Abstract
The aim of our study is to determine whether chronic obstructive pulmonary disease (COPD) is an independent risk factor for ischemic heart disease and whether this association is related with a greater prevalence of classical cardiovascular risk factors. Ours is a case-control cross-sectional study design. Cases were hospital patients with ischemic heart disease in stable phase, compared with control hospital patients. All patients underwent post-bronchodilator (PBD) spirometry, a standardized questionnaire, and blood analysis. COPD was defined as per GOLD PBD forced expiratory volume in the first second (FEV1)/forced vital capacity (FVC) < 0.70. In our series of patient cases (n = 204) and controls (n = 100), there were 169 men in the case group (83%) and 84 in the control group (84%). Ages were 67 and 64 years, respectively (P < 0.05). There were no significant differences by weight, body mass index (BMI), packyears, leukocytes, or homocysteine. The abdominal perimeter was significantly greater in cases (mean 101 cm ± standard deviation [SD] 10 versus 96 cm ± 11; P < 0.000). Both groups also had significant differences by C-reactive protein (CRP), fibrinogen, and hemoglobin values. In univariate analysis, increased risks for cases to show with individual classical cardiovascular risk factors were seen, with odds ratio (OR) 1.86 and 95% confidence interval (CI) (1.04–3.33) for diabetes mellitus, dyslipidemia (OR 2.10, 95% CI: 1.29–3.42), arterial hypertension (OR 2.47, 95% CI: 1.51–4.05), and increased abdominal perimeter (OR 1.71, 95% CI: 1.06–2.78). Percent predicted PBD FEV1 was 97.6% ± 23% in the patient group and 104% ± 19% in the control group (P = 0.01), but the prevalence of COPD was 24.1% in cases and 21% in controls. Therefore, COPD was not associated with ischemic heart disease: at the crude level (OR 1.19, 95% CI: 0.67–2.13) or after adjustment (OR 1.14, 95% CI:0.57–2.29). In conclusion, COPD was not associated with ischemic heart disease. The greater prevalence of classical cardiovascular risk factors in COPD patients could explain the higher occurrence of ischemic heart disease in these patients.
Keywords: chronic obstructive pulmonary disease, cardiovascular disease, systemic inflammation, comorbidity
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality throughout the world. It is currently considered the fourth most common cause of death, and the burden it generates on health care systems is enormous.1 Comorbidities are frequent throughout the lifespan of COPD patients, and are ultimately responsible for many deaths. In COPD patients admitted to hospital, for severe cases, respiratory failure is the most common cause of death, and for mild and moderate cases, cardiovascular disease (CVD) and lung cancer rank high as causes of death.2
It is a research priority to establish whether there is a common pathogenesis of CVD and COPD, or whether these are associated but independent processes.3 A number of articles have been recently published highlighting systemic inflammation in COPD patients and suggesting that inflammation could favor the appearance of atherosclerotic disease in COPD patients.4,5 This theory would assume a causal relationship of COPD in CVD. However, there are no definitive data yet to confirm that chronic airflow obstruction, and its accompanying systemic inflammation, causes CVD.
Different studies have demonstrated that forced expiratory volume in the first second (FEV1) is a predictor of coronary disease in smokers as well as in nonsmokers.6 Supporting this, several observational studies published in the last two decades have reported an association between a reduction of FEV1 and increased cardiovascular risk. They are further supported by findings in more basic studies that directly analyze vascular damage by means of magnetic resonance, tomography, or ultrasound.7–9 However, this association is not as straightforward in clinical studies with standard spirometry confirming a COPD diagnosis by a post-bronchodilator (PBD) FEV1/forced vital capacity (FVC) ratio less than 0.70 has not always been confirmed. Briefly, other risk factors were not adequately evaluated; in some cases, symptoms of bronchitis without spirometry were taken into account, and in others the COPD definition was based on having been labeled by International Classification of Diseases, 9th Edition (ICD-9) codes for COPD, emphysema, or chronic bronchitis, but without spirometric confirmation.6,10,11
In a recent observational study carried out in our setting, we found that COPD patients present a prevalence of cardiovascular risk factors greater than that reported in the general population for similar age strata; a higher prevalence of CVD was likewise observed, but although age and classic cardiovascular risk factors were related to increased cardiovascular morbidity, we did not observe a relationship between CVD severity and airflow limitation.12
The objective of this research is to determine, by means of a case-control study, whether COPD is an independent risk factor of ischemic heart disease, or whether the increase in CVD observed in COPD patients is related to classical cardiovascular risk factors.
Material and methods
Study population
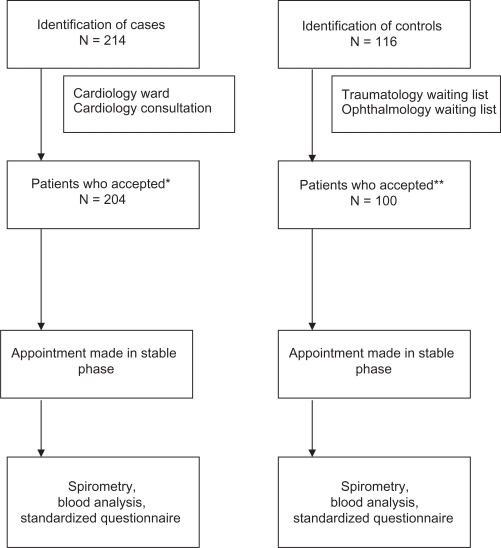
Ours is a case-control cross-sectional study of subjects that met the inclusion criteria described in Table 1. The study population domain included patients aged 40 years and older in the outpatient consultations and the cardiology unit (for cases), and from the surgical waiting lists of ophthalmology for cataracts and traumatology for knee prosthesis (for controls). All participating patients were considered to be in a stable phase for at least 1 month prior to recruitment. Given that in our setting COPD is still a far less frequent disease in women than men, reference control group was frequency-matched to the gender and age distribution of cases. The flow chart of patients considered to participate in the study is presented in Figure 1. This study followed the Strobe statement for observational studies.13
Table 1.
|
Inclusion criteria Male and female patients older than 40. Cases: patients with ischemic heart disease, meaning those patients who within the last 12 months had at least one episode of:
Controls: hospital patients with no history of ischemic heart disease, frequency matched to similar age and gender to the case group. |
|
Exclusion criteria Patients with chronic or acute liver disease. Patients with diseases that cause an increase in inflammatory markers:
|
Figure 1.
Flow chart of participants.
Notes: *10 patients in case group declined to participate; **16 patients in control group declined to participate; In both cases these patients were changed for subjects with similar age and the same sex.
Ethical considerations
This study was conducted in accordance with the ethical principles established in the Declaration of Helsinki (Edinburgh revision, 2000). In the first visit, and prior to data collection, all study subjects gave their informed, written consent. The study was approved by the Ethics and Clinical Research Committee of the Hospital Universitario of Guadalajara.
Diagnostic tests
In all participants, a blood sample was obtained to measure inflammatory markers (high-sensitivity C-reactive protein [SCRP] and fibrinogen, hemogram, glucose, homocysteine, and blood cholesterol). Standard spirometry was performed, and tests were repeated again 30 minutes after administering 400 μg of salbutamol and 80 μg of ipratropium bromide. Spirometries were carried out in accordance with current international quality standards with a spirometer (Masterlab; Jaeger, Hoechberg Germany). COPD was defined as a PBD FEV1/FVC ratio of less than 0.70 (GOLD and American Thoracic Society/European Respiratory Society criteria.1,15 Finally, study subjects completed a standardized questionnaire on clinical data, smoking data, drug treatments, and concomitant diseases.
Sample size
Considering an expected prevalence of GOLD-defined COPD in controls of 9%, and expecting a three-fold higher frequency of COPD in cases, given an alpha error of 5% and power of 90%, we required 200 cases and 100 controls to obtain statistical significance.16,17 Calculations assuming larger prevalences of COPD in controls or lower in cases would result in more powerful statistics.
Data analysis
Initially, a statistical descriptive analysis of crude data and in-depth quality control was conducted. Continuous data are presented as means and standard deviation (SD) or with 95% confidence intervals (CIs). Differences by groups were analyzed with Student’s t-test for unpaired samples, or using the chi-squared test for dichotomous variables. A P value lower than 0.05 was considered significant. To estimate the magnitude of the effect or the association, we used the odds ratio (OR) as the ratio between the odds of exposure (ie, COPD) observed in cases versus the one in controls. Likewise, we calculated the corresponding confidence interval of the OR.
Finally, a logistic regression analysis was undertaken to evaluate the effect of covariates on the response variable, adjusting for confounding factors.
Results
We studied a series of 204 patient cases with ischemic heart disease and 100 controls, whose gender distribution was similar by design (male 83% versus 84%). Ages were 67 and 64 years, respectively (P < 0.05). In addition, no significant differences by weight, body mass index (BMI), pack-years, leukocytes, or homocysteine were observed. The abdominal perimeter was significantly greater in cases (mean 101 cm ± SD 11 versus 96 cm ± 11; P < 0.000), and using a cutoff of 88 cm in women and 102 cm in men, this difference persisted for the number of patients who presented a greater abdominal perimeter in the CVD group compared with the control group, although as mentioned above, weight and BMI were not statistically different (Table 2).18
Table 2.
Demographic, clinical characteristics, and selected biochemistry for cases and controls
| Cases (204) | Controls (100) | P | |
|---|---|---|---|
| Age | 67 (10) | 64 (9) | <0.05 |
| Male (%) | 169 (83%) | 84 (84%) | |
| Weight (Kg) | 76 (13) | 78 (12) | 0.26 |
| BMI (Kg/m2) | 27.7 (4.8) | 28.2 (4.3) | 0.44 |
| Smokers n (%) | 149 (73%) | 66 (66%) | |
| Current | 32 (16%) | 32 (32%) | |
| Ex-smokers | 117 (57%) | 31 (31%) | |
| Nonsmokers | 55 (27%) | 34 (34%) | |
| Pack/year | 33 (25) | 37 (23) | 0.24 |
| Abdominal perimeter (cm) | 101 (10) | 96 (11) | 0.000 |
| Abdominal perimeter* | 115 (56.4%) | 43 (43%) | 0.028 |
| Diabetes mellitus | 62 (30.4%) | 19 (19%) | 0.035 |
| Dyslipidemia | 117 (57.4%) | 39 (39%) | 0.003 |
| Arterial hypertension | 123 (60.3%) | 38 (38%) | 0.000 |
| FEV1 post (L) | 2.66 (0.75) | 2.88 (0.88) | 0.05 |
| FEV1 post (%) | 97.6 (23) | 104 (19) | 0.01 |
| FVC post (L) | 3.44 (0.89) | 3.79 (1.24) | 0.01 |
| FVC post (%) | 104 (20) | 109 (19) | 0.04 |
| FEV1/FVC post (%) | 76 (11) | 79 (10) | 0.02 |
| CRP (mg/L) | 4.80 (7.89) | 2.75 (2.85) | 0.001 |
| Hemoglobin (g/dl) | 14.2 (1.5) | 15.3 (1.6) | 0.000 |
| Leukocytes (x1000/l) | 7.73 (2.15) | 7.62 (2.2) | 0.67 |
| Homocysteine (μmol/L) | 12.07 (5) | 12.9 (5) | 0.18 |
| Fibrinogen (mg/dL) | 338 (91) | 298 (67) | 0.000 |
Note:
Number of subjects with abdominal perimeter greater than 102 cm in men and 88 cm in women (16).
Abbreviations: BMI, body mass index; CRP, C-reactive protein; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity.
Both groups had also significant differences in CRP, fibrinogen, and hemoglobin values. In univariate analysis, increased risks with individual classical cardiovascular risk factors were seen, with OR 1.86 and 95% CI (1.04–3.33) for diabetes mellitus, dyslipidemia (OR 2.10, 95% CI: 1.29–3.42), arterial hypertension (OR 2.47, 95% CI: 1.51–4.05), prior smoking history (OR 1.39, 95% CI: 0.83–2.34), overweight (OR 1.24, 95% CI: 045–3.30), moderate obesity (OR 1.69, 95% CI: 0.67–4.30), severe obesity (OR 1.71, 95% CI: 0.63–4.68), and increased abdominal perimeter (OR 1.71, 95% CI: 1.06–2.78). After adjustment, age, arterial hypertension, abdominal perimeter, obesity, and treatment with statins remained significant (Table 3). Percent predicted PBD FEV1 was 97.6% ± 23 in the patient group and 104% ± 19 in the control group (P = 0.01), but the prevalence of COPD was 24.1% in cases and 21% in controls. Therefore, COPD was not associated with ischemic heart disease at the crude level (OR 1.19, 95% CI: 0.67–2.13) or after adjustment by cardiovascular risk factors (OR 1.19, 95% CI: 0.57–2.29). Most of COPD were mild and moderate according to GOLD criteria. Only four patients had a FEV1 below 50%. Our plan did not propose an analysis with different levels of severity, and our sample size does not allow such analysis.
Table 3.
Crude and adjusted ORs (95% CI) for classical cardiovascular risk factors and COPD defined according to GOLD
| Crude OR (95% CI) | Adjusted OR (95% CI) | ||
|---|---|---|---|
| Age | 1.41 (0.87–2.28) | 1.03 (1.00–1.06) | |
| Male (%) | 1.48 (0.48–1.75) | 1.51 (0.62–3.66) | |
| Smoker n (%) | 1.39 (0.83–2.34) | 1.71 (0.88–3.36) | |
| Diabetes mellitus | 1.86 (1.04–3.33) | 0.65 (0.33–1.27) | |
| Arterial hypertension | 2.47 (1.51–4.05) | 2.14 (1.17–3.89) | |
| Dyslipidemia | 2.10 (1.29–3.42) | 0.97 (0.50–1.90) | |
| High abdominal perimeter | 1.71 (1.06–2.78) | 2.81 (1.45–5.47) | |
| Obesity: | (BMI: 25–30) | 1.24 (0.45–3.30) | 2.27 (0.67–7.72) |
| (BMI: 30–35) | 1.69 (0.67–4.30) | 3.97 (1.22–12.92) | |
| (BMI: ≥35) | 1.71 (0.63–4.68) | 8.70 (2.24–33.7) | |
| Treatment with statins | 0.44 (0.30–0.62) | 0.22 (0.11–0.44) | |
| COPD | 1.19 (0.67–2.13) | 1.14 (0.57–2.29) | |
Abbreviations: BMI, body mass index (Kg/m2); OR, odds ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease.
Discussion
The most relevant finding of our study is that COPD, defined as a PBD FEV1/FVC ratio of less than 70%, was not associated with ischemic heart disease. Although patients with ischemic heart disease had significantly lower lung function than controls, they also had more often classical cardiovascular risk factors. The classical cardiovascular risk factors were associated with an increase in CVD disease, but a decrease in lung function was not.
Previous studies
In a recent observational study carried out in our setting, COPD patients presented a greater prevalence of cardiovascular and ischemic cardiopathy risk factors than the general population. We did not observe, however, a direct relationship with the deterioration of FEV1 or with having been diagnosed with COPD using spirometric criteria.12 Our data had the limitations inherent of observational studies and can therefore only be considered exploratory. These limitations should be extrapolated to all observational studies published to date. Recently, Johnston et al have reported an association between poor lung function and greater cardiovascular risk in a population-based study.19 However, when the results were adjusted for other classical risk factors such as age, sex, race, hypertension, diabetes, cholesterol, fibrinogen and tobacco habit, the association between lung function and cardiovascular risk held fast only with the restrictive patients in the nonsmoker group, with the restrictive patients and COPD stages 2–4 in the group of ex-smokers, and with none of the active smokers. It is necessary to keep in mind that although the adjustment for other variables that could generate confusion was notable, these adjustments present limitations as they did not include other vascular risk factors, such as lack of physical activity, obesity, alcohol intake, poor diet, or presence of sleep respiratory disorders, whose relevance has yet to be determined in COPD patients. In addition, it is extremely difficult in observational studies to evaluate how these factors interrelate.20 For example, tobacco habit, which is practically constant in these patients, in addition to being independently related to cardiovascular disease, is also associated with an increase in blood pressure. Moreover, the limited physical activity that these patients frequently develop can lead to excess weight gain, a situation which occurs in more than 50% of our COPD population.21 Taking into account the limitations of observational studies, and until prospective longitudinal studies are completed, case-control studies can augment our information in this area.
In a study of cases and controls done in the United Kingdom with 2699 COPD patients (46% active smokers), the patients presented a greater number of comorbidities than asthma patients. This study was not adequately adjusted for other risk factors, so it is therefore not possible to evaluate the impact of COPD on the cardiovascular risk of these patients.22 In another series of cases and controls, the COPD patients had a greater history of tobacco habit and a greater prevalence of cardiovascular pathologies, heart failure, neoplasia, and neurological and digestive pathologies (3.7 chronic processes in the COPD group versus 1.8 in the control group).23 Lastly, in a retrospective study completed in Canada with a cohort of patients diagnosed with COPD, Curkendall et al found that after adjusting for cardiovascular risk factors, the degree of severity of the disease, established according to clinical criteria, constituted a risk factor for the presentation of myocardial infarction and death due to CVD.24 Nevertheless, the case definition included a heterogeneous group of patients including those diagnosed with COPD, emphysema, or chronic bronchitis in accordance with the ICD-9, but with no spirometric evaluation following the GOLD criteria.
From another point of view, Soriano et al have described recently that the prevalence of airflow limitation (AL) in patients with CVD was substantial and that most of these patients were not diagnosed appropriately.25 However, they found that the prevalence of AL in population participants with CVD (19.2%; 95% CI: 8.1–30.7) was only slightly higher than that of population participants without it (17.5%; 95% CI: 14.0–21.0) (P > 0.05).23 This prevalence is quite similar to that observed in our study.
Interpretation of findings
Unlike the reports of other studies, we have not observed a direct relationship between the presence of GOLD spirometric COPD criteria and a presence of cardiovascular complications. On the contrary, we did find that obesity, abdominal perimeter, or high blood pressure, diabetes mellitus, and dyslipemia constituted risk factors for ischemic cardiopathy.
Although slightly lower values of FEV1 and FVC were observed in CVD patients when compared with the control group, various factors can explain these findings and our discrepancy with previous series, as follows:
COPD spirometric criteria (PBD FEV1/FVC ratio < 70%) is arbitrary and does not necessarily reflect the existence of a real disease. In fact, when the prevalence of COPD is evaluated in population studies, the actual impact of COPD can be drastically reduced when the lower limit of normal (LLN) is used.26–28 Any study that uses GOLD criteria can be conditioned by the limited clinical relevance of the FEV1/FVC ratio in certain senior age population groups. In our series, the prevalence of COPD by GOLD criteria was greater than in the general Spanish population, but the difficulty of properly evaluating these values has recently been demonstrated in a study carried out in our country, mostly in older populations. Not surprisingly, prevalence by LLN criteria was lower in both groups, but the difference between both groups was greater.29
Reduced spirometric volumes are not always associated with airflow obstruction. In a recent population study completed in Paris with 121,965 subjects, an association was observed between deterioration in lung function and the presence of metabolic syndrome, which was related to the increase in abdominal perimeter, regardless of age, sex, tobacco habit, alcohol consumption, level of education, BMI, physical activity, or history of CVD.30 This increase in abdominal perimeter is a well known cardiovascular risk factor. The reduction of FVC that was observed in this study can lead to restrictive spirometries or accentuate the functional deterioration (FEV1 and FVC) in obstructive patients. In these cases, the decrease in spirometric values would only be an epiphenomenon, and our interpretation that the obstruction of the airflow is a risk factor “per se” would be erroneous.31 In our series, we observed an inverse relationship between FEV1 and FVC values and abdominal perimeter; these data suggest that this aspect can be relevant in explaining the association between functional deterioration and the risk for CVD.
COPD could favor cardiovascular risk, but due to mechanisms that are collateral to the obstruction itself and not necessarily due to a mechanism of systemic inflammation produced by the obstructive disease. During the last few years, it has been proposed that COPD could produce systemic inflammation and that this inflammation could be the link between COPD and an increase in CVD in these patients.32 In this sense, more attention has been centered on CRP. Although COPD patients can present higher CRP values, various factors can condition this increase.33 In a study by Aronson, the highest levels of CRP in COPD patients were in those who presented associated obesity.34 In addition to excess weight, other factors that are frequently observed in COPD patients, such as an abdominal fat mass or sedentary lifestyle, can elevate CRP values in addition to being related with a greater risk for metabolic syndrome.21,35–37 It is therefore not surprising that COPD patients have more CVD, as there are multiple cardiovascular risk factors associated with the disease, but when this increase in risk is adjusted for the classical cardiovascular risk factors, an additional effect of airway obstruction is not confirmed.
Study limitations
The prevalence of cardiovascular risk factors is not uniform among countries. Diet and lifestyle in the Mediterranean population have possibly influenced the results and may limit the extrapolation of these results to other geographical areas. Given that neither observational studies nor those of cases and controls would allow for this factor to be adequately adjusted, only the completion of longitudinal studies in different settings would make this clarification.
The size of the sample does not exclude definitively that there is a weak effect of COPD on the CVD. However, our data do not support that COPD is a relevant risk factor and, if any, it would be a small one. Only a longitudinal study with sufficient person–time experience can answer this question properly. We were indeed concerned with sample size, as ours is a single-center study. A posteriori, after an interim analysis, we estimated it was not worth increasing further our obtained sample, as the power gain would have been little.
One possible risk in the study of these characteristics is the generation of selection bias due to lack of patient collaboration. To correct this factor, when pre-selected patients refused participation in the study, various attempts were made to convince said subjects to participate. When this was not possible, another patient with similar age and sex characteristics was selected as a replacement.
For controls, we used other hospital patients within our domain catchment population. Given our interest in an aging population, we considered that other options such as using a general population sample might have led to an excessive number of failures and a potential responder bias. Hospital patient bias (Berkson’s bias) may occur when hospital controls are used in a case-control study. If the controls (ophthalmic and trauma outpatients) are hospitalized due to exposure that is also related to the disease under study (COPD) then the measure of effect may be weakened, ie, biased towards the null hypothesis of no association. It is highly unlikely that CVD or COPD, our prime interest, were the reason for consultations in our ophthalmic or trauma patients. Therefore, we believe that controls are appropriate for the purpose of the study, because all information indicate a priori that it does not affect for a greater or lesser prevalence of COPD within groups.
Finally, as this study was of cases and controls, it can be difficult to establish the time sequence between exposure and the disease, but given that COPD is a disease with a long evolution, we consider this situation to be infrequent. However, the lack of a longitudinal follow-up can condition the proper evaluation of the impact of different CVD risk factors. For the same reason, other risk factors such as physical activity or alcohol intake were not included in the analysis. In a nonlongitudinal study, the impact of treatment on risk could not be adequately evaluated between the two groups despite our observation of a significant relation with the use of statins.
In conclusion, in this study of cases (patients with ischemic cardiopathy) and control subjects (those without ischemic cardiopathy), carried out in a Mediterranean population, we have not observed that COPD diagnosis according to GOLD criterion conditions greater vascular risk when adjusted for classic risk factors.
Acknowledgments
The authors express thanks for the collaboration of auxiliary nurse Inmaculada Monasor and nurse Esperanza Señor and to Dr Joan B Soriano from Fundación Caubet Cimera (Illes Balears, Spain) for his contribution in the statistical analysis.
Footnotes
Grant support
This work was supported by a research grant from Neumomadrid. Preliminary data from this research was presented at the ERS 2009 International Conference in Vienna.
Author contributions
JLI had the original idea, designed the protocol of the study, drafted the report, and obtained funding. AM and EG recruited patients and performed all clinical tests. PL and JMR contributed to the design of the protocol of the study. All coauthors contributed to and approved this manuscript.
Disclosure
The authors declare no conflict of interest regarding this study.
References
- 1.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: Gold executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE, For the Lung Health Study Research Group The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233–239. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 3.Hokanson JE. COPD and coronary heart disease: challenges in understanding the natural history of common complex chronic diseases. COPD. 2009;6:149–151. doi: 10.1080/15412550902994106. [DOI] [PubMed] [Google Scholar]
- 4.Izquierdo Alonso JL, Arroyo-Espliguero R. Chronic obstructive pulmonary disease and cardiovascular risk. Arch Bronconeumol. 2005;41:410–412. doi: 10.1016/s1579-2129(06)60254-1. [DOI] [PubMed] [Google Scholar]
- 5.Sin DD, Man SF. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc. 2005;2:8–11. doi: 10.1513/pats.200404-032MS. [DOI] [PubMed] [Google Scholar]
- 6.Hole J, Watt GC, Davey-Smith G, et al. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313:711–715. doi: 10.1136/bmj.313.7059.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao D, Higgins M, Bryan NR, et al. Lower pulmonary function and cerebral subclinical abnormalities detected by MRI: the Atherosclerosis Risk in Communities study. Chest. 1999;116:150–156. doi: 10.1378/chest.116.1.150. [DOI] [PubMed] [Google Scholar]
- 8.Newman AB, Naydeck BL, Sutton-Tyrrell K, et al. Coronary artery calcification in older adults to age 99: prevalence and risk factors. Circulation. 2001;104:2679–2684. doi: 10.1161/hc4601.099464. [DOI] [PubMed] [Google Scholar]
- 9.Iwamoto H, Yokoyama A, Kitahara Y, et al. Airflow limitation in smokers is associated with subclinical aterosclerosis. Am J Respir Crit Care Med. 2009;179:35–40. doi: 10.1164/rccm.200804-560OC. [DOI] [PubMed] [Google Scholar]
- 10.Jousilahti P, Vartiainen E, Tuomilehto J, et al. Symptoms of chronic bronchitis and the risk of coronary disease. Lancet. 1996;348:567–572. doi: 10.1016/S0140-6736(96)02374-4. [DOI] [PubMed] [Google Scholar]
- 11.Schünemann HJ, Dorn J, Grant BJB, et al. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest. 2000;118:656–664. doi: 10.1378/chest.118.3.656. [DOI] [PubMed] [Google Scholar]
- 12.Lucas-Ramos P, Izquierdo-Alonso JL, Rodríguez-González Moro JM, et al. Asociación de factores de riesgo cardiovascular y EPOC. Resultados de un estudio epidemiológico (estudio ARCE) Arch Bronconeumol. 2008;238:233–238. doi: 10.1016/s1579-2129(08)60037-3. [DOI] [PubMed] [Google Scholar]
- 13.Thelle DS. STROBE and STREGA: instruments for improving transparency and quality of reporting scientific results. Eur J Epidemiol. 2009;24:7–8. doi: 10.1007/s10654-008-9303-x. [DOI] [PubMed] [Google Scholar]
- 14.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 15.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 16.Sobradillo-Peña V, Miravitlles M, Gabriel R, et al. Geographic variations in prevalence and underdiagnosis of COPD: results of the IBERPOC multicentre epidemiological study. Chest. 2000;118:981–989. doi: 10.1378/chest.118.4.981. [DOI] [PubMed] [Google Scholar]
- 17.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 18.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 19.Johnston AK, Mannino DM, Hagan GW, et al. Relationship between lung function impairment and incidence or recurrence of cardiovascular events in a middle-aged cohort. Thorax. 2008;63:599–605. doi: 10.1136/thx.2007.088112. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 21.Pitta F, Troosters T, Spruit MA, et al. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Care Med. 2005;171:972–977. doi: 10.1164/rccm.200407-855OC. [DOI] [PubMed] [Google Scholar]
- 22.Soriano JB, Visick GT, Muellerova H, et al. Patterns of comorbidities in newly diagnosed COPD and asthma in the primary care. Chest. 2005;128:2099–2107. doi: 10.1378/chest.128.4.2099. [DOI] [PubMed] [Google Scholar]
- 23.Mapel DW, Hurley JS, Frost FJ, et al. Health care utilization in chronic obstructive pulmonary disease. A case-control study in a health maintenance organization. Arch Intern Med. 2000;160:2653–2658. doi: 10.1001/archinte.160.17.2653. [DOI] [PubMed] [Google Scholar]
- 24.Curkendall S, DeLuise C, Jones JK, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16:63–70. doi: 10.1016/j.annepidem.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Soriano JB, Rigo F, Guerrero D, et al. High prevalence of undiagnosed airflow limitation in patients with cardiovascular disease. Chest. 2010;137:333–340. doi: 10.1378/chest.09-1264. [DOI] [PubMed] [Google Scholar]
- 26.Vollmer WM, Gíslason T, Burney P, et al. Comparison of spirometry criteria for the diagnosis of COPD: results from the BOLD study. Eur Respir J. 2009;34:588–597. doi: 10.1183/09031936.00164608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enright PL, Ruppel GL. Don’t use the flawed fixed ratio to diagnosis COPD. Respiratory Care. 2009;54:1500. [PubMed] [Google Scholar]
- 28.Swanney MP, Ruppel G, Enright PL, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclasification of airway obstruction. Thorax. 2008;63:1046–1051. doi: 10.1136/thx.2008.098483. [DOI] [PubMed] [Google Scholar]
- 29.Soriano JB, Ancochea J, Miravitlles M, et al. Recent trends in COPD prevalence in Spain: a repeated cross-sectional survey 1997–2007. Eur Respir J. doi: 10.1183/09031936.00138409. Epub 2009 Dec 8. [DOI] [PubMed] [Google Scholar]
- 30.Leone N, Courbon D, Thomas F, et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179:509–516. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- 31.Enright P. Overindulgence/overweight/reduced vital capacity/reduced longevity. Am J Respir Crit Care Med. 2009;179:432–433. doi: 10.1164/rccm.200901-0140ED. [DOI] [PubMed] [Google Scholar]
- 32.Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107:1514–1519. doi: 10.1161/01.cir.0000056767.69054.b3. [DOI] [PubMed] [Google Scholar]
- 33.Wilson PWF, Nam BH, Pencina M, et al. C-reactive protein and risk of cardiovascular disease in men and women from the Framingham Heart Study. Arch Intern Med. 2005;165:2473–2478. doi: 10.1001/archinte.165.21.2473. [DOI] [PubMed] [Google Scholar]
- 34.Aronson D, Roterman I, Yigla M, et al. Inverse association between pulmonary function and C-reactive protein in apparently healthy subjects. Am J Respir Crit Care Med. 2006;174:626–632. doi: 10.1164/rccm.200602-243OC. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Aymerich J, Lange P, Benet M, et al. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61:772–778. doi: 10.1136/thx.2006.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutten EA, Breyer MK, Spruit MA, et al. Abdominal fat mass contributes to the systemic inflammation in chronic obstructive pulmonary disease. Clin Nutr. 2010 Jun 2; doi: 10.1016/j.clnu.2010.04.007. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 37.Watz H, Waschki B, Kirsten A, et al. The metabolic syndrome in patients with chronic bronchitis and COPD. Chest. 2009;136:1039–1046. doi: 10.1378/chest.09-0393. [DOI] [PubMed] [Google Scholar]