Since november, 1962, 12 renal homografting procedures, exclusive of a previously reported isograft 1 between identical twins, have been performed at the University of Colorado Medical Center. During this period numerous observations have been made which bear on various aspects of the complex care of these patients, including (a) technical refinements in the procurement, preservation, and implantation of the kidneys, (b) selection of donor sources, and (c) prevention, recognition, and reversal of the rejection process. An evaluation of the early results in these cases provides hope that renal homografts may be performed with a higher success rate than previously thought possible.
Materials and Methods
Case Material
The cases have been divided into two categories (Table). The first group of ten patients received homografts from living donors. The second group consisted of two patients for whom the renal homografts were obtained from recently deceased cadavers. The patients were all males ranging from 12 to 50 years of age. Renal failure was due to end-stage chronic glomerulonephritis in ten patients. One patient was referred after unintentional surgical removal of his only kidney. The oldest patient had polycystic disease. Uremia had been present for many months or years except in the patient with inadvertent nephrectomy. In all except Patient No. 9 multiple preoperative hemodialyses were required as often as 24 times (case 1).
Table.
Cases of Renal Homotransplantation*
| Patient No. and Age (Yr) |
Donor to Recipient Blood Types |
Date of Thymectomy |
Date of Splenectomy |
Left Nephrectomy |
Right Nephrectomy |
Date of Transplant |
Age (Yr) and Relation of Donor |
Duration of Ischemia |
Rejection Attempt |
Outcome | Renal Function 6-1-63 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Homograft with Living Donor | |||||||||||
| 1 (12 | B+-B+ | 10-23-62 | 11-4-62 | 11-4-62 | 11-4-62 | 11-24-62 | 35 (mother) | 34 min | 14 days | Alive | Good |
| 2 (38) | B+-A+ | 1-17-63 | 1-31-63 | 1-31-63 | Not done | 1-31-63 | 32 (sister) | 28 min | 27 days | Alive | Good |
| 3 (21) | A+-A+ | 1-24-63 | 2-2-63 | 2-2-63 | 2-9-63 | 2-9-63 | 21 (fraternal twin) | 24 min | 0 | Alive | Good |
| 4 (34) | A+-AB+ | 2-12-63 | 2-12-63 | 2-12-63 | 2-12-63 | 2-25-63 | 30 (wife) | 36 min | 17 days 42 days |
Alive | Good |
| 5 (50) | A+-A+ | 2-26-63 | 2-26-63 | Not done | Not done | 3-26-63 | 42 (unrelated) | 71 min† (2 arteries) | Hard to assess | Died 10 days sepsis | ........ |
| 6 (23) | O+-O+ | 3-22-63 | 3-22-63 | 4-17-63 | 4-24-63 | 4-17-63 | 22 (brother) | 35 min | 21 days | Alive | Good |
| 7 (25) | O+-A+ | 0 | 5-3-63 | 5-3-63 | Not done | 5-3-63 | 32 (wife) | 41 min (2 arteries) | 14 days | Alive | BUN level 38 mg% |
| 8 (29) | O+-O+ | 0 | 5-8-63 | Congenitally Absent | 4-25-63 Elsewhere |
5-8-63 | 20 (brother) | 61 min (2 arteries) | 4 days | Alive | Good |
| 9 (30) | O+-A+ | 2-14-63 | 2-14-63 | 5-10-63 | 5-10-63 | 5-10-63 | 30 (fraternal twin) | 28 min | 5 days | Alive | Good |
| 10 (47) | O+-O− | 0 | 5-15-63 | 5-15-63 | 5-15-63 | 5-15-63 | 32 (unrelated) | 38 min | 4 days | Alive | Good |
| Homograft with Cadaver Donor | |||||||||||
| 11 (21) | A+-O+ | 0 | 0 | Not done | Not done | 3-19-63 | 32 (unrelated) | 106 min from death | Hard to assess | Died 24 days sepsis | ........ |
| 12 (29) | A+-A+ | 3-27-63 | 3-27-63 | 4-18-63 | 4-18-63 | 4-19-63 | 47 (unrelated) | 124 min from death | Anuric‡ | Died hemorrhage 40 days postoperatively | ........ |
All donors and recipients were males unless otherwise indicated.
Cardiac arrest during transplantation.
Homograft removed 12 days; ruptured.
Donor Selection
Familial relationships of donors to the 12 patients are indicated in the Table. There was one mother-to-son and five sibling donations, including two from fraternal twins. In the other six patients there was no genetic relationship. Three kidneys were contributed by females to males.
With living donors, evaluation of renal function was not exhaustive in the absence of a history of renal disease. Blood urea nitrogen (BUN) levels and creatinine clearance were determined. An intravenous pyelogram was obtained. Aortography was carried out in eight of the ten living donors as recommended by Goodwin 2 and Hume.3 The information obtained was useful in selecting the donor kidney. In two patients a double renal arterial supply was found on one side, prompting use of the contralateral kidney. In a third patient, two renal arteries were found to the kidney which was eventually used and three to the contralateral kidney. In a fourth patient, double renal arteries were seen on both sides, with a large and diminutive pair on the left side and two medium-sized arteries on the right. The right kidney was selected for technical reasons. When convenient, donors were selected of the same major blood type as the recipient. However, in six of the 12 cases, there were major blood group incompatibilities (Table).
In two cases living donors were obtained from rather unusual sources. A 42-year-old male volunteered a kidney to a 50-year-old man with polycystic renal disease as the result of a newspaper appeal by the patient’s wife. A second kidney was donated by a convict from the Colorado State Prison. His recovery from nephrectomy was rapid. Twelve days after the operations he disappeared from the ward and has not been seen since.
The cadaveric kidneys were both obtained from patients dying of central nervous system (CNS) disease. The first was a 32-year-old man who was thought to have a brain tumor. Subsequent complete autopsy revealed the presence of acute bacterial endocarditis with CNS sepsis. The contralateral kidney, studied at autopsy, had no evidence of involvement with septic emboli. The second cadaveric kidney was obtained from a 47-year-old patient who died of subarachnoid hemorrhage. Both patients were hypotensive for several hours before death.
Preservation of Renal Tissue During Homografting
The kidneys were protected from the effects of ischemia in three ways, all involving cooling. The five living donors whose major blood groups were the same as those of the recipient patients received total body coding to 30 to 32 C (86 to 89.6 F). In addition, they were given 2 mg per kilogram of body weight of heparin sodium intravenously ten minutes before the renal artery was occluded. Immediately after removal of the kidney, a neutralizing intravenous dose of hexadimethrine bromide was administered. The five living donors who had major blood groups different from those of the recipients did not receive total body hypothermia or heparin sodium. The kidney was removed and immediately perfused with several hundred milliliters of cooled lactated Ringer’s solution at 5 to 15 C (41 to 59 F) to which 1 gm of procaine chloride and 50 mg of heparin sodium per liter had been added.4 The two cadaveric kidneys were obtained by a recently described method of extracorporeal total body hypothermic perfusion 5 which was instituted within a few minutes after death. As soon as the cadaver perfusion had been started, the abdomen was opened and dissection of the prospective homograft done.
Technical Considerations
Donor nephrectomy was performed through an extraperitoneal lateral incision in living donors and through a midline abdominal incision in cadavers. Great care was taken to avoid inadvertent occlusion of the renal artery or vein during dissection. Special precautions were taken to preserve the blood supply to the renal pelvis and ureter.1 An attempt was made to remove all adventitia at the point where the renal artery and vein were transected in order to facilitate subsequent anastomoses.
The recipient bed was prepared by an extraperitoneal approach through an oblique lower abdominal incision on the left in seven patients and on the right in five. The technique of Murray and Harrison 6 was used with placement of the left donor kidney in the right iliac fossa (Fig 1) and the right donor kidney in the left iliac fossa, thereby reversing the anteroposterior relationships of the renal pedicle. The venous, arterial, and ureteral anastomoses were performed in that order. In five of the 12 patients the presence of venous valves in the external iliac vein were noted and the end-to-side anastomoses placed either above or below these structures. End-to-end arterial anastomoses using 6–0 silk were performed to the hypogastric artery in the nine patients with single renal arteries (Fig 1). In three patients there were two renal arteries and the anastomoses were performed to suitable branches of the hypogastric artery. The necessity for double arterial anastomoses materially increased the ischemia time (Table). In almost all patients there was a disparity in size between the vessels used for arterial anastomosis. Under these circumstances, the smaller of the arteries was dilated with the tip of a hemostat. Ureterocystostomy was performed with a sub-mucosal tunnel and nipple technique (Fig 1). Urethral drainage was used for 24 to 72 hours postoperatively.
Fig 1.
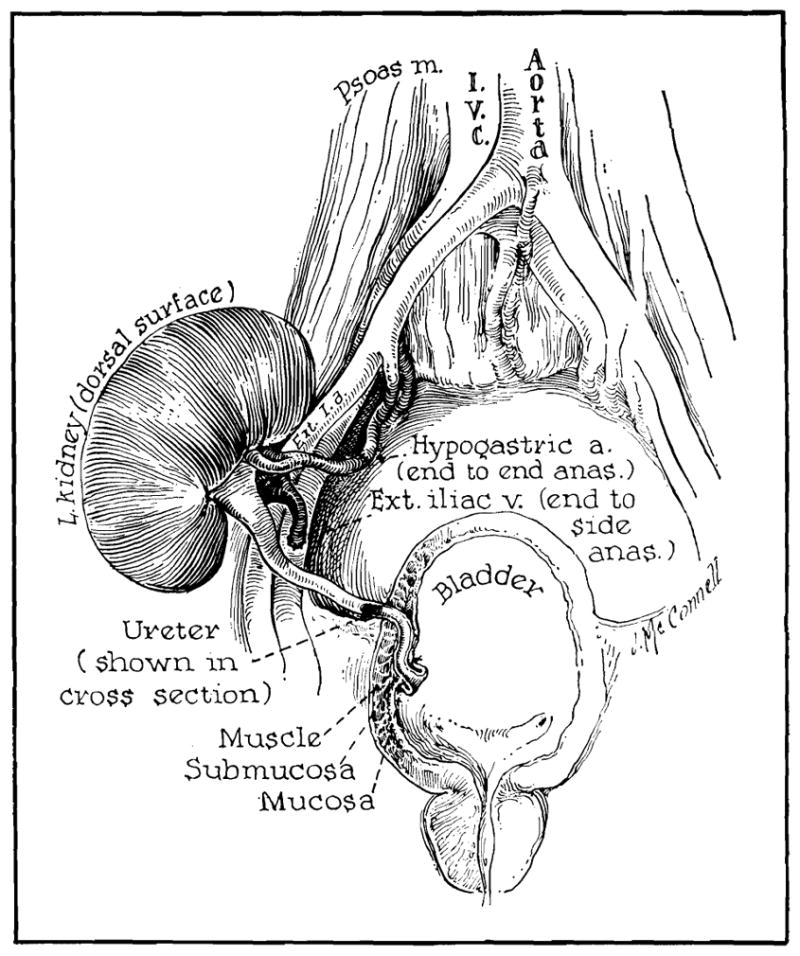
Position of renal homograft in recipient site. Peritoneal cavity is not entered. Note tunnel and nipple technique used for ureteral implantation.
During revascularization of the kidney, 250 mg/kg of mannitol were given intravenously. If urine flow subsequently was demonstrated from the ureteral tip, an additional 30 to 50 gm of mannitol was administered in 20% solution at 4 to 6 ml per minute and ipsilateral nephrectomy was performed through the same incision if this had not been done previously (Table). In two patients the contralateral kidney was also removed on the day of transplantation. In four patients concomitant splenectomy was performed through a separation incision (Table).
Methods to Prevent Rejection
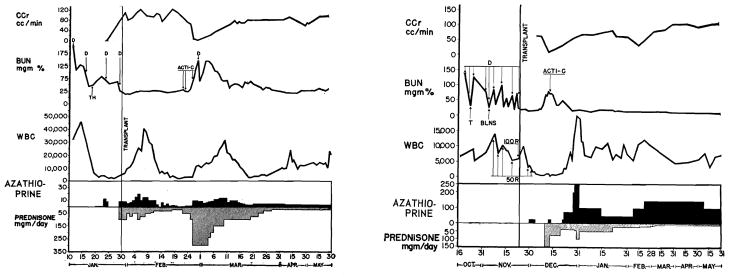
In principle, all patients except the patient in case 1 received similar antirejection therapy. The first patient was treated with 300 roentgens (r) of total body irradiation (at depth) preoperatively. Fifty r were given 17, 14, 11, and 5 days before transplantation, culminating in a final dose of 100 r on the day before operation. Four and six days after transplantation, additional doses of 50 r were given (Fig 2), shielding the renal transplant on the second of these occasions. The x-ray doses were given with a 250 kv unit at 30 ma, half-value layer 2.5 mm copper, with a Thoraeus filter at a distance of 207 cm to the near surface in lateral position. Maximum tissue thickness was 19 cm and the dosage rate at the midline was 3.48 r per minute. Despite the resultant suppression of white blood cell (WBC) count to less than 500/cu mm, vigorous rejection began after two weeks (Fig 2). Therapy was changed to azathioprine (Imuran), actinomycin C, and prednisone with apparent reversal of the rejection.
Fig. 2.
Left, Record of patient 1 shows vigorous rejection attempt two weeks after operation despite profound leukopenia induced with 400 r total body irradiation. Azathioprine dose given in total milligrams per day because patient gained weight rapidly postoperatively (22 to 41 kg). (D=dialysis. T=thymectomy. BLNS=bilateral nephrectomy and splenectomy. r=dose in roentgens. Acti-C=intravenous Actinomycin C. CCr=creatinine clearance.) Right, record of patient 2 shows rejection beginning after two weeks. Left nephrectomy and splenectomy done same day as transplantation. Right kidney has not been removed. (TH = thymectomy.)
Therapy for the last 11 patients consisted of a combination of azathioprine, actinomycin C, and prednisone. The basic azathioprine therapy was started 1 to 19 days before transplantation in a dosage of 3 to 8 mg/kg per day. On the day of surgery and for two or three days thereafter the dosage was increased to 6 to 15 mg/kg per day. In patients who were unable to take the drug orally it was administered intravenously in isotonic sodium chloride solution to which one molecular equivalent of sodium hydroxide and 2 mg/ml of azathioprine were added. Thereafter the dosage was reduced to 3 to 6 mg/kg per day. WBC counts were obtained daily and the dosage of azathioprine was regulated accordingly in the attempt to avoid the production of leukopenia. In three patients, prednisone in a dosage of 30 to 100 mg per day was started before surgery and continuued after. In the other nine, steroids were withheld until the threat of rejection appeared. Actinomycin C was added to the regimen as discussed in the next section.
In addition, portions of the lymphoid system were excised. Eight patients had both splenectomy and thymectomy performed at varying intervals before or at the time of transplantation (Table). Three of the 12 patients had splenectomy alone. The one patient who received no adjuvant surgical procedure (case 11) received a renal homograft during a bout of uncontrollable gastrointestinal bleeding at a time when his condition was too precarious to allow consideration of concomitant splenectomy.
Reversal of Rejection
A most important factor of success in these cases was the ability to halt and reverse the attempted rejection which occurred in 11 of the 12 patients, from 4 to 27 days after transplantation. The earliest and most subtle evidence that rejection was at hand was persistent elevation of the blood pressure and fluid retention. Shortly thereafter there was increased excretion of protein in the urine, elevations in BUN and creatinine levels, depression of creatinine clearance, and oliguria or anuria. These events were accompanied by fever, often to 41 C (105.8 F).
As soon as the diagnosis of rejection was made, oral prednisone was increased to 150 to 400 mg per day in those patients already receiving smaller quantities or was immediately started at this dosage level in the others. The high steroid doses were continued for as long as 14 days and then slowly reduced to maintenance therapy of 10 to 20 mg per day. A first course of 400-gamma actinomycin C was given intravenously and repeated every five to seven days thereafter until renal function returned to normal. During these emergency measures, azathioprine was continued, ideally, without any reduction in dose. The most serious problems of management occurred in those patients in whom the advent of rejection coincided with the development of leukopenia. When the dose of azathioprine was lowered or stopped under these circumstances to avoid fatal agranulocytosis, rejection proceeded with great vigor (Fig 2) despite the presence of a depressed WBC count. Salt and fluid restriction were instituted and antihypertensive drug therapy started. In a few days restoration of adequate renal function began in nine of the ten patients who had received kidneys from living donors (Fig 2). There has been only one subsequent mild rejection attempt (case 4) which was easily controlled.
Results
Deaths
There were three deaths in the series of 12 patients. In the group of ten patients receiving kidneys from living donors there was one fatality. A 50-year-old man with polycystic kidney disease received a renal transplant from an unrelated volunteer donor on March 26, 1963. Splenectomy and thymectomy had been performed one month previously. Development of a mediastinal hematoma had complicated his convalescence, causing an unrecognized hemorrhagic pericarditis. During the transplantation operation cardiac arrest developed just before the renal arterial anastomosis was completed. Open cardiac massage resulted in restitution of circulation. Urine flow from the homograft was delayed for 12 hours, but was 2,000 to 4,000 ml per day thereafter. The patient received excessive doses of azathioprine before and the first few days after transplantation with a consequent marked leukopenia first observed on the seventh postoperative day. He died of mediastinitis and generalized sepsis on the tenth postoperative day. The histologic findings of rejection were not present in the renal homograft, although there was evidence of tubular necrosis which was probably related to the prolonged ischemia at the time of transplantation.
The other two deaths occurred in patients who received cadaveric kidneys. The first of these was a 21-year-old man in whom urine flow was delayed for eight hours after transplantation. Unfortunately, the transplantation wound was made a short distance from an infected dialysis site in the right groin. The transplant wound first drained copious amounts of lymph. After ten days it became grossly infected and had to be widely opened for drainage. Ultimately the patient developed a Pseudomonas septicemia and died of generalized sepsis. The bacterial complication was rendered uncontrollable by the development of leukopenia with a WBC count of 300/cu mm. Terminally, function of the renal homograft deteriorated. At autopsy there was no evidence of rejection in the homograft, although there was extensive renal parenchymal destruction, secondary to arteriolitis, which was histologically comparable to that seen in malignant hypertension.
The other patient receiving a cadaveric kidney also died. He was a 29-year-old man who had previously undergone splenectomy and thymectomy. The renal graft, which was revascularized 124 minutes after the death of the donor, never functioned satisfactorily. The maximum urine flow was 5 cc per hour. Eleven days after transplantation he was reported to have fallen out of bed and 24 hours later it was necessary to remove the homograft which had ruptured. Following this, he developed a hemolytic staphylococcal septicemia. Two weeks after removal of the homograft, at a time when he was being considered for a second homotransplantation, a mediastinal abscess developed from the site of the previous thymectomy. He was maintained on hemodialyses during efforts to heal his wounds. On May 28, 40 days after his original homografting procedure, the connections of the chronic dialysis shunt became dislodged as he slept and he hemorrhaged to death from his indwelling arterial catheter. The renal homograft, which was removed 12 days after its implantation, was grossly swollen and showed extensive infiltration with mononuclear cells. The picture was histologically compatible with rejection.
Complications
The nonfatal complications of hypertension, fluid retention, and fever which accompany a threatened rejection have already been mentioned. Perhaps the most dramatic non atal complication occurred in case 4. The patient had a massive pulmonary embolus five weeks and two days after transplantation which was successfully removed by pulmonary embolectomy. Peripheral thrombophlebitis without embolism occurred in cases 2 and 11. Many of the patients had numerous superficial wound infections prior to renal transplantation resulting from multiple hemodialysis. Healing of these indolent wounds usually occurred rapidly after the homografts had been placed. Three of the patients (cases 2, 3, and 8) developed psychiatric disorders in the postoperative period, long-term psychiatric care being required in one. One patient developed a leak at the ureterocystostomy (case 7). He was re-explored six days after the original operation and reimplantation was satisfactorily carried out.
Postoperative Renal Function
The retarded or absent early renal function in the three patients who died has already been mentioned. The nine surviving patients all had an immediate dramatic diuresis followed by a period of normal renal function lasting from 4 to 27 days and which was interrupted by an attempted rejection in all except case 3 (Fig 3). After reversal of the rejection there was rapid return of renal function to essentially normal levels. This has been most thoroughly studied in the first five patients who survived. These patients have been followed for 1½ to 6½ months and all have normal BUN levels, creatinine level, and creatinine clearance. The graphic record of three of these patients is illustrated in Fig 2 and 3. The last four patients have been operated on in the last four weeks and all have just emerged from rejection crises. Three of these four patients have experienced return of normal renal function, while the fourth has been indolent in his response to the antirejection therapy and still has a BUN level of 38 mg%.
Fig 3.
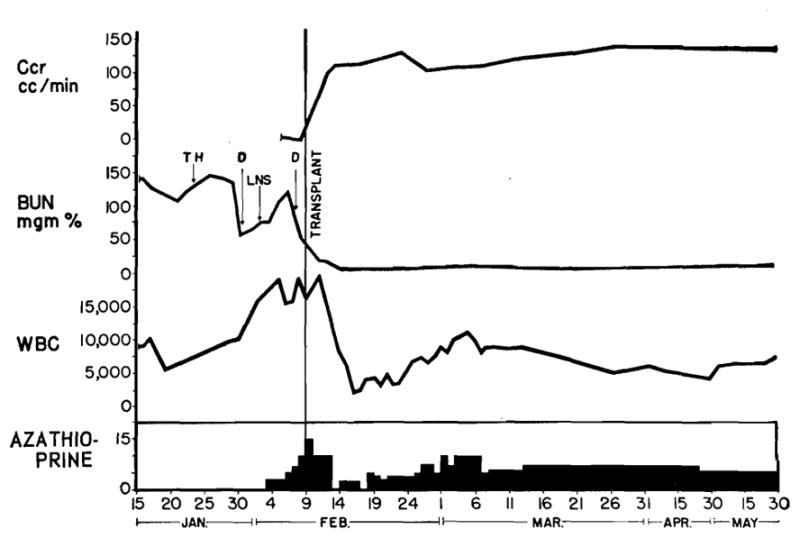
Patient 3 only one in whom rejection crisis was not encountered. (D=dialysis. TH=thymectomy. LNS=left nephrectomy and splenectomy.) Right splenectomy was performed at time of transplantation.
Comment
The use of renal homografts in humans has not usually been successful. Recently, Goodwin and Martin2 accumulated published and unpublished data on renal homografts from various parts of the world. Of almost 200 cases available for analysis in this collected series, only six patients are known to have lived as long as one year. Most commonly, death occurred within a few weeks or months after surgery, since less than 10% of the patients whose cases have been recorded lived for as long as three months.
Most of the following remarks will serve to focus attention on some of the preoperative and postoperative details which may have contributed to the somewhat higher early success rate in the present series. In the patients receiving kidneys from living donors, nine of ten are well from one to six months after surgery and all of these have adequate renal function as of June 1, 1963. The problems to be discussed can be broadly classified into two categories: technical and biological.
There has been a tendency to believe that the technical factors are of secondary importance. Those holding this point of view allude to the almost uniform success of renal transplantation between identical twins, despite great differences in the trauma inflicted upon the donated kidney in terms of ischemia and organ temperature during its devascularization. An alternative view could be supported easily, based upon the contention that the margin of safety in identical twins (isografts) is incomparably greater than with true homografts. Even though severe, but reversible, renal injury occurs, steady improvement may be expected, since a secondary parenchymal insult does not occur when isografts are used. In contrast, the recovery of homograft function is almost inevitably interrupted by a rejection phase. The ability of the graft to remain viable is almost surely related, at least to some extent, to the quality of the tissue at the time of the rejection crisis. It is perhaps significant that the three failures in the present series occurred in the patients who received the most severely traumatized homografts. Greatest trauma occurred in two from cadavers and in a third after an excessive period of ischemia which resulted from the necessity of interrupting the vascular anastomoses to treat a cardiac arrest. Although urine flow was observed in two of the three patients, renal function was greatly retarded.
Aside from efforts to minimize the period of ischemia during transfer of the graft, other measures were taken to protect the donated tissue. Care was exercised in mobilization and dissection of the kidney to avoid inadvertent occlusion of the vessels. Cooling was provided by either total body hypothermia or cold perfusion. The donated organ was also heparinized, either by giving heparin sodium systemically to the donor1 or by direct perfusion into the renal artery with cold solution after completion of nephrectomy.4 Finally, the osmotic diuresis, which would be anticipated as the result of a high degree of urea concentration, was reinforced with mannitol intravenously administered to the recipient patient during and after the vascular anastomoses. Optimally prepared kidneys invariably exhibited a massive diuresis, starting within 5 to 20 minutes after reconstitution of renal blood flow. BUN level was usually restored to normal within 24 to 72 hours. Normal renal function then continued until the beginning of rejection.
The kidney having been transplanted with the minimum of operative injury, continued function of the homograft is dependent upon the adequacy of the antirejection therapy. The most important single agent is azathioprine,7 a purine analogue. The drug is used essentially as reported in the pioneer work of Murray and Calne and their associates,7, 8 with the exception of a few possibly important details. Studies in our laboratory have indicated that homograft survival is greatly increased in dogs treated with azathioprine for two or three weeks before, as well as after, transplantation in comparison to a comparable group pretreated for one or two days. Consequently, eight of the 12 patients received the drug for 4 to 19 days before operation.
Originally, it was thought that it would be desirable to gradually suppress the WBC count with azathioprine during the early postoperative period so that the appearance of rejection would coincide with the development of leukopenia. This proved to be the most costly error in the management of the present series of cases. The effectiveness of therapy is not directly related to the degree of bone marrow depression and the necessity for withdrawal of the drug because of agranulocytosis at a time when it is most needed does nothing but decrease the chances for success. Variants of this error were committed in the three patients who died. Sepsis eventually developed in all, as well as deterioration of renal function.
In spite of the value of azathioprine, it is doubtful that survival of the homograft would have been possible in most of the cases without the use of secondary drug therapy. Particularly in those patients with violent rejection crises, the clinical picture of acute renal failure, fever, and tenderness in the transplant area was so alarming as to leave little hope for recovery. The additional administration of high doses of prednisone and actinomycin C regularly resulted in the reversal of these events. Unfortunately, the combined use of actinomycin C and prednisone precludes an accurate clinical evaluation of the effect of either agent alone. However, Goodwin9 has described a case in which the only additional drug used was prednisone in a child undergoing rejection while under treatment with mechlorethamine hydrochloride (nitrogen mustard) and cyclophosphamide. In our laboratory it has been possible to test the effect of prednisone on nine dogs undergoing rejection while on azathioprine therapy, in eight of which evidence of reversal of rejection occurred. Also in dogs, Murray8 has reported the reversal of rejection using actinomycin C in combination with continuing azathioprine therapy.
The single most important factor contributing to early success is proper management of the rejection crisis. If the patient has been treated with a relatively stable dose of azathioprine, it is possible to continue this therapy with the addition of the secondary drugs mentioned above. At this point, the greatest virtue is patience. While it is tempting to suspect mechanical factors as a cause for the clinical situation, it is now our opinion that the essential anatomic integrity of the transplant must be taken on faith at the time of the almost inevitable attempted graft repudiation. Instrumentation is scrupulously avoided. Furthermore, the temptation is great to make impetuous changes in the azathioprine therapy in an effort to suppress the WBC count. This has seemed to be undesirable. Although azathioprine dosages can frequently be increased, the dose should be selected with a view to avoiding profound bone marrow depression. With this general approach, the seriously depressed renal function has consistently returned, often after many agonizing days of waiting.
From the above remarks it is evident that the critical alterations in the immunologic mechanism were brought about by pharmacological means. Deserving of additional comment is the role of thymectomy and splenectomy in mitigating the vigor of the rejection response. If these additional operative procedures have any value, it is certainly adjuvant in scope, since neither splenectomy nor thymectomy can potentiate homograft survival in the adult experimental animal when used alone. In our laboratory, efforts to demonstrate an increased survival in dogs treated with azathioprine by the addition of splenectomy and thymectomy have not produced statistically convincing evidence of the value of extirpation of these lymphoid masses. Our use of these procedures should, therefore, be interpreted as a clinical trial rather than a recommendation for their use. The ultimate role of thymectomy and splenectomy will require long-term follow-up and careful evaluation of late results. This admonition is particularly germane, since thymectomy has proved to be a relatively dangerous operation in the presence of uremia. Two patients have developed mediastinal hematomas after this procedure which led to death before transplantation could be carried out. Mediastinal abscesses complicated the course of two additional patients.
Despite the necessity for caution in recommending adjuvant splenectomy and thymectomy, considerable experimental information is available which suggests the need for evaluation of these measures under highly controlled circumstances. Both old and more recent experimental studies have demonstrated that the antibody response to many antigens is reduced after splenectomy.10, 11 Wissler and his colleagues12 believe that the spleen is the most active portion of the lymphoid system in response to antigens given intravenously. They have suggested that sensitized lymphoid tissue within the spleen can ultimately leave this organ and repopulate other lymphoid centers with the establishment of specific clones in widespread locations.
Less evidence can be cited that the thymus occupies an important role in maintaining immunologic reactivity in the adult state, since the thymus cannot be demonstrated to contribute to the antibody response to antigens in grown experimental animals.13 In adult mice, Miller14 has shown that thymectomy, combined with total body irradiation, can result in homograft tolerance of a high degree, far exceeding that resulting from irradiation alone. This finding suggests that the thymus may, in adult life, resume the preceptor function which it is thought to provide for immunologic development in the fetal and newborn state,15 providing there is first a temporary suppression of the mature lymphopoietic system.14 Miller’s observations, however, remain to be confirmed both in mice and in higher species. The thymus glands removed in the present series of patients all showed atrophy to such a degree that the resumption of an important functional role by this fibrotic, fat-infiltrated tissue is difficult to imagine. The validity of application of Miller’s concept to human renal homotransplantation will have to wait long-term follow-up of cases such as these.
Summary
Twelve patients with terminal uremia were treated with renal homotransplantation. The homografts were obtained from blood relatives in six cases, unrelated living donors in four, and from recently deceased cadavers in the other two cases. The presence of major blood group incompatibilities or differences of sex in the donor and recipient patients did not adversely influence the outcome.
Particular attention was focused on two aspects of the transplantation problem. First, an effort was made to improve the quality of the homograft tissue by reducing its period of ischemia during transfer and by reducing its metabolic requirements during this period by one of three cooling techniques. Second, it was attempted to improve the methods used for prevention and reversal of the rejection crisis which interrupted the recovery of every patient except one. The greatest progress appeared to be in the proper manipulation of drugs so that they were maximally effective at the time of greatest need–during the height of the attempted rejection. In addition, a clinical study was initiated in these patients to determine if surgical excision of the thymus and spleen is of value in mitigating the vigor of the immunologic response to the foreign tissue. The worth, if any, of these adjuvant surgical measures will have to wait long-term follow-up of the present series of cases.
Nine of the ten patients who received renal homografts from living donors have good renal function from two weeks to 6½ months postoperatively. The tenth patient, as well as both recipients of cadaveric kidneys, succumbed with a combined picture of inadequate renal function and sepsis.
Addendum
On Dec 15, 1963, the patients in cases 1, 2, 3, 6, and 10 are alive with normal renal function 7 to 13 months after surgery. All five of the patients have had prednisone discontinued and all receive smaller maintenance doses of azathioprine.
Four additional patients had died. The patient in case 4 succumbed 118 days after surgery of an unrecognized paravertebral gutter abscess. The patient in case 7 died of a perinephric abscess surrounding the homograft 79 days after transplantation. The patient in case 8 died unexpectedly 62 days after transplantation of an unexplained central nervous system disorder, characterized by the acute onset of coma and convulsions with death 24 hours later. The patient in case 9 died six months and 24 days after transplantation as a consequence of a massive gastrointestinal hemorrhage from a duodenal ulcer which was unsuccessfully treated with vagotomy, suture transfixion of the ulcer, and pyeloroplasty. The last patient was still on maintenance doses of prednisone. In all but the last case, renal function was essentially unimpaired prior to death.
Since submission of this report, use of thymectomy has been discontinued because of excessive morbidity encountered. Splenectomy is still employed. When it is necessary to use donors and recipients of different ABO blood groups, incompatibilities are accepted only when they confirm to that scheme already defined for the use of mismatched blood, transfusions in which O is a universal donor and AB the universal recipient. Thus O to A, O to B, and so forth would still be used, as well as A to AB and B to AB. Transfers such as A to O, B to O, and B to A would no longer be considered.
Acknowledgments
Drs. Gilbert Hermann, Oliver Stonington, Martin Hutt, David Rifkind, David Talmage, I.C.S. Knight, Mathew Block, and Robert Virtue aided in this study.
The azathioprine used in this study was provided as Imuran (577-322) by Dr. Donald S. Searle of Burroughs- Wellcome Co., Tuckahoe, NY. Actinomycin C was provided as Sanamycin by Dr. Donald Whitfield of FBA Pharmaceuticals, Haywards Heath, Sussex, England.
Footnotes
Read before the session on immune processes and microbiology of the Third Multiple Discipline Research Forum during the 112th Annual Meeting of the American Medical Association, Atlantic City, NJ, June 20, 1963.
References
- 1.Starzl TE, et al. Renal Transplantation in Identical Twins. Arch Surg (Chicago) 1963;86:600. doi: 10.1001/archsurg.1963.01310100084013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodwin WE, Martin DC. Transplantation of Kidney, Read before the American Urological Association, St. Louis. J Urol. 1963 May 15; to be published. [Google Scholar]
- 3.Hume DM, et al. Renal Homotransplantation in Man in Modified Recipients, Read before the American Surgical Association, Phoenix, Ariz, April 5, 1963. Ann Surg. 1963;158:608. doi: 10.1097/00000658-196310000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, et al. Renal Homografts in Patients with Major Donor-Recipient Blood Group Incompatibilities. Surgery. to be published. [PMC free article] [PubMed] [Google Scholar]
- 5.Marchioro TL, Waddell WR, Starzl TE. Use of Extracorporeal Cadaver Perfusion for Preparation of Organ Homografts. Surg Forum. 1963;14:174. [PMC free article] [PubMed] [Google Scholar]
- 6.Murray JE, Harrison JH. Surgical Management of 50 Patients With Kidney Transplants Including 18 Pairs of Twins. Amer J Surg. 1963;105:205. doi: 10.1016/0002-9610(63)90292-7. [DOI] [PubMed] [Google Scholar]
- 7.Calne RY, Murray JE. Inhibition of Rejection of Renal Homografts in Dogs by Burroughs Wellcome 57–322. Surg Forum. 1961;12:118. [PubMed] [Google Scholar]
- 8.Murray JE, et al. Kidney Transplantation in Modified Recipients. Ann Surg. 1962;156:337. doi: 10.1097/00000658-196209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodwin WE, et al. Human Renal Transplantation: I. Clinical Experiences With Six Cases of Renal Homotransplantation. J Urol. 1963;89:13. doi: 10.1016/S0022-5347(17)64491-4. [DOI] [PubMed] [Google Scholar]
- 10.Hektoen L. Further Observations on Effects of Roentgenization and Splenectomy on Antibody Formation. J Infect Dis. 1920;27:23. [Google Scholar]
- 11.Rowley DA. Formation of Circulating Antibodies in Splenectomized Human Being Following Intravenous Injection of Heterologous Erythrocytes. J Immun. 1950;65:515. [PubMed] [Google Scholar]
- 12.Gunderson CH, et al. Tissue and Cellular Changes Associated With Antibody Formation in Rat Spleen. JAMA. 1962;180:1038. [PubMed] [Google Scholar]
- 13.Harris TN, Rhodes J, Stokes J. Study of Thymus and Spleen in Formation of Antibody in Rabbit. J Immun. 1948;58:27. [PubMed] [Google Scholar]
- 14.Miller JFAP. Immunologic Significance of Thymus of Adult Mouse. Nature (London) 1962;195:1318. [Google Scholar]
- 15.Miller JFAP. Immunity and Thymus. Lancet. 1963;2:43. [Google Scholar]