Abstract
We assessed the physicochemical properties of the surface microlayer (SML: first 900 μm) and its underlying water (ULW: 0.2–0.5-m depth) and compared the composition and activity of their bacterial communities in six lakes located across an altitude gradient. Activity was assessed at both the community level, by measuring leucine bulk incorporation, and at the single-cell level, by using microautoradiography. Catalyzed reporter deposition fluorescence in situ hybridization was used to quantitatively assess the structure of the bacterial assemblage. Dissolved organic matter at the SML was significantly enriched in small-size molecules as compared to the ULW. Bacterial abundance in the SML ranged from 3.2 × 105 cells mL−1 to 3.2 × 106 cells mL−1 and was enriched in four out of six lakes when compared to the ULW. The SML and ULW showed lake-specific differences in bacterial community composition, although in most cases, both layers were dominated by Betaproteobacteria. This group also contributed the most to total activity in both layers in all lakes, followed by Actinobacteria. Despite large differences in environmental conditions among lakes, the fraction of active neustonic bacteria was very similar in most of them. Both bulk and single-cell activities are not necessarily lower in the SML than in the ULW, and well-adapted bacteria exist in the extreme conditions found in this habitat.
In marine and freshwater environments, the air–water boundary or surface microlayer (SML) constitutes an important interface between the atmosphere and the underlying water (ULW) that has distinct physicochemical properties compared to its contiguous environments (Carlson 1982; Hardy 1982). Biological and chemical processes taking place at the SML are important because they control, among others, the gaseous exchange between the atmosphere and the hydrosphere and vice versa (Conrad and Seiler 1988; Upstill-Goddard et al. 2003). The SML also acts as a collector of natural and anthropogenic dissolved substances and particles, including microorganisms, which are deposited and even accumulated in this habitat (Crawford et al. 1982; Maki 1993). Organisms of the SML are collectively known as neuston (Naumann 1917), and the bacterial community is known as bacterioneuston. The neuston has often been characterized as being more numerous than the plankton (Norkrans 1980; Hardy 1982); however, information regarding the abundance of bacterioneuston compared to bacterioplankton is still incomplete and sometimes contradictory. Although several studies have considered the abundance of bacterioneuston (Dietz et al. 1976; Hermansson and Dahlbäck 1983; Joux et al. 2006), direct comparisons among aquatic systems are difficult or impossible because of the different sampling devices used that strongly influence the thickness of the microlayer sampled and consequently the results obtained (Agogué et al. 2004).
The metabolic activity of bacterioneuston is believed to be lower than of bacterioplankton (Dietz et al. 1976). This has been usually attributed to different environmental stress factors found in the SML, such as high incident solar ultraviolet (UV) radiation, variable water temperatures and salinity (in marine systems), as well as high concentrations of toxic organic substances in some cases (Norkrans 1980; Maki 1993). Traits such as pigmentation, deoxyribonucleic acid (DNA) with high guanosine and cytosine content, frequent presence of extracellular polymers, or enhanced growth rates are thought to be advantageous for bacteria colonizing the SML (Maki 1993).
Recently, several new cultivable bacteria have been isolated from the SML of marine environments (Agogué et al. 2005), and also freshwater lakes are known to host a unique bacterioneuston (Cypionka et al. 2006). However, despite these isolation and description approaches, information on bacterial community composition of the SML is scarce (Franklin et al. 2005; Cunliffe et al. 2008; Obernosterer et al. 2008), particularly in lakes (Hervàs and Casamayor 2009). For example, species of Crenarchaeota have been found in the neuston of several alpine lakes in the Pyrenees, Spain (Auguet and Casamayor 2008), but it is unknown how common these microorganisms are in other lakes. Furthermore, we know very little about which members of the bacterial community in the SML of lakes are active and which factors control their activity, though this knowledge is crucial to determine the success of colonization by these microorganisms.
In this study, we quantitatively characterized the bacterial community composition of the SML and the ULW in six lakes and assessed the relationship of specific bacterial groups to environmental parameters. Additionally, we examined the activity of the bacterial assemblage at the community level and at the single-cell level in the SML and the ULW. Our sampling strategy was based on selecting lakes across an altitude gradient to represent a broad range of environmental conditions and ultimately to identify factors potentially controlling the bacterioneuston. In fact, several parameters, such as the concentration of dissolved organic carbon (DOC) and inorganic nutrients, water temperature, and incident UV radiation, change significantly across the altitudinal gradient (Laurion et al. 2000). Considering the difficulty of constraining the potentially different dynamics and previous history before sampling, we sampled the lakes (after several attempts) during a calm weather period in July to minimize this variability. To our knowledge, this is the first simultaneous characterization of the quantitative composition and the activity of bacteria in the SML of lakes.
Methods
Sampling
Between 17 and 25 July 2007, we sampled the SML and the ULW of six lakes located across an altitude gradient from 913 to 2799 m above sea level in the central Alps (Table 1). Sampling was done from a boat around noon during stable weather conditions (i.e., neither rain nor continuous wind speed > 5 m s−1). Three of the lakes are located below the tree line, which is situated at ~ 2000 m above sea level (a.s.l.) in this region, whereas the others are located above tree line. The lakes are small (area < 0.15 km2), and their maximum length, a proxy for wind fetch, is < 0.9 km (Table 1). A more detailed description of the lakes sampled (except for Seefelder Wildsee) can be found in Laurion et al. (2000).
Table 1.
Geographic coordinates, altitude, lake area, catchment area, and maximum length of the six sampled lakes
| Lake | Latitude (N) |
Longitude (E) |
Altitude (m a.s.l.) |
Area (km2) |
Catchment (km2) |
Maximum length (km) |
|---|---|---|---|---|---|---|
| PIB | 47°11′ | 10°53′ | 913 | 0.134 | 2.65 | 0.81 |
| WSS | 47°19′ | 11°11′ | 1177 | 0.061 | 6.90 | 0.65 |
| OBS | 46°59′ | 11°24′ | 1590 | 0.120 | 11.63 | 0.77 |
| GKS | 47°13′ | 11°00′ | 2417 | 0.017 | 0.30 | 0.20 |
| ROT | 47°14′ | 11°00′ | 2485 | 0.009 | 0.33 | 0.16 |
| SOS | 46°57′ | 10°56′ | 2799 | 0.035 | 0.18 | 0.40 |
PIB, Piburger See; WSS, Seefelder Wildsee; OBS, Obernberger See; GKS, Gossenköllesee; ROT, Rotfelssee; and SOS, Schwarzsee ob Sölden.
Water from the SML was collected from the upper 900 μm of the lake surface with a modified screen sampler (Agogué et al. 2004) consisting of an acid-stable plastic frame and an aluminum screen with regularly distributed holes of 1-mm diameter. The thickness of lake surface sampled was calculated by dividing the volume recovered from the sampler by the area of the screen. This measurement was repeated several times in the laboratory to obtain an average. To account for potential spatial heterogeneity of the SML within each lake, we collected a composite sample in the central zone of the lake from at least 15 different randomly selected points. This sampling strategy was also necessary to obtain enough volume for all analyses and to obtain replicates. Before and after sampling, the device was washed with diluted hydrochloric acid and subsequently rinsed several times with Milli-Q water to reduce organic contamination. The DOC concentration in the last washing step with Milli-Q water was measured on several occasions to check for potential organic contamination of the device. These tests showed that organic contamination was not probable. The ULW was sampled at 0.2-m depth with a modified Schindler-Patalas sampler (32 cm × 14 cm, 5 liters), thus collecting the water layer between 0.2- and ~ 0.5-m depth.
Physicochemical parameters
The water temperature of the SML and ULW was measured with a glass thermometer (± 0.1°C) immediately after collecting the samples. Conductivity and pH were measured with an LF196 (WTW) and an Orion 960 meter (Thermo Fischer Scientific), respectively. For the analysis of total dissolved phosphorous (TDP), water samples were placed in polyethylene bottles (HCl-cleaned and rinsed several times with Milli-Q and sample water) and transported to the laboratory at in situ temperature, where they were filtered through a glass-fiber filter (Whatman, GF/F). The samples were stored at 4°C until further analysis within 24 h. The concentration of TDP was determined spectrophotometrically using the molybdate method after digestion with sulfuric acid and hydrogen peroxide.
To analyze DOC and total dissolved nitrogen (TDN), the sample was directly collected in precombusted (4 h at 450°C) glass bottles with glass stoppers (100 mL, Schott) and filtered within 4 h through two glass-fiber filters (Whatman, GF/F; precombusted for 2 h at 450°C) using a stainless-steel syringe holder. The filters and the syringe holder were previously rinsed with 20 mL of Milli-Q and 10 mL of sample water. Filtered samples were collected in precombusted (4 h at 450°C) glass vials (40 mL, Shimadzu), acidified with HCl to pH 2, and stored in the dark at 4°C until further analysis (within 48 h). Both DOC and TDN concentrations were measured with a total organic carbon analyzer (Shimadzu TOC-Vc series) equipped with a total nitrogen module. The calibration of the instrument for DOC analysis was done with potassium hydrogen phthalate in the range of 0.4 to 4 mg L−1, and calibration for the TDN analysis was done with potassium nitrate in the range of 0.1 to 2 mg L−1 (for both calibrations: four-point calibration curves; r2 = 0.999). Concentrations of DOC and TDN were detected simultaneously after combustion and catalytic oxidation of the injected sample. Three to five injections were analyzed for each sample. A consensus reference material (CRM) for DOC (batch 5 FS-2005: 0.57 mg C L−1) provided by the Rosenstiel School of Marine and Atmospheric Science, Division of Marine and Atmospheric Chemistry, University of Miami, was run in parallel. Results differed from the CRM given value by 5%, and the coefficient of variation was better than 2%.
Optical characterization of the dissolved organic matter (DOM)
Samples for DOM absorption measurements were filtered as described for DOC analysis and placed in a 10-cm quartz cuvette. Next, the sample was scanned in a double-beam spectrophotometer (Hitachi U-2001) between 250 nm and 750 nm. Milli-Q water was used as reference for the samples, and the absorption coefficients at specific wavelengths (absλ) were calculated as absλ = (Dλ × ln 10)/L, were Dλ is the absorbance at the considered wavelength, and L is the path length (m) of the cuvette. To account for scattering effects by colloids, we corrected the absolute absorption coefficient (aλ) by the absorption at 750 nm. The ratio between the absorption at 254 nm and 365 nm was calculated to assess the dominant relative molecular size of DOM (DeHaan 1993). Further, the DOC-specific UV absorption at 254 nm (SUVA) was used as a proxy for the aromaticity degree of the DOM (Weishaar et al. 2003).
Bacterial abundance
Three independent samples for bacterial abundance were fixed with formaldehyde (2% final concentration) immediately after sampling. Within 24 h, samples were filtered onto black polycarbonate filters (Millipore GBTP, 0.22 μm) and stained with 4′,6-diami-dino-2-phenylindole (DAPI, Molecular Probes) according to Porter and Feig (1980). At least 400 cells in more than 10 microscopic fields per filter were counted using an epifluorescence microscope (Zeiss Axiophot) equipped with a filter set for DAPI (Zeiss No. 1).
Bulk incorporation rates of leucine
Leucine incorporation was measured following the method of Simon and Azam (1989) using [4,5-3H]-L-leucine (Amersham, specific activity = 17.7 GBq mmol−1) at a saturating final concentration of 20 nmol L−1. One formaldehyde-fixed control and duplicate samples (15–40 mL) were incubated for 1.5 h at in situ temperature in the dark. Incubations were stopped by adding formaldehyde (2% final concentration); samples were then filtered onto 0.22-μm polycarbonate filters (Millipore GTTP). The filters were rinsed twice with 5 mL of trichloroacetic acid (5%) for 5 min before being dissolved by adding 6 mL of scintillation cocktail (Ready-safe, Beckman Coulter). Radioactivity was measured after 15 h with a scintillation counter (Beckman LS 6000IC). Cell-specific leucine incorporation rates per day were calculated by dividing the concentration of incorporated leucine (expressed in 10−18 mol) by the number of active bacterial cells assessed by microautoradiography (see below).
Incubation for microautoradiography
Two independent samples were incubated with [4,5-3H]-L-leucine (same specific activity as for bacterial production) at in situ temperature for 2 h. Control samples were fixed with formaldehyde (2% final concentration) 20 min before addition of the substrate and incubated in parallel with the samples. Incubation was stopped with formaldehyde (2% final concentration), and samples were fixed overnight at 4°C. Afterward, samples were filtered onto 0.22-μm white polycarbonate filters (Millipore GTTP) and rinsed with 5 mL of particle-free Milli-Q water. Filters were stored at −20°C for further processing.
In situ hybridization and tyramide signal amplification
Thawed filters were embedded in low-gelling-point agarose (0.2%) and then permeabilized with lysozyme and achromopeptidase as previously described in Pérez and Sommaruga (2006). Hybridizations were carried out as described by Pernthaler et al. (2002a). The following group-specific 5′-horseradish peroxidase (HRP)–labeled oligonucleotide probes (Thermo-Hybrid) were used: domain Bacteria (EUBI-III); Alphaproteobacteria (ALF986); Betaproteobacteria (BET42a); Actinobacteria (HGC69a); Gammaproteobacteria (GAM42a); Cytophaga-like (CF319a); and domain Archaea (ARCH915). Except for the Archaea probe (Stahl and Amann 1991), all others have been previously described in Pérez and Sommaruga (2006). The formamide concentration in the hybridization buffer was 55%, except for the probes HGC69 and ARCH915 (35%). Hybridization was done at 35°C for 5 h, whereas amplification was done in the dark for 15 to 30 min at 37°C in a 0.5-mL reaction vial containing amplification buffer and tyramide-Alexa 488 in a 1 : 100 ratio. Additionally, we made fluorescent in situ hybridization (FISH) with the Cy3-monolabeled probe ARCH915 following the protocol described by Glöckner et al. (1996) because we noticed that filamentous bacteria in our samples yielded double positives with both probes EUBI-III and ARCH915 when the catalyzed reporter deposition (CARD) FISH conditions described in Auguet and Casamayor (2008) were applied to probe ARCH915. Hybridized filter sections were stored at −20°C until they were processed for microautoradiography.
Microautoradiography
For microautoradiography, we slightly modified the procedure described in Tabor and Neihof (1982). Briefly, the agarose-embedded hybridized cells were transferred to gelatine-coated slides, the filter sections were peeled-off, and the cells were coated with a molten Kodak NTB photographic emulsion. Immediately, the slides were transferred onto a cold plate for a few minutes to harden the emulsion. The exposure of the slides was carried out at 4°C in the dark between 72 and 96 h in light-tight boxes containing silica gel. The optimal exposure times for the different lakes were determined empirically in preliminary tests to detect a maximum of labeled cells.
Development and fixation of the slides were done according to the description of the manufacturer. After fixation, the slides were rinsed with Milli-Q water for at least 4 min before being stained with an antifading solution containing DAPI at a final concentration of 1 μg mL−1. The slides were examined with a Zeiss Axioplan microscope equipped with a 100-W Hg lamp and Zeiss filter sets for DAPI, and for Alexa 488 (no. 9). Cells were counted in at least 20 different randomly selected microscopic fields, and for every field, counts were recorded for (1) DAPI-positive cells, (2) probe-specific-positive cells (representing the relative abundance of a given bacterial group), (3) DAPI + autoradiography-positive cells (representative for active cells of the whole bacterial community), and (4) probe specific + autoradiography-positive cells (representative for the fraction of active cells within a bacterial group). At least 400 DAPI-positive cells were counted per sample.
Data analysis
To determine whether an environmental or a microbial parameter was enriched or depleted in the SML compared to the ULW, the ratio of the values of the parameter in these two layers was calculated (Maki 1993). Differences between SML and ULW were tested using Wilcoxon-signed rank tests with an overall significance level of 0.05. Furthermore, relationships among parameters within the whole data set were examined using Spearman rank correlation. Statistical analyses were done using SigmaStat (Systat Software).
Results
Physicochemical parameters
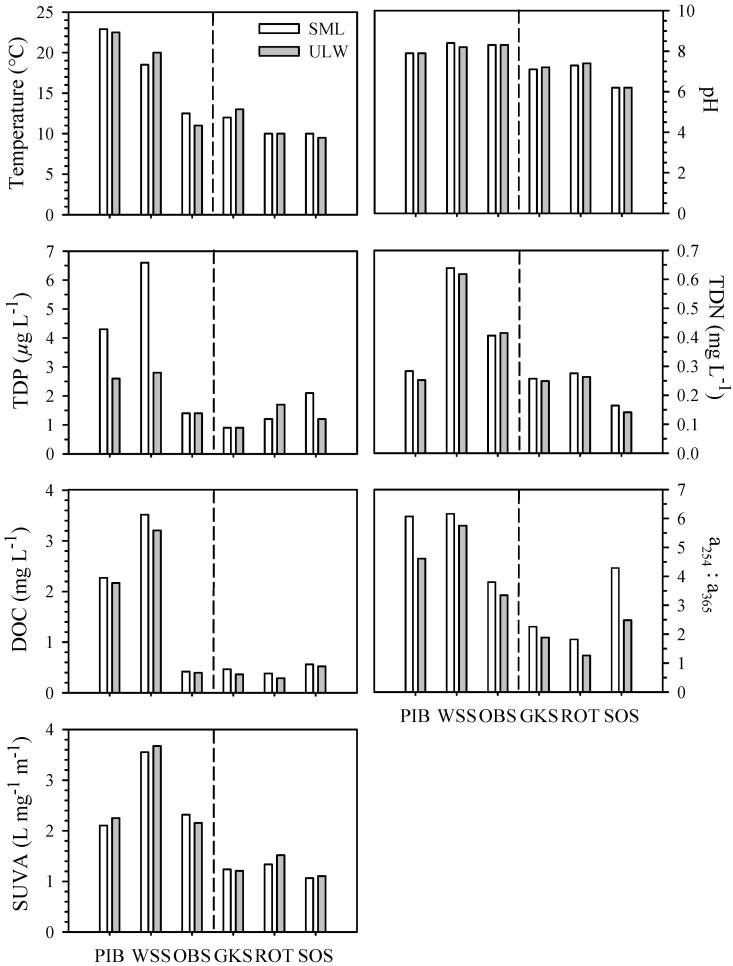
Differences in water temperature between the SML and the ULW within the six lakes did not exceed 1°C (Fig. 1). The highest temperature (22.9°C) was measured in the SML of Piburger See (PIB), a lake located at 913 m above sea level (a.s.l.), whereas the lowest one (9.5°C) was measured in the ULW of the alpine lake Schwarzsee ob Sölden (SOS), located at 2799 m a.s.l. As expected, we found a negative relationship between water temperature and lake altitude for both layers (SML: r = −0.98, p < 0.01; ULW: r = −0.94, p < 0.05).
Fig. 1.
Physicochemical parameters in the surface microlayer (SML) and the underlying water (ULW) of the six lakes. Lakes are ordered according to altitude from 913 m to 2799 m above sea level (a.s.l.). The dashed line indicates the location of the tree line (about 2000 m a.s.l.) in this region of the central Alps. Abbreviations: Total dissolved phosphorus (TDP); total dissolved nitrogen (TDN); dissolved organic carbon (DOC); DOM absorption ratio between 254 nm and 365 nm (a254 : a365); DOC-specific UV absorption at 254 nm (SUVA); Piburger See (PIB); Wildsee Seefeld (WSS); Obernberger See (OBS); Gossenköllesee (GKS); Rotfelssee (ROT); Schwarzsee ob Sölden (SOS).
Concentrations of TDP in the SML ranged from 0.9 to 6.6 μg L−1, and in the ULW from 0.9 to 2.8 μg L−1, but they were not significantly different between layers (Fig. 1). The SML of PIB, SOS, and Wildsee Seefeld (WSS) was enriched in TDP (Table 2), whereas no enrichment was found in the other lakes.
Table 2.
Ratio of main environmental and biological parameters between the surface microlayer (SML) and the underlying water (ULW) in the six lakes
| Lake | DOC | a254 : a365 | TDP | TDN | ABU | BULK | FBact | BET | HGC | ALF | CYT | BETact | HGCact | ALFact | CYTact |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PIB | 1.05 | 1.32 | 1.65 | 1.12 | 1.12 | 1.41 | 1.48 | 1.79 | 1.81 | 0.67 | 1.01 | 1.11 | 3.61 | 0.62 | — |
| WSS | 1.10 | 1.07 | 2.36 | 1.03 | 1.38 | 1.40 | 0.77 | 1.32 | 0.71 | 1.58 | 0.91 | 1.03 | 0.74 | 1.33 | — |
| OBS | 1.06 | 1.14 | 1.00 | 0.98 | 0.95 | 0.37 | 0.56 | 0.93 | 0.83 | 0.71 | 1.23 | 0.68 | 1.33 | 0.44 | — |
| GKS | 1.28 | 1.20 | 1.00 | 1.03 | 0.81 | 2.91 | 0.42 | 1.05 | 0.60 | 0.72 | 0.94 | 0.94 | 0.73 | 0.48 | 2.28 |
| ROT | 1.32 | 1.45 | 0.71 | 1.05 | 1.24 | 0.42 | 1.35 | 1.27 | 0.81 | 1.79 | 0.78 | 1.26 | 0.56 | — | 0.68 |
| SOS | 1.08 | 1.73 | 1.75 | 1.17 | 1.42 | 0.86 | 2.27 | 1.18 | 0.78 | 1.46 | 1.16 | 1.22 | 0.68 | — | — |
DOC, dissolved organic carbon; a254 : a365, DOM absorption ratio between 254 nm and 365 nm; TDP, total dissolved phosphorus, TDN, total dissolved nitrogen; ABU, bacterial abundance; BULK, bulk incorporation rates of leucine per active cell; FBact, fraction of active bacteria; BET, Betaproteobacteria; HGC, Actinobacteria; ALF, Alphaproteobacteria; CYT, Cytophaga-like group; BETact, active Betaproteobacteria; HGCact, active Actinobacteria; ALFact, active Alphaproteobacteria; CYTact, active Cytophaga-like group. (—) ratio not assessable.
Concentrations of TDN (Fig. 1) ranged from 0.14 to 0.64 mg L−1, and the highest concentration was found in the SML of WSS. TDN concentrations were fairly similar between the two layers. Nevertheless, a slight enrichment was found in the SML of SOS (17%) and of PIB (12%) (Table 2).
DOC concentrations differed by over an order of magnitude among lakes; the highest concentration (3.51 mg L−1) was found in the SML of WSS, located at 1177 m a.s.l., and the lowest concentration (0.29 mg L−1) was found in the ULW of the alpine Rotfelssee (ROT), located at 2485 m a.s.l. (Fig. 1). Significantly higher (Wilcoxon-signed rank test) DOC concentrations were found in the SML than in the ULW (Fig. 1), and the strongest enrichments in DOC (~ 30%) were found in Gossenköllesee (GKS) and ROT (Table 2).
Optical characteristics of the DOM
The highest value of the a254 : a365 ratio was measured in the SML of WSS (6.16), followed by PIB (6.07), which were those lakes located at low elevation (Fig. 1). The SML of all lakes showed significantly higher a254 : a365 ratios (Wilcoxon-signed rank test) than the ULW (Fig. 1). For example, the a254 : a365 ratio in the SML of SOS was 73% higher than in the ULW. In both layers, the a254 : a365 ratio was positively correlated to DOC (SML: r = 0.94, p < 0.05; ULW: r = 0.94, p < 0.01).
As expected, the DOM of the lakes located below the tree line had a higher absorption coefficient at 254 nm (SUVA) than those above tree line (Fig. 1). The values of SUVA coefficient were not significantly different between the SML and the ULW. In the SML, SUVA values were positively correlated to pH (r = 1.00, p < 0.01) and TDN concentrations (r = 1.00, p < 0.01), whereas in the ULW, SUVA values were negatively related to lake altitude (r = −0.88, p < 0.05) and positively related to TDP concentrations (r = 0.88, p < 0.05).
Bacterial abundance and community composition
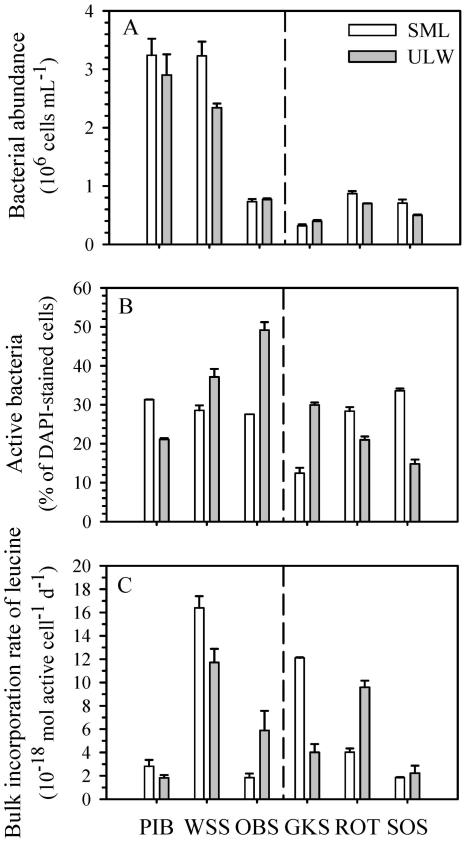
The bacterial assemblage in all lakes was dominated by free-living cells. Bacterial abundance ranged from 3.2 × 105 cells mL−1 in the SML of GKS to 3.2 × 106 cells mL−1 in the SML of WSS and PIB (Fig. 2A), but no consistent enrichment or depletion was observed among the six lakes (Table 2). In the ULW, bacterial abundance was positively correlated to TDP concentrations (r = 0.88, p < 0.05) and to the SUVA (r = 0.88, p < 0.05).
Fig. 2.
(A) Bacterial abundance, (B) percentage of active bacteria, and (C) bulk incorporation rates of leucine per active cell in the surface microlayer (SML) and the underlying water (ULW) of the six lakes. The lakes are ordered according to altitude, from 913 m to 2799 m above sea level (a.s.l.). The dashed line indicates the location of the tree line (about 2000 m a.s.l.) in this region of the central Alps. Values represent the mean ± 1 SD (n = 3 for bacterial numbers and n = 2 for the percentage of active bacteria and bulk incorporation rates of leucine). Piburger See (PIB); Wildsee Seefeld (WSS); Obernberger See (OBS); Gossenköllesee (GKS); Rotfelssee (ROT); Schwarzsee ob Sölden (SOS).
Between 75% and 94% of the DAPI-stained cells in all six lakes belonged to the domain Bacteria, and the lowest detection rates were observed in PIB (76.2% for the SML and 75.2% for the ULW). The numbers of ARCH915-positive cells ranged from 1.0% of DAPI-stained cells in the ULW of PIB to a maximum of 8.9% of DAPI-stained cells in the ULW of SOS (data not shown). On average, 96% of the cells identified as Bacteria were targeted with the four group-specific probes we used.
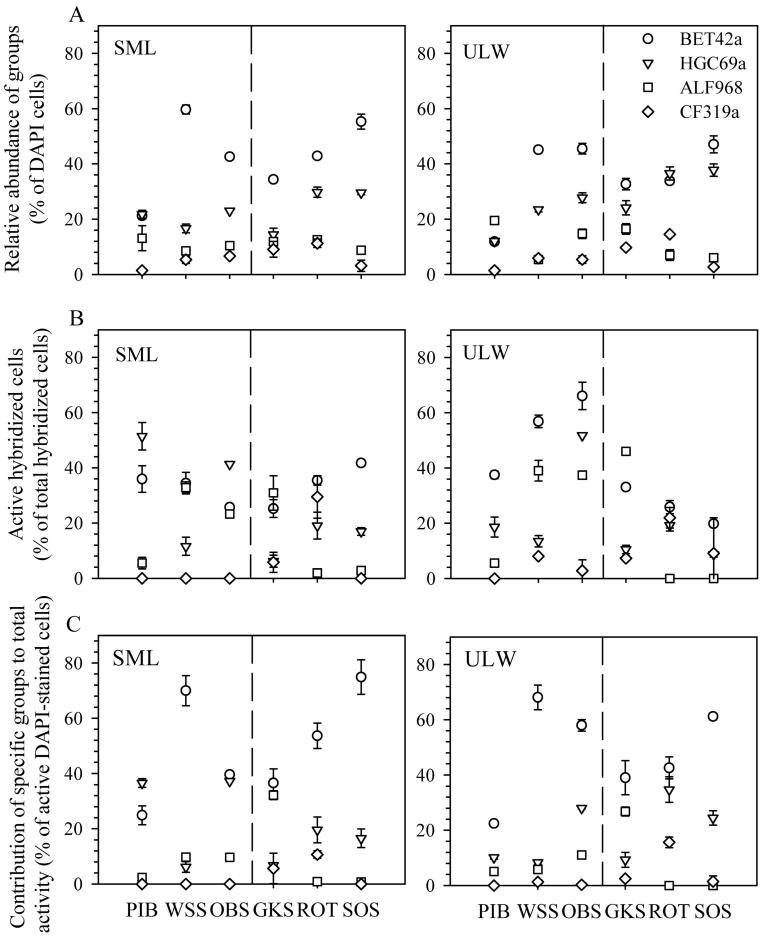
The bacterial assemblage of both layers was dominated by Betaproteobacteria in four out of six lakes. In the SML of PIB, Actinobacteria were almost as abundant as Betaproteobacteria, whereas in the ULW of PIB and ROT, Alphaproteobacteria and Actinobacteria, respectively, were slightly more numerous than Betaproteobacteria (Fig. 3A). The highest relative abundance of Betaproteobacteria (59.7% of DAPI counts) was found in the SML of WSS, whereas the lowest (11.8% of DAPI counts) was found in the ULW of PIB (Fig. 3A). Actinobacteria was the second most abundant group (except in the ULW of PIB and GKS), with relative abundances ranging from 12.3% to 37.7% of DAPI-stained cells (Fig. 3A). Alphaproteobacteria and Cytophaga-like groups were present in both layers, but their relative abundance in the SML was < 12% (Fig. 3A).
Fig. 3.
(A) Relative abundance of the main bacterial groups targeted by the probes Betaproteobacteria (BET42a), Actinobacteria (HGC96a), Alphaproteobacteria (ALF968), and Cytophaga-like (CF319a). (B) Active fraction within the specific bacterial groups. (C) Relative contribution of the specific bacterial groups to the total activity in the surface microlayer (SML) and the underlying water (ULW) of the six lakes. Values represent the mean ± 1 SD (n = 2). The lakes are ordered according to altitude, from 913 m to 2799 m above sea level (a.s.l.). The dashed line indicates the location of the tree line (about 2000 m a.s.l.) in this region of the central Alps. Piburger See (PIB); Wildsee Seefeld (WSS); Obernberger See (OBS); Gossenköllesee (GKS); Rotfelssee (ROT); Schwarzsee ob Sölden (SOS).
The SML of four out of six lakes was enriched in Betaproteobacteria, with the strongest enrichment (79%) observed in PIB, whereas in five lakes out of six, the relative abundance of Actinobacteria was lower in the SML than in the ULW. Particularly, in the SML of GKS, the relative abundance of Actinobacteria was 40% lower than in the ULW (Table 2). There was no consistent enrichment or depletion in the relative abundance of Alphaproteobacteria and Cytophaga-like groups in the SML of the lakes considered (Table 2).
A negative relationship between the Cytophaga-like group and the a254 : a365 ratio (r = −0.83, p < 0.05) was found in the SML, whereas in the ULW, the relative abundance of Actinobacteria was positively correlated to altitude (r = 0.94, p < 0.05).
Bacterial activity at the community and the group level
The percentage of active bacteria in the SML represented ~ 30% (± 3%) of DAPI-stained cells for all lakes, except for GKS (12.5%), whereas in the ULW, this fraction ranged from 15% in SOS to 49% in Obernberger See (OBS) (Fig. 2B). The active fraction of the bacterial community was enriched in the SML of PIB (48%), ROT (35%), and SOS (127%), whereas the SML of the other three lakes was depleted in active bacteria (WSS: 23%, OBS: 44%, and GKS: 58%) (Fig. 2B; Table 2).
Leucine bulk incorporation rates did not show any consistent trend between the SML and the ULW when data from all lakes were pooled (Fig. 2C). Incorporation rates were higher in the SML than in the ULW of WSS (40%) and GKS (191%), but lower in OBS (by 63%) and ROT (by 58%) (Fig. 2C; Table 2).
Between 25% and 42% of all Betaproteobacteria were active in the SML (Fig. 3B), and between 20% and 66% were active in the ULW. Enrichments in active Betaproteobacteria were found in the SML of PIB (11%), ROT (26%), and SOS (22%), whereas a depletion of 32% was found in OBS (Table 2). Furthermore, Betaproteobacteria was the bacterial group that contributed most to total activity (Fig. 3C) in both the SML and the ULW of all lakes (except in the SML of PIB). For example, in the SML of SOS and WSS, Betaproteobacteria represented 75% and 70%, respectively, of all active cells (Fig. 3C).
The SML of PIB and OBS was enriched in active Actinobacteria (3.61 and 1.33, respectively), but a depletion was found in the SML of the other lakes (Fig. 3B; Table 2). Regarding their contribution to total activity (Fig. 3C), Actinobacteria was generally the second group in importance, particularly in the ULW, except for GKS. No significant trend was found when data of active bacterial groups of all lakes were pooled.
Discussion
Does the SML represent a distinct physicochemical environment?
Previous comparisons of physicochemical parameters between the SML and the ULW in marine and freshwater environments have detected enrichments in inorganic and organic nutrients at the air–water interface (Maki and Remsen 1989; Münster et al. 1997). However, a direct comparison among these studies is difficult because of the use of diverse sampling devices and techniques (reviewed by Maki 1993). In this study, we used the same sampling technique for sampling physicochemical and microbial parameters to be able to directly compare the SML with the ULW. Moreover, considering the relatively small area (< 0.15 km2) and low wind fetch (< 0.9 km) of the lakes, as well as their location in relatively steep catchments, it is probable that the SML is more protected from frequent disruptions than in larger, wind-exposed aquatic systems. Further, our sampling was done during a period of calm weather (stable conditions at least 2 d before and during the sampling), and in consequence, we argue that differences in the physicochemical characteristics of the SML reflect real differences and are not biased by sampling conditions.
Within a single lake, nearly all measured physicochemical parameters differed between the SML and the ULW (Table 2), indicating the existence of a distinct surface microlayer with unique physicochemical characteristics in all lakes. However, the absolute values of most parameters differed greatly among lakes. Only the DOC concentration and the relative molecular size of DOM were significantly higher in the SML of all lakes compared to the ULW (Table 2; Fig. 1). The DOC-specific UV absorption (SUVA) also differed strongly among lakes (Fig. 1), indicating different sources and, thus, composition of the DOM. The highest SUVA value was found in Wildsee Seefeld (WSS), the only lake in our survey that had a developed belt of emergent macrophytes.
Differences in the chemical composition of the SML have been found when comparing different aquatic systems (Gasparovic et al. 1998), but also within an ecosystem. For example, the SML from a small coastal lake differed in its reflection spectrum due to spatial heterogeneities in the optical characteristics and concentration of chromophoric DOM (Kozarac et al. 2005). In Piburger See (PIB), which is located in a forested catchment, 39% of the total phosphorus and 18% of the nitrogen load derive from airborne material that is heterogeneously distributed in the lake surface (Psenner 1984). Therefore, it is important to obtain a representative sample by collecting the SML at different points within a system.
Bacterial abundance, community composition, and activity
Bacterial abundance was slightly enriched in the SML of four out six lakes (Table 2), which is in agreement with the higher DOC concentration found in this layer (Fig. 1; Table 2). Similarly, enrichments in bacterial abundance have also been found in the SML of other marine and freshwater environments (Kuznetsova et al. 2004; Kalwasinska and Donderski 2005; Joux et al. 2006). Crawford et al. (1982) observed higher enrichment in bacterial cells in the SML of oligotrophic lakes than of eutrophic ones. However, the enrichment in bacterial abundance observed in our study did not seem to be related to the trophic degree of the lake.
The highest bacterial abundance found in the two lowest altitude lakes (i.e., Piburger See and Wildsee Seefeld) was not unexpected because several parameters such as water temperature and nutrients (DOC and TDP concentrations) changed substantially across the altitude gradient. In fact, these two lakes recorded the highest water temperatures, as well as DOC and TDP concentrations (Figs. 1, 2).
Most of the information available on the composition of bacterioneuston is based on nonquantitative techniques such as cultivation (Mudryk and Skorczewski 2000; Joux et al. 2006) and/or physiological tests (Carlucci et al. 1985; Maki and Remsen 1989). Molecular techniques such as 16S rRNA clone libraries or single-strand conformation polymorphism (SSCP) have been used for qualitative comparisons between bacterioneuston and bacterioplankton in marine environments (Franklin et al. 2005; Cunliffe et al. 2008; Obernosterer et al. 2008), and more recently also in freshwater bodies (Auguet and Casamayor 2008; Hervàs and Casamayor 2009). Nevertheless, there have been no quantitative studies on the composition and activity of the bacterioneuston as compared to the bacterioplankton, though such studies are essential for determining the bacterial groups that can “successfully” colonize or dominate the SML in freshwaters.
We found Betaproteobacteria to generally dominate the bacterial community of both layers and to be enriched in the SML of most lakes (Fig. 3A). At first glance, this is striking considering the potential difference in bacterial dynamics among these very different lakes. However, Betaproteobacteria are a ubiquitous group in freshwater ecosystems (Zwart et al. 2002) and often dominate the bacteria assemblage of mountain lakes (Pérez and Sommaruga 2006; Nelson 2009). Some of their members have been found in very remote alpine lakes located at high altitude (Sommaruga and Casamayor 2009), which is in agreement with the notion that members of this group are transported through the atmosphere by aerosols (Amato et al. 2007). Furthermore, members of Betaproteobacteria respond rapidly to the supply of nutrients (Pérez and Sommaruga 2006), which could be an advantageous strategy for an active colonization at the air–water interface. Recently, a high similarity between airborne neustonic and planktonic members of Betaproteobacteria was found in a lake of the Pyrenees, Spain (Hervàs and Casamayor 2009), suggesting that airborne members of this group successfully colonize the SML of lakes. The SML of high-altitude lakes acts as an interceptor of atmospheric deposition of diverse origin (Psenner 1999). For example, aerosols containing Saharan dust have been found to influence the composition of the bacterial community in lakes of the Sierra Nevada, Spain (Reche et al. 2009). At the latitude of the lakes in our study, Sahara intrusions occur at lower frequency than in the Mediterranean region, but three to four such events were recorded in Tyrol in 2007 and 2008 (Hörtnagl and Sommaruga unpubl.).
Another important group within the freshwater bacterioplankton, and particularly in mountain lakes, is Actinobacteria (Warnecke et al. 2005), which was the second most abundant one in our study (Fig. 3A), and it was particularly enriched in the ULW of most lakes. In fact, both the abundance of Actinobacteria and their relative contribution to the bacterioplankton in the ULW increased with altitude (Fig. 3A). This is in agreement with the study of Warnecke et al. (2005), who previously assessed the bacterioplankton of some of the lakes in our study. By contrast, the strong enrichment of Actinobacteria, particularly of active cells observed in the SML of Piburger See (Table 2), which was the lowest lake in our series, suggests a different origin of this group.
Unlike the results of Auguet and Casamayor (2008) for high mountain lakes of the Pyrenees, we found that Archaea represented a rather small fraction of the bacterioneuston and bacterioplankton in the sampled lakes. Our results are in agreement with the study of Pernthaler et al. (1998) done in Gossenköllesee (GKS), where they reported that Archaea were only abundant in the bacterioplankton during autumn and hardly present thereafter. Nevertheless, we caution interpretations on the abundance values detected by probe ARCH915 because this probe seems to unspecifically bind to a variable fraction of Eubacteria cells when following both the protocol for FISH (Pernthaler et al. 2002b) and for CARD-FISH (Auguet and Casamayor 2008). In fact, the use of the stringency conditions reported by Auguet and Casamayor (2008) led to an unacceptable number of double positives in our samples.
Among the bacterial assemblages of the six lakes, the most abundant bacterial group (i.e., Betaproteobacteria) was also the most active in both layers, whereby the contribution of this group to the total activity differed strongly among lakes (Fig. 3A,C). For instance, in the oligotrophic Schwarzsee ob Sölden, the relative contribution of active Betaproteobacteria to the total activity in the SML and the ULW was comparable to that found in the mesotrophic Wildsee Seefeld. Though this is at first glance counterintuitive, the comparison is not straightforward because there are not necessarily the same members within the Betaproteobacteria contributing to the high activity in these two lakes.
Although previous studies have reported lower metabolic activities of the bacterioneuston compared to the underlying bacterioplankton (reviewed by Maki 1993), we could not find a general trend in our data set that supports this argument (Fig. 2). Interestingly, different trends in the fraction of active cells were observed in the ULW of lakes located below and above the tree line. In lakes located below the tree line, the fraction of active bacteria increased with increasing altitude, while in the lakes located above the tree line, this fraction decreased with increasing altitude (Fig. 2B). At present, we do not have an explanation for the different trends, but it would be important to assess the dominant and active bacteria in the ULW and SML of different aquatic ecosystems with higher taxonomical resolution.
Despite large differences in environmental conditions among the six lakes, the fraction of active bacteria was very similar in the SML of most of them. For instance, 30% ± 3% of the bacteria in the SML of five out of six lakes incorporated leucine (Fig. 2B). This result is striking considering the large difference in water temperature (13.4°C) and in other parameters such as DOC and TDP among these lakes, and it implies that many of the bacteria in the SML are well adapted to the extreme conditions prevailing in this habitat. This is in agreement with the idea that bacterioneuston is more tolerant of stressful environmental conditions than bacteria from bulk waters (Maki 1993). On the other hand, significant differences in cell-specific leucine incorporation rates were observed between both layers, reflecting the particularities of each lake (Fig. 2C). For example, cell-specific bacterial activity was apparently stimulated by the high concentration and availability of DOC and nutrients in the SML of Wildsee Seefeld (Figs. 1, 2C).
One factor that changes considerably in lakes located across an altitude gradient is the level of UV exposure (Laurion et al. 2000). UV radiation can alter the DOM pool (e.g., by photodegradation) and subsequently bacterial activity (Pérez and Sommaruga 2007). Indeed, we expected that photodegradation of DOM could result in higher bacterial activity at the SML, but this was not always observed (Fig. 2). One possible explanation is that exposure history of DOM and its reactivity to solar UV radiation in the SML differed among lakes. On the other hand, it could be difficult to detect a stimulatory effect because the higher absorption of UV radiation by surface films compared to underlying waters (Carlson 1982) can also inhibit bacterial activity (Sommaruga et al. 1997) despite enriched nutrient concentrations. For instance, the level of UV exposure of the SML and ULW is very different among lakes. Thus, whereas in Piburger See, the 50% attenuation depth of UV-B at 305 nm is found at 0.15 m, in the alpine lake Schwarzsee ob Sölden, it is approximately 10 times deeper (Laurion et al. 2000).
In summary, our results show that the SML and the ULW of lakes do not only differ in many physicochemical parameters, but also in bacterial community composition and bacterial activity at the community and group level. The community composition of both layers consisted of common freshwater bacterial groups and was dominated in most cases by Betaproteobacteria. The abundance of this group was generally higher in the SML than in the ULW and was also the most active one in both layers. Finally, the SML seems to reflect the peculiarities of the different lakes and the various common freshwater bacterial groups that are metabolically active despite the extreme conditions at the air–water interface.
Acknowledgments
We thank J. Franzoi and W. Müller for analyzing the nutrients and dissolved organic carbon, R. Psenner for helpful comments, and the Tyrolean department of the Central Institute for Meteorology and Geodynamics, the Department of Meteorology (University of Innsbruck), and the Tiroler Wasserkraft AG (TIWAG) for providing meteorological data. This work was supported by the Austrian Science Fund (FWF) through a research project (P19245-BO3) to R. S.
References
- Agogué H, Casamayor EO, Bourrain M, Obernosterer I, Joux F, Herndl GJ, Lebaron P. A survey on bacteria inhabiting the sea surface microlayer of coastal ecosystems. FEMS Microbiol. Ecol. 2005;54:269–280. doi: 10.1016/j.femsec.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Agogué H, others Comparison of samplers for the biological characterization of the sea surface microlayer. Limnol. Oceanogr. Meth. 2004;2:213–225. [Google Scholar]
- Amato P, Parazols M, Sancelme M, Laj P, Mailhot G, Delort AM. Microorganisms isolated from the water phase of tropospheric clouds at the Puy de Dome: Major groups and growth abilities at low temperatures. FEMS Microbiol. Ecol. 2007;59:242–254. doi: 10.1111/j.1574-6941.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- Auguet JC, Casamayor EO. A hotspot for cold Crenarchaeota in the neuston of high mountain lakes. Environ. Microbiol. 2008;10:1080–1086. doi: 10.1111/j.1462-2920.2007.01498.x. doi:10.1111/j.1462-2920.2007.01498.x. [DOI] [PubMed] [Google Scholar]
- Carlson DJ. Surface microlayer phenolic enrichments indicate sea-surface slicks. Nature. 1982;296:426–429. doi:10.1038/296426a0. [Google Scholar]
- Carlucci AF, Craven DB, Henrichs SM. Surface-film microheterotrophs: Amino acid metabolism and solar radiation effects on their activities. Mar. Biol. 1985;85:13–22. doi:10.1007/BF00396410. [Google Scholar]
- Conrad R, Seiler W. Influence of the surface microlayer on the flux of nonconservative trace gases (CO, H2, CH4, N2O) across the ocean–atmosphere interface. J. Atmos. Chem. 1988;6:83–94. doi:10.1007/BF00048333. [Google Scholar]
- Crawford RL, Johnson L, Martinson M. Bacterial enrichment in surface films of freshwater lakes. J. Great Lake Res. 1982;8:323–325. [Google Scholar]
- Cunliffe M, Schafer H, Harrison E, Cleave S, Upstill-Goddard R, Murrell JC. Phylogenetic and functional gene analysis of the bacterial and archaeal communities associated with the surface microlayer of an estuary. ISME J. 2008;2:776–789. doi: 10.1038/ismej.2008.28. doi:10.1038/ismej.2008.28. [DOI] [PubMed] [Google Scholar]
- Cypionka H, Babenzien H-D, GLöckner F, Amann R. The genus Nevskia. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editors. The prokaryotes. Springer; 2006. pp. 1152–1155. [Google Scholar]
- DeHaan H. Solar UV-light penetration and photodegradation of humic substances in peaty lake water. Limnol. Oceanogr. 1993;38:1072–1076. [Google Scholar]
- Dietz AS, Albright LJ, Tuominen T. Heterotrophic activities of bacterioneuston and bacterioplankton. Can. J. Microbiol. 1976;22:1699–1709. doi: 10.1139/m76-251. [DOI] [PubMed] [Google Scholar]
- Franklin MP, McDonald IR, Bourne DG, Owens NJP, Upstill-Goddard RC, Murrell CJ. Bacterial diversity in the bacterioneuston (sea surface microlayer): The bacterioneuston through the looking glass. Environ. Microbiol. 2005;7:723–736. doi: 10.1111/j.1462-2920.2004.00736.x. doi:10.1111/j.1462-2920.2004.00736.x. [DOI] [PubMed] [Google Scholar]
- Gasparovic B, Kozarac Z, Saliot A, Cosovic B, Möbius D. Physicochemical characterization of natural and ex-situ reconstructed sea-surface microlayers. J. Coll. Interf. Sci. 1998;208:191–202. doi: 10.1006/jcis.1998.5792. doi:10.1006/jcis.1998.5792. [DOI] [PubMed] [Google Scholar]
- Glöckner FO, Amann R, Alfreider A, Pernthaler J, Psenner R, Trebesius K, Schleifer KH. An in situ hybridization protocol for detection and identification of planktonic bacteria. Syst. Appl. Microbiol. 1996;19:403–406. [Google Scholar]
- Hardy JT. The sea surface microlayer: Biology, chemistry and anthropogenic enrichment. Prog. Oceanogr. 1982;11:307–328. doi:10.1016/0079-6611(82)90001-5. [Google Scholar]
- Hermansson M, Dahlbäck B. Bacterial activity at the air/water interface. Microb. Ecol. 1983;9:317–328. doi: 10.1007/BF02019021. doi:10.1007/BF02019021. [DOI] [PubMed] [Google Scholar]
- Hervàs A, Casamayor EO. High similarity between bacterioneuston and airborne bacterial community compositions in a high mountain lake area. FEMS Microbiol. Ecol. 2009;67:219–228. doi: 10.1111/j.1574-6941.2008.00617.x. doi:10.1111/j.1574-6941.2008.00617.x. [DOI] [PubMed] [Google Scholar]
- Joux F, Agogué H, Obernosterer I, Dupuy C, Reinthaler T, Herndl GJ, Lebaron P. Microbial community structure in the sea surface microlayer at two contrasting coastal sites in the northwestern Mediterranean Sea. Aquat. Microb. Ecol. 2006;42:91–104. doi:10.3354/ame042091. [Google Scholar]
- Kalwasinska A, Donderski W. Neustonic versus planktonic bacteria in eutrophic lake. Pol. J. Ecol. 2005;53:571–577. [Google Scholar]
- Kozarac Z, Risovic D, Frka S, Möbius D. Reflection of light from the air/water interface covered with sea-surface microlayers. Mar. Chem. 2005;96:99–113. doi:10.1016/j.marchem.2004.12.003. [Google Scholar]
- Kuznetsova M, Lee C, Aller J, Frew N. Enrichment of amino acids in the sea surface microlayer at coastal and open ocean sites in the North Atlantic Ocean. Limnol. Oceanogr. 2004;49:1605–1619. [Google Scholar]
- Laurion I, Ventura M, Catalan J, Psenner R, Sommaruga R. Attenuation of ultraviolet radiation in mountain lakes: Factors controlling the among- and within-lake variability. Limnol. Oceanogr. 2000;45:1274–1288. [Google Scholar]
- Maki JS. The air–water interface as an extreme environment. In: Ford TE, editor. Aquatic microbiology: An ecological approach. Blackwell; 1993. pp. 409–439. [Google Scholar]
- Maki JS, Remsen CC. Examination of a freshwater surface microlayer for diel changes in the bacterioneuston. Hydrobiologia. 1989;182:25–34. doi:10.1007/BF00006365. [Google Scholar]
- Mudryk ZJ, Skorczewski P. Occurrence and activity of lipolytic bacterioneuston and bacterioplankton in the estuarine Lake Gardno. Estuar. Coast. Shelf Sci. 2000;51:763–772. doi:10.1006/ecss.2000.0719. [Google Scholar]
- Münster U, Heikkinen E, Knulst J. Nutrient composition, microbial biomass and activity at the air–water interface of small boreal forest lakes. Hydrobiologia. 1997;363:261–270. doi:10.1023/A:1003106713091. [Google Scholar]
- Naumann E. Contribution to the knowledge of pond nanoplankton. II. Freshwater neuston. Biol. Zentralbl. 1917;37:98–106. In German. [Google Scholar]
- Nelson CE. Phenology of high-elevation pelagic bacteria: The roles of meteorologic variability, catchment inputs and thermal stratification in structuring communities. ISME J. 2009;3:13–30. doi: 10.1038/ismej.2008.81. doi:10.1038/ismej.2008.81. [DOI] [PubMed] [Google Scholar]
- Norkrans B. Surface microlayers in aquatic environments. Adv. Microb. Ecol. 1980;4:51–85. [Google Scholar]
- Obernosterer I, others Biochemical characteristics and bacterial community structure of the sea surface microlayer in the South Pacific Ocean. Biogeosciences. 2008;5:693–705. [Google Scholar]
- Pérez MT, Sommaruga R. Differential effect of algal- and soil-derived dissolved organic matter on alpine lake bacterial community composition and activity. Limnol. Oceanogr. 2006;51:2527–2537. [Google Scholar]
- Pérez MT, Sommaruga R. Interactive effects of solar radiation and dissolved organic matter on bacterial activity and community structure. Environ. Microbiol. 2007;9:2200–2210. doi: 10.1111/j.1462-2920.2007.01334.x. doi:10.1111/j.1462-2920.2007.01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernthaler A, Pernthaler J, Amann R. Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl. Environ. Microbiol. 2002a;68:3094–3101. doi: 10.1128/AEM.68.6.3094-3101.2002. doi:10.1128/AEM.68.6.3094-3101.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernthaler A, Preston CM, Pernthaler J, DeLong EF, Amann R. Comparison of fluorescently labeled oligonucleotide and polynucleotide probes for the detection of pelagic marine bacteria and archaea. Appl. Environ. Microbiol. 2002b;68:661–667. doi: 10.1128/AEM.68.2.661-667.2002. doi:10.1128/AEM.68.2.661-667.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernthaler J, GLöckner FO, Unterholzner S, Alfreider A, Psenner R, Amann R. Seasonal community and population dynamics of pelagic bacteria and archaea in a high mountain lake. App. Environ. Microbiol. 1998;64:4299–4306. doi: 10.1128/aem.64.11.4299-4306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter KG, Feig YS. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 1980;25:943–948. [Google Scholar]
- Psenner R. The proportion of empneuston and total atmospheric inputs of carbon, nitrogen and phosphorus in the nutrient budget of a small mesotrophic lake (Piburger See, Austria) Int. Rev. Ges. Hydrobiol. 1984;69:23–39. doi:10.1002/iroh.19840690103. [Google Scholar]
- Psenner R. Living in a dusty world: Airborne dust as a key factor for Alpine lakes. Water Air Soil Poll. 1999;112:217–227. doi:10.1023/A:1005082832499. [Google Scholar]
- Reche I, Ortega-Retuerta E, Romera O, Pulido-Villena E, Morales-Baquero R, Casamayor E. Effects of Saharan dust inputs on bacterial activity and composition in freshwater ecosystems. Limnol. Oceanogr. 2009;54:869–879. [Google Scholar]
- Simon M, Azam F. Protein content and protein synthesis rates of planktonic marine bacteria. Mar. Ecol. Prog. Ser. 1989;51:201–213. doi:10.3354/meps051201. [Google Scholar]
- Sommaruga R, Casamayor EO. Bacterial ‘cosmopolitanism’ and importance of local environmental factors for community composition in remote high-altitude lakes. Freshwater Biol. 2009;54:994–1005. doi: 10.1111/j.1365-2427.2008.02146.x. doi: 10.1111/j.1365-2427.2008.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommaruga R, Obernosterer I, Herndl GJ, Psenner R. Inhibitory effect of solar radiation on thymidine and leucine incorporation by freshwater and marine bacterioplankton. Appl. Environ. Microbiol. 1997;63:4178–4184. doi: 10.1128/aem.63.11.4178-4184.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl DA, Amann R. Development and application of nucleic acid probes in bacterial systematics. In: Stackebrandt E, Goodfellow M, editors. Sequencing and hybridization techniques in bacterial systematics. Wiley; 1991. pp. 205–248. [Google Scholar]
- Tabor PS, Neihof RA. Improved microautoradiographic method to determine individual microorganisms active in substrate uptake in natural waters. Appl. Environ. Microbiol. 1982;44:945–953. doi: 10.1128/aem.44.4.945-953.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upstill-Goddard RC, Frost T, Henry GR, Franklin M, Murrell JC, Owens NJP. Bacterioneuston control of air–water methane exchange determined with a laboratory gas exchange tank. Glob. Biogeochem. Cy. 2003;17:1–15. [Google Scholar]
- Warnecke F, Sommaruga R, Sekar R, Hofer JS, Pernthaler J. Abundances, identity, and growth state of Actinobacteria in mountain lakes of different UV transparency. Appl. Environ. Microbiol. 2005;71:5551–5559. doi: 10.1128/AEM.71.9.5551-5559.2005. doi:10.1128/AEM.71.9.5551-5559.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weishaar JL, Aiken GR, Bergamaschi BA, Fram MS, Fujii R, Mopper K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003;37:4702–4708. doi: 10.1021/es030360x. doi:10.1021/es030360x. [DOI] [PubMed] [Google Scholar]
- Zwart G, Crump BC, Agterveld MP, Hagen F, Han SK. Typical freshwater bacteria: An analysis of available 16S rRNA gene sequences from plankton of lakes and rivers. Aquat. Microb. Ecol. 2002;28:141–155. doi:10.3354/ame028141. [Google Scholar]