia P, Pipe A, Greenberg L. The use of anabolic-androgenic steroids by Canadian students. Clin J Sport Med. 1996;6:9–14. doi: 10.1097/00042752-199601000-00004. [DOI] [PubMed] [Google Scholar]
- Meyer G, Ferres-Torres R, Mas M. The effects of puberty and castration on hippocampal dendritic spines of mice. A Golgi study. Brain Res. 1978;155:108–112. doi: 10.1016/0006-8993(78)90309-8. [DOI] [PubMed] [Google Scholar]
- Moser MB, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc Natl Acad Sci U S A. 1994;91:12673–12675. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram DR, George AJ. Anabolic steroids. Bailliere’s Clin Endocrinol Metab. 2000;14:55–69. doi: 10.1053/beem.2000.0053. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol. 2006;494:635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- Nuriya M, Jiang J, Nemet B, Eisenthal KB, Yuste R. Imaging membrane potential in dendritic spines. Proc Natl Acad Sci U S A. 2006;103:786–790. doi: 10.1073/pnas.0510092103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HGJ, Katz DL. Affective and psychotic symptoms associated with anabolic steroid use. Am J Psychiat. 1988;145:487–490. doi: 10.1176/ajp.145.4.487. [DOI] [PubMed] [Google Scholar]
- Pope HGJ, Katz DL. Psychiatric and medical effects of anabolic-androgenic steroid use. A controlled study of 160 athletes. Arch Gen Psychiat. 1994;51:375–382. doi: 10.1001/archpsyc.1994.03950050035004. [DOI] [PubMed] [Google Scholar]
- Primus R, Kellogg C. Gonadal hormones during puberty organize environment-related socia0l interaction in the male rat. Horm Behav. 1990;24:311–323. doi: 10.1016/0018-506x(90)90012-m. [DOI] [PubMed] [Google Scholar]
- Rall W. Dendritic spines, synaptic potency, and neuronal plasticity. In: Woody CD, et al., editors. Cellular Mechanisms Subserving Changes in Neuronal Activity. Brain Information Service; Los Angeles: 1974. pp. 13–21. [Google Scholar]
- Rasia-Filho AA, Londero RG, Achaval M. Effects of gonadal hormones on the morphology of neurons from the medial amygdaloid nucleus of rats. Brain Res Bull. 1999;48:173–183. doi: 10.1016/s0361-9230(98)00160-9. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Sisk CL. Pubertal and seasonal plasticity in the amygdala. Brain Res. 2001;889:71–77. doi: 10.1016/s0006-8993(00)03111-5. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Schulz KM, Zehr JL. Puberty: A finishing school for male social behavior. Ann NY Acad Sci. 2003;1007:189–198. doi: 10.1196/annals.1286.019. [DOI] [PubMed] [Google Scholar]
- Smith MD, Jones LS, Wilson MA. Sex differences in hippocampal slice excitability: role of testosterone. Neuroscience. 2002;109:517–530. doi: 10.1016/s0306-4522(01)00490-0. [DOI] [PubMed] [Google Scholar]
- Thiblin I, Parlklo T. Anabolic androgenic steroids and violence. Act Psychiatr Scan. 2002;106:125–128. doi: 10.1034/j.1600-0447.106.s412.27.x. [DOI] [PubMed] [Google Scholar]
- Trommald M, Jensen V, Andersen P. Analysis of dendritic spines in rat CA1 pyramidal cells intracellularly filled with a fluorescent dye. J Comp Neurol. 1995;353:260–274. doi: 10.1002/cne.903530208. [DOI] [PubMed] [Google Scholar]
- Wesson DW, McGinnis MY. Stacking anabolic androgenic steroids (AAS) during puberty in rats: a neuroendocrine & behavioral assessment. Pharm Biochem Behav. 2006;83:410–419. doi: 10.1016/j.pbb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Wood RI, Coolen LM. Integration of chemosensory and hormonal cues is essential for sexual behavior in the male Syrian hamster: role of the medial amygdaloid nucleus. Neurosci. 1997;78:1027–1035. doi: 10.1016/s0306-4522(96)00629-x. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Yankova M, Hart SA, Woolley CS. Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: a serial electron-microscopic study. Proc Natl Acad Sci U S A. 2001;98:3525–3530. doi: 10.1073/pnas.051624598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. J Neurobiol. 2006;66:578–590. doi: 10.1002/neu.20251. [DOI] [PubMed] [Google Scholar]


