Abstract
Chronic pain and PTSD are known to hold substantial comorbidity following traumatic injury. Although pharmacological agents have been examined in the treatment of pain and PTSD individually, little is known regarding the relationship of medication use with functioning in patients with comorbid conditions. This research examined the relationships of pain, PTSD, and medication use across physical and psychosocial functioning in patients with chronic pain following motor vehicle injury (N = 234). Separate analyses were conducted for opioids, SSRIs, and sedative/anxiolytics, respectively. Several relevant effects were noted: 1.) Pain evidenced strong associations with reduced functioning across both physical and psychosocial domains 2.) Opioid use held interactive relationships with PTSD across both functioning domains. Specifically, opioids were associated with greater physical impairment in patients without comorbid PTSD. Opioids also were related to greater psychosocial impairment in patients without PTSD while PTSD was associated with greater impairment in patients not using opioids 3.) Opioid use evidenced a marginal interaction with pain on psychosocial functioning. Opioids were associated with greater psychosocial impairment among patients with high pain, and high pain was associated with greater impairment among opioid users 4.) SSRIs held a marginal interaction with PTSD such that PTSD was related to poorer psychosocial functioning only among individuals not using an SSRI 5.) Anxiolytic use evidenced a marginal interaction with PTSD on physical functioning although no between-group differences were noted. These data suggest that PTSD symptomology may be an important consideration in determining treatment modality for patients experiencing pain subsequent to traumatic injury.
Keywords: chronic pain, PTSD, medication, motor vehicle accidents, opioid, SSRI, anxiolytic
1. Introduction
Chronic pain and posttraumatic stress disorder (PTSD) are frequently observed to be comorbid following traumatic injury (Bryant et al., 1999; Hickling and Blanchard, 1992). Data suggest that ongoing pain is associated with increased risk for PTSD (e.g., Blanchard et al., 1996; Zatzick et al., 2007), and pain patients with PTSD evidence greater levels of subjective pain, affective distress, and functional disability than do those without (e.g., Duckworth and Iezzi, 2005; Geisser et al., 1996; Sherman et al., 2000). As such, several authors have proposed that pain and PTSD may interact to maintain and exacerbate dysfunction (Asmundson et al., 2002; McLean et al., 2005; Sharp and Harvey, 2001). Despite evidence of a dynamic relationship between chronic pain and PTSD, research examining the association between patient functioning and pharmacological agents used in the treatment of these conditions has largely overlooked the potential impact of this comorbidity.
While controversial, the use of opioid medications in the treatment of chronic, non-malignant pain has increased significantly over the past three decades (Cudill-Slosberg, 2004). The efficacy of opioids in alleviating acute pain is well established, but less is known regarding their utility in treating chronic pain or their relationship with patient functioning over extended periods of use (Ballantyne and Shin, 2008). With respect to trauma exposure, some data suggests that pain patients with comorbid PTSD use analgesic medications at higher rates than their non-PTSD counterparts (e.g., Schwartz et al., 2006). Understanding the interrelationships of chronic pain, PTSD, and opioid use with patient functioning becomes clinically relevant given that comorbid psychiatric disorders are known to increase risk of abuse and dependency among persons with chronic pain (Edlund et al., 2007).
Other pharmacological agents have been examined for use in the treatment of both chronic pain and PTSD. Selective serotonin reuptake inhibitors (SSRIs) are recommended as the first-line pharmacological intervention for PTSD according to practice guidelines from the American Psychiatric Association (2004). SSRIs also have been examined for use in the treatment of chronic pain, but support for their efficacy in this population is limited (see reviews by McCleane, 2008; Dworkin et al., 2007). Alternatively, sedative and anxiolytic medications are sometimes prescribed to alleviate symptoms associated with both PTSD and chronic pain. Recommendations for their use in either condition, however, are qualified by the addictive properties of many anxiolytic agents (e.g., APA, 2004; Sanders et al., 2005). Review of these literatures reveal varying levels of support for the use of SSRI and anxiolytic/sedative medications in the treatment of chronic pain and PTSD individually, but again, the relationship between these pharmacological agents and functioning among patients with comorbid pain and PTSD remains unexamined.
The aim of the present research was to explore the unique and interactive associations of pain severity, PTSD, and medication use across domains of physical and psychosocial functioning using a sample of motor vehicle accident (MVA) survivors with chronic pain. Separate analyses are presented for opioid analgesic, SSRI, and anxiolytic/sedative medications in an effort to delineate specific effects.
2. Methods
2.1. Procedure
Data were collected as part of a research clinic specializing in the assessment and treatment of PTSD following an MVA (references omitted for anonymous review). Patients were referred through a variety of professional contacts including physicians, chiropractors, psychologists, and other healthcare providers. Public service announcements and local advertisements also were used for recruitment. Exclusion criteria for the larger research clinic included 1.) MVAs which failed to meet standards for Criterion-A for PTSD (i.e., the MVA did not pose a serious threat and/or the individual did not respond with fear, helplessness, or horror; APA, 1994), 2.) evidence of significant cognitive impairment as indicated by a score of 23 or below on the Mini-Mental State Examination (MMSE; Folstein et al., 1975), and 3.) evidence of current substance abuse, psychosis (e.g., delusions, hallucinations), and/or acute suicidality.
Following the provision of informed consent, patients received a comprehensive psychiatric assessment which included the MMSE, a structured interview detailing the specifics of the MVA (Blanchard and Hickling, 1997), and an assessment of current psychiatric diagnoses. All diagnostic assessments were conducted by advanced graduate students supervised by the fourth author. Patients were given a number of additional self-report measures pertaining to medication use, pain severity, and global functioning following completion of the interview. Questionnaire packets were completed by the patient in their home and returned to the clinic in person or via mail. All procedures received approval from the institutional review board at SUNY Buffalo.
A total of 511 individuals were scheduled for assessment. Of these, eight were excluded owing to MVAs which failed to meet Criterion-A for PTSD. Eighteen evidenced significant cognitive impairment, seven reported ongoing substance abuse, nine were diagnosed with severe psychopathology (e.g., psychosis), and two reported suicidal ideation requiring hospitalization. A final patient was excluded due to insufficient English for completion of the assessment.
2.1. Participants
Patients included in the present research were limited to those reporting chronic pain as a result of MVA-related injuries. Chronic MVA-related pain was operationalized as ongoing pain persisting for a minimum of three months following the traumatic injury (IASP, 1986). Of the 466 individuals included in the larger clinic research, 156 failed to meet criteria for chronic pain. Two individuals reported chronic pain which preceded the MVA and were excluded from these analyses. An additional 74 patients failed to complete the psychiatric assessment resulting in a final sample of 234. Noncompleters did not differ from those in the final sample with respect to age, gender, education level, employment status, or household income (all p > .05). Ethnic and racial minorities (minority: yes/no) were overrepresented among non-completers, however (χ2(1) = 4.03; p = .05).
Demographic information is provided in Table 1. Mean age of participants in this sample was 43 years (SD = 10.8) with the majority of patients being Caucasian (83.3%) and female (77.4%). Approximately 30% of the sample reported completion of a 4-year or an advanced degree. Although total household income evidenced a relatively even distribution across the sample, approximately one half of patients (n = 123) reported being unemployed or receiving disability compensation as a consequence of the accident.
Table 1.
Demographic characteristics and associations with SIP physical and SIP psychosocial scores (N=234)a
| n | % | Physical | Psychsoc | |
|---|---|---|---|---|
| Sex | -.06 | -.18*** | ||
| Female | 181 | 77.4 | ||
| Male | 53 | 22.6 | ||
| Raceb | .19** | .03 | ||
| Caucasian | 195 | 83.3 | ||
| African American | 31 | 13.2 | ||
| Hispanic | 4 | 1.7 | ||
| Other | 4 | 1.7 | ||
| Education | -.12 | -.13* | ||
| High school or less | 48 | 20.5 | ||
| Some undergraduate | 78 | 33.3 | ||
| 2-year degree | 38 | 16.2 | ||
| 4-year degree | 39 | 16.7 | ||
| Post-graduate | 31 | 13.2 | ||
| Employmentc | .41*** | .18* | ||
| Employed | 94 | 40.2 | ||
| Unemployed | 86 | 36.8 | ||
| Disability | 52 | 22.2 | ||
| Income | -.29** | -.14* | ||
| Below 10,000 | 36 | 15.4 | ||
| 10 – 20,000 | 43 | 18.4 | ||
| 20 – 30,000 | 37 | 15.8 | ||
| 30 – 40,000 | 24 | 10.3 | ||
| 40 – 50,000 | 33 | 14.1 | ||
| 50 – 60,000 | 19 | 8.1 | ||
| Over 60,000 | 30 | 12.8 |
Some categories do not sum to 234 given incomplete reporting
Race coded as minority status (1 = yes; 0 = no) for correlations with SIP physical and psychosocial scores
Correlations between employment and SIP physical and SIP psychosocial scores calculated as eta coefficients
<.05
<.01
<.001
2.3. Measures
2.3.1. Pain Severity
Intensity of subjective pain (high, 1; low, 0) was quantified using the pain severity subscale of the West Haven – Yale Multidimensional Pain Inventory (WHYMPI; Kerns et al., 1985). The pain severity subscale of the WHYMPI contains three items rated on a 0 to 6 Likert scale. Raw pain severity is calculated as the mean of these items with higher scores indicating more severe pain. The pain subscale has demonstrated significant associations with other measures of physical pain as well as adequate internal consistency and test-retest reliability. Kerns et al. (1985) report an average score of 4.52 (SD = 1.04) on the pain severity subscale among a normative sample of patients with chronic, heterogeneous pain (N = 300). For the present research, individuals scoring greater than 4.52 on the pain severity subscale were categorized as “high-pain” patients. Those scoring less than 4.52 were categorized as “low-pain” patients.
2.3.2. PTSD
A diagnosis of PTSD (present, 1; absent, 0) was established using the Clinician-Administered PTSD Scale (CAPS; Blake et al., 1990). CAPS items correspond to the 17 cardinal DSM-IV symptoms for PTSD and evaluate both the frequency and severity of symptoms, each rated on a 0 to 4 Likert scale. A 1-2 rule was employed to establish PTSD diagnoses such that any symptom with a frequency rating of at least 1 and an intensity rating of at least 2 was considered clinically significant (Blake et al., 1990). Patients reporting a minimum of one reexperiencing symptom, three avoidance/numbing symptoms, and two hyperarousal symptoms were given a diagnosis of PTSD, per DSM-IV criteria. The CAPS is widely considered the “gold standard” for establishing PTSD diagnoses (Forbes et al., 2001; Zayfert et al., 2002) and demonstrates strong concordance with the Structured Clinical Interview for DSM-IV (First, et al., 1995). CAPS interviews were conducted by advanced graduate students supervised by the fourth author. Interviews were videotaped, and 73 (30.9%) cases from the present sample were randomly selected for independent review. Diagnostic agreement between raters in this sample was excellent (κ = .94).
2.3.3. Medication Use
Patients were asked to report all current medications using standardized forms following the psychiatric interview. Although official medical records were not available to the research clinic, patients were asked to copy information directly from the labels of their current prescriptions. Medication use was coded as present (1) or absent (0) for each category of drug. For the purposes of the current research, opium derivatives prescribed for the treatment of chronic pain were categorized as opioid analgesics. Medications categorized as SSRIs were restricted specifically to selective serotonin uptake inhibitors. Related antidepressant medications (e.g., venlafaxine) were not included in this category. Medications categorized as anxiolytic/sedatives included a heterogeneous collection of benzodiazepines, psycholeptics, and GABAA agonists. Because the intent of the research was to examine the impact of medication on functioning following a serious MVA, medications prescribed prior to the target MVA did not qualify for inclusion in these analyses.
2.3.4. Patient Functioning
Patient functioning was assessed on a continuous scale using the Sickness Impact Profile (SIP; Bergner et al., 1976), a 136-item measure examining impairment across both physical and psychosocial domains. Patients respond to a series of behaviorally anchored items that “describe you today and are related to your state of health.” The physical subscale of the SIP contains 45-items pertaining to body care and management, mobility, and ambulation (e.g., I walk shorter distances or stop to rest often). The psychosocial subscale, in contrast, contains 48-items pertaining to emotional behavior, social interaction, alertness behavior, and communication (e.g., I am doing fewer social activities with groups of people). Scores for each subscale are calculated by summing weighted values for each endorsed item and dividing by the total possible score. Scores range from 0 to 100, with higher scores indicating poorer functioning. SIP subscales have evidenced adequate test-retest reliability across a number of studies (Physical = .87-.90; Psychosocial = .79-.81; DeBruin et al. 1992; Deyo, 1986). As a reference for the present research, Friedland and Dawson (2001) reported a mean SIP physical score of 14.2 among a sample of MVA survivors admitted to a large tertiary care center (N = 99). Mean SIP psychosocial scores in the Friedland and Dawson sample was 17.5.
2.4. Data Analysis
The relationship of chronic pain, PTSD, and medication use with global functioning was examined using a series of 2 (Pain: high, low) × 2 (PTSD: present, absent) × 2 (Medication: present, absent) ANCOVA models. Given that practice guidelines for both chronic pain and PTSD (APA, 2004; Dworkin et al, 2007) recommend an additive approach to pharmacotherapy (e.g., primary agent followed by additional medications based on treatment response), use of medications across multiple categories was included as a covariate in all ANCOVA models. Multiple medication status was coded as a dichotomous variable (use across multiple categories, 1; otherwise, 0) for these analyses. Demographic characteristics evidencing unique associations with SIP physical and SIP psychosocial scores also were considered for inclusion as additional covariates.1 The magnitude of associations between demographic characteristics and primary study variables are presented as Pearson r (r; continuous/dichotomous vs. continuous), eta (η; categorical vs. continuous), and phi (Φ; categorical vs. categorical) coefficients as appropriate.
ANCOVA models were conducted for opioid, SSRI, and anxiolytic medications separately across both physical and psychosocial domains. Interaction effects were probed using post-hoc comparison procedures which account for discrepancies in sample size and variance across groups (Games and Howell, 1976). Prior to analyses, variables were examined to determine accuracy of data entry and concordance with the statistical assumptions of ANCOVA (Tabachnick and Fidell, 2001). All variables approximated normality (i.e., skew and kurtosis ≤ 2) with no evidence of significant outliers. Effect sizes were calculated as partial eta squared (ηP2) which reflect the proportion of variance in the outcome measure attributable to a given factor(s). Values of .01, .06, and .14 are typically associated with small, medium, and large effect sizes, respectively (Cohen, 1988). Bivariate associations between primary model variables are presented in Table 2.
Table 2.
Means, standard deviations, and intercorrelations for primary study variables
| SIPphys | SIPpsy | Pain | PTSD | Opioid | SSRI | Anxiolytic | |
|---|---|---|---|---|---|---|---|
| SIPphys | - | ||||||
| SIPpsy | .60** | - | |||||
| Pain | .52** | .32** | - | ||||
| PTSD | .07 | .28** | .16* | - | |||
| Opioid | .27** | .22** | .27** | .05 | - | ||
| SSRI | .09 | .13* | .13* | .10 | .08 | - | |
| Anxiolytic | .10 | .06 | -.03 | .03 | .08 | .14* | - |
| | |||||||
| M | 59.57 | 27.86 | .41 | .59 | .46 | .21 | .15 |
| SD | 11.34 | 18.80 | .49 | .49 | .50 | .41 | .35 |
Note: SIPphys = SIP physical subscale; SIPpsy = SIP psychosocial subscale; PTSD = Posttraumatic Stress Disorder; SSRI = selective serotonin reuptake inhibitor; Anxiolytic = anxiolytic/sedative-hypnotic medication
<.05
<.001
3. Results
3.1. Sample Characteristics
Although participation in the present study was restricted to individuals with pain persisting for a minimum of three months, the average duration of pain for the sample was 30.4 months (SD = 50.5). Injuries to the neck and back were common with 86.4% of patients reporting ongoing neck and shoulder pain and 78.4% reporting ongoing back pain. Eighty-five percent of patients reported pain in their extremities and 76.3% reported ongoing headaches following the MVA. Mean SIP physical and psychosocial scores for the present sample were 14.5 (SD = 11.3) and 27.9 (SD = 18.8) respectively. Associations with demographic characteristics indicate that SIP physical scores were associated with minority status (yes, 1; no, 0; r = .19; p = .004) and income (r = -.29; p <.001). Employment status also evidenced an association with physical functioning (η = .41; p <.001) such that functioning decreased across employed, unemployed, and disability patients, respectively. Psychosocial functioning was associated with sex (female, 1; male, 0; r = -.18; p = .006), education (r = -.13; p = .05), and income (r = -.14; p = .04). Employment status evidenced an association with psychosocial functioning (η = .18; p <.02) such that degree of functioning decreased across those patients who were employed, unemployed, and on disability respectively.
Ninety-seven individuals (41.5%) were categorized as high-pain based on scores from the WHYMPI pain subscale. Mean pain severity scores for the high and low-pain groups were 5.27 (SD = 0.47) and 3.29 (SD = 1.01), respectively. Comparison across demographic characteristics indicate that pain groups differed with respect to minority status (χ2(1) = 5.92; Φ =.16; p = .02), education (χ2(4) = 11.12; Φ = .29; p = .03), and employment (χ2(2) = 29.60; Φ = .36; p <.001). Specifically, ethnic and racial minorities were overrepresented in the high-pain group. High-pain patients also were less likely to report graduate education (adj residual z = -2.3, p = .02), less likely to be employed (adj residual z = -4.7, p <.001) and more likely to be on disability (adj residual z = 4.6, p <.001) than were low-pain patients. Patient age, sex, and income were not associated with pain group (all p >.05).
One hundred thirty-seven patients (58.5%) were diagnosed with PTSD based on CAPS interviews. PTSD status held no relationship with any demographic variable (all p >.05).
One hundred eight individuals (46.2%) reported prescriptions for opioid analgesics. Analgesic prescriptions included hydrocodone (n = 57), propoxyphene (n = 35), oxycodone (n = 13), codeine (n = 10), tramadol (n = 10), fentanyl (n = 3), methadone (n = 1), and pentazocine (n = 1).2 Fifty patients (21.4%) reported using an SSRI. SSRI prescriptions included sertraline (n = 21), paroxetine (n = 10), citalopram (n = 7), fluoxetine (n = 7), and escitalopram (n = 5). Thirty-four individuals (14.5%) reported use of anxiolytic/sedative medication. Anxiolytic/sedative prescriptions included alprazolam (n = 13), zolpidem (n = 8), clonazepam (n = 6), diazepam (n = 4), lorazepam (n = 2), and temazepam (n = 1). Forty-two individuals (17.9%) reported using pharmacological agents across multiple categories of medication (see Table 3). Comparison across demographic characteristics indicate that employment status was associated with opioid (χ2(2) = 6.05; Φ = .16; p = .05), SSRI (χ2(2) = 6.30; Φ = .17; p = .04), and anxiolytic (χ2(2) = 8.54; Φ = .19; p = .01) use. Patients using opioid medications were less likely to be employed than were non-opioid users (adj residual z = -2.2, p = .03). Patients using SSRIs were less likely to be employed (adj residual z = -2.0, p = .05) and more likely to be receiving disability (adj residual z = 2.2, p = .03) than non-users. Individuals using anxiolytic/sedative medications also were less likely to be employed (adj residual z = -2.6, p = .01) and more likely to be on disability (adj residual z = 2.4, p = .02) than were patients without anxiolytics. Age, sex, minority status, education, and income were unrelated to medication use (all p > .05).
Table 3.
Medication use by category
| Medications | n | % | ||
|---|---|---|---|---|
| Opioid - | SSRI - | Anx - | 92 | 39.3% |
| Opioid - | SSRI - | Anx + | 11 | 4.7% |
| | ||||
| Opioid - | SSRI + | Anx - | 19 | 8.1% |
| Opioid - | SSRI + | Anx + | 4 | 1.7% |
| | ||||
| Opioid + | SSRI - | Anx - | 70 | 29.9% |
| Opioid + | SSRI - | Anx + | 11 | 4.7% |
| | ||||
| Opioid + | SSRI + | Anx - | 19 | 8.1% |
| Opioid + | SSRI + | Anx + | 8 | 3.4% |
Note: SSRI = selective serotonin reuptake inhibitor; Anx = anxiolytic/sedative-hypnotic medication
+ presence; - absence
3.2. Physical Functioning
3.2.1. What is the relationship of pain, PTSD, and opioid use with physical functioning?
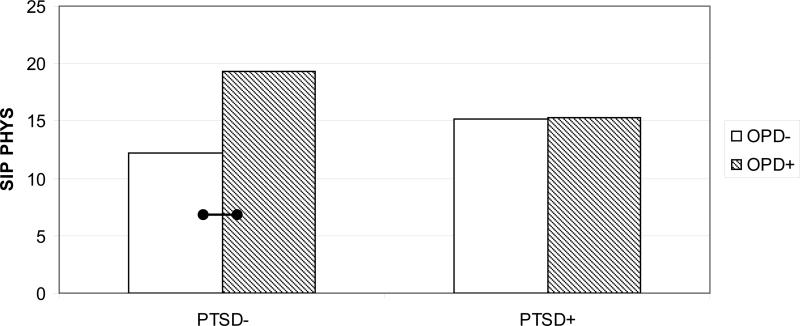
Results for the physical functioning models are presented in Table 4. Examination of the opioid data indicate a significant main effect of pain on physical functioning after covarying for minority status and multiple medication use (p <.001, ηP2 = .21). As expected, patients classified as high-pain demonstrated poorer physical functioning (M = 20.78; SE = 1.08) than did those classified as low-pain (M = 10.23; SE = .86). A significant PTSD × Opioid interaction (p = 0.01, ηP2 = .03) also was observed (see Fig. 1). Among individuals without PTSD, patients using opioid medications evidenced poorer functioning (n = 42; M = 19.31; SE = 1.50) that those who were not using opioids (n = 55; M = 12.23; SE = 1.57; p <.01). No difference in functioning was noted among patients with PTSD who reported using opioids (n = 66; M = 15.28; SE = 1.30) versus those who were not (n = 71; M = 15.20; SE = 1.22). No differences in physical functioning across PTSD diagnostic status were observed among the subset of patients taking opioid medications or among those not using opioids.
Table 4.
Opioid, SSRI, and anxiolytic ANCOVA models for association of pain, PTSD, and medication use with SIP Physical subscale
| Opioid | SSRI | Anxiolytic | ||||
|---|---|---|---|---|---|---|
| F | η P2 | F | η P2 | F | η P2 | |
| Minoritya | 4.37* | .02 | 2.82 | .01 | 4.48* | .02 |
| Multiple medsa | .81 | <.01 | 1.55 | .01 | .65 | <.01 |
| | ||||||
| Pain | 58.33*** | .21 | 44.09*** | .16 | 36.34*** | .14 |
| PTSD | .15 | <.01 | 1.86 | .01 | 1.00 | <.01 |
| Med | 6.04* | .03 | .02 | <.01 | .33 | <.01 |
| Pain × PTSD | .02 | <.01 | .23 | <.01 | .96 | <.01 |
| Pain × Med | .82 | <.01 | <.01 | <.01 | .12 | <.01 |
| PTSD × Med | 6.33* | .03 | 2.62 | .01 | 3.60† | .02 |
| Pain × PTSD × Med | .90 | <.01 | .15 | <.01 | 3.20 | .01 |
Note: PTSD = Posttraumatic Stress Disorder; SSRI = selective serotonin reuptake inhibitor; Anxiolytic = anxiolytic/sedative-hypnotic medication
Minority status and multiple meds entered as a covariates; multiple meds indicates use of medications across multiple categories (1 = yes; 0 = no)
<.07
<.05
**<.01
<.001
Fig. 1.
Interaction of PTSD and opioid medication on SIP Physical; PTSD = Posttraumatic Stress Disorder; SIP PHYS = Sickness Impact Profile - Physical; Note. Higher SIP scores indicate lower levels of physical functioning; Bars connected by lines indicate mean differences in physical functioning
 p<.01
p<.01
 p<.05
p<.05
3.2.2. What is the relationship of pain, PTSD, and SSRIs with physical functioning?
Examination of physical functioning with respect to SSRI use again evidenced a main effect of pain (p <.001, ηP2 = .16) adjusting for covariates (high-pain: M = 21.48; SE = 1.34; low-pain: M = 10.06; SE = 1.15). No other variable in this model was associated with differences in physical functioning.
3.2.3. What is the relationship of pain, PTSD, and anxiolytic/sedative use on physical functioning?
Consistent with previous analyses, examination of physical functioning with respect to anxiolytic use evidenced a main effect of pain (p <.001, ηP2 = .14) adjusting for covariates (high-pain: M = 22.03; SE = 1.69; low-pain: M = 10.11; SE = 1.18). A marginal PTSD × anxiolytic interaction also emerged in these data (p = .06; ηP2 = .02) although no significant pairwise effects were noted.3 No other effects were observed in this model.
3.3. Psychosocial Functioning
3.3.1. What is the relationship of pain, PTSD, and opioid use with psychosocial functioning?
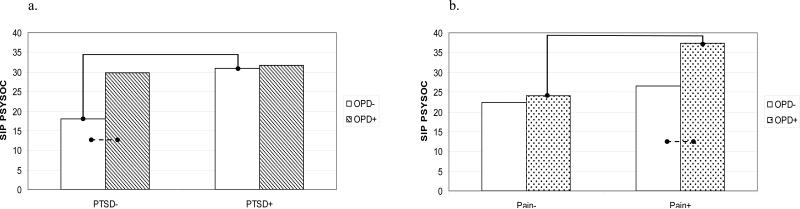
Results for the psychosocial functioning models are presented in Table 5. Examination of the opioid data revealed a significant PTSD × Opioid interaction (p = .03; ηP2 = .02; see Fig. 2a). For individuals with no diagnosis of PTSD, patients using opioid medications evidenced poorer psychosocial functioning (n = 42; M = 29.67; SE = 2.60) that those who were not using opioids (n = 55; M = 18.03; SE = 2.75; p <.05). No difference in psychosocial functioning was observed among patients with PTSD using opioids (n = 66; M = 31.74; SE = 2.27) relative to those not using (n = 71; M = 30.91; SE = 2.13). Among the subset of patients not using opioid medications, those with PTSD evidenced poorer psychosocial functioning than those without (p <.01). In contrast, the subset of patients who reported using opioids demonstrated equally poor functioning irrespective of PTSD diagnosis.
Table 5.
Opioid, SSRI, and anxiolytic ANCOVA models for associations of pain, PTSD, and medication use with SIP Psychosocial subscale
| Opioid | SSRI | Anxiolytic | ||||
|---|---|---|---|---|---|---|
| F | η P2 | F | η P2 | F | η P2 | |
| Sex | 11.58*** | .05 | 9.16** | .04 | 9.99** | .04 |
| Education | .76 | <.01 | .79 | <.01 | .77 | <.01 |
| Multiple meds | .85 | <.01 | .47 | <.01 | 1.06 | <.01 |
| | ||||||
| Pain | 12.92*** | .06 | 14.05*** | .06 | 13.48*** | .06 |
| PTSD | 9.60** | .04 | 2.90 | .01 | 7.02** | .03 |
| Med | 5.96* | .03 | 1.05 | <.01 | .36 | <.01 |
| Pain × PTSD | .18 | <.01 | .06 | <.01 | .19 | <.01 |
| Pain × Med | 3.45† | .02 | .30 | <.01 | 1.28 | .01 |
| PTSD × Med | 4.97* | .02 | 3.36† | .02 | .14 | <.01 |
| Pain × PTSD × Med | .62 | <.01 | .18 | <.01 | .42 | <.01 |
Note: PTSD = Posttraumatic Stress Disorder; SSRI = selective serotonin reuptake inhibitor; Anxiolytic = anxiolytic/sedative-hypnotic medication
a Sex, education level, and multiple meds entered as a covariates; multiple meds indicates use of medications across multiple categories (1 = yes; 0 = no)
<.07
<.05
<.01
<.001
Fig. 2.
Interaction of opioid analgesic use with a.) PTSD and b.) pain severity on SIP Psychosocial; PTSD = Posttraumatic Stress Disorder; SIP PSYSOC= Sickness Impact Profile - Psychosocial; Note. Higher SIP scores indicate lower levels of psychosocial functioning; Bars connected by lines indicate mean differences in psychosocial functioning
 p<.01
p<.01
 p<.05
p<.05
A marginal Pain × Opioid interaction also was observed in these data (p = .07; ηP2 = .02; see Fig. 2b). High-pain patients using opioid medications (n = 60; M = 37.31; SE = 2.39) evidenced greater psychosocial impairment than did high-pain patients not using opioids (n = 37; M = 26.62; SE = 2.95; p <.05). Low-pain patients with (n = 48; M = 24.10; SE = 2.46) and without opioids (n = 89; M = 22.32; SE = 1.87) reported comparable levels of functioning. Among the subset of patients using opioid medications, those with high levels of pain evidenced greater impairment than those with low levels (p <.01). Pain status held no relationship with psychosocial functioning among patients not using opioids.
3.3.2. What is the relationship of pain, PTSD, and SSRIs with psychosocial functioning?
Examination of the SSRI model again revealed a significant effect of pain on psychosocial functioning (p <.001, ηP2 = .06) adjusting for covariates (high-pain: M = 34.89; SE = 2.37; low-pain: M = 23.50; SE = 2.01). A marginal PTSD × SSRI interaction also was observed in these data (p = .07, ηP2 = .02). Post-hoc examination of this interaction (see Fig. 3) suggests that among patients not using an SSRI, those with PTSD evidenced poorer psychosocial functioning (n = 103; M = 32.53; SE = 1.73) than those without PTSD (n = 81; M = 22.12; SE = 2.17; p <.01). Among individuals using an SSRI, however, patients with PTSD (n = 34; M = 30.87; SE = 3.38) evidenced no differences in psychosocial functioning than those without PTSD (n = 16; M = 31.27; SE = 4.66). No differences in psychosocial functioning across SSRI use were observed within the subset of patients diagnosed with PTSD or among those without PTSD diagnoses.
Fig. 3.
Interaction of PTSD and SSRI medication on SIP Psychosocial; PTSD = Posttraumatic Stress Disorder; SSRI = selective serotonin reuptake inhibitor; SIP PSYSOC = Sickness Impact Profile -Psychosocial; Note. Higher SIP scores indicate lower levels of psychosocial functioning; Bars connected by lines indicate mean differences in psychosocial functioning
 p<.01
p<.01
 p<.05
p<.05
3.3.3. What is the relationship of pain, PTSD, and anxiolytic/sedative use on psychosocial functioning?
Examination of psychosocial functioning with respect to anxiolytic use revealed a main effect of pain (p <.001, ηP2= .06) adjusting for covariates (high-pain: M = 35.47; SE = 3.00; low-pain: M = 22.50; SE = 2.12). These analyses also indicated a unique effect of PTSD (p = .01, ηP2 = .03) such that individuals with PTSD evidenced poorer psychosocial functioning (M = 33.53; SE = 2.23) than those without PTSD (M = 24.43; SE = 2.12). Anxiolytic/sedative use was not associated with functioning in these data.
4. Discussion
The aim of the present research was to explore the unique and interactive associations of chronic pain, PTSD, and medication use across indices of physical and psychosocial functioning. Patients classified as high-pain in this sample evidenced poorer physical functioning than did those with lower levels of pain irrespective of medication use, medication type, or PTSD status. In contrast, the relationship between opioid use and physical functioning was dependant on PTSD diagnosis. Opioid use was associated with greater physical impairment among patients without PTSD but was unrelated to functioning among those with PTSD. PTSD diagnostic status evidenced no association with physical functioning either among opioid or non-opioid groups. Pain severity was the only significant factor associated with physical functioning within the SSRI and anxiolytic/sedative models although a marginal interaction of PTSD and anxiolytic use was observed.
Pain severity evidenced equally robust associations with psychosocial impairment whereas PTSD demonstrated more broad effects within this domain of functioning. Opioid use again evidenced an interactive association with PTSD whereby use was related to greater psychosocial impairment specifically among patients without PTSD. A diagnosis of PTSD also was associated with greater psychosocial impairment among patients not using opioids. For opioid users, equally poor functioning was observed irrespective of PTSD symptomology. A marginal pain by opioid interaction also emerged whereby high-pain patients using opioids evidenced poorer psychosocial functioning than both high-pain patients without opioids and low-pain patients with opioids. Associations between SSRI use and psychosocial functioning also were marginally dependent on PTSD. Among the subset of individuals not using an SSRI, patients with PTSD evidenced greater psychosocial impairment than did those without PTSD. No other group differences were noted in these analyses. Data pertaining to anxiolytic/sedative use evidenced main effects of pain and of PTSD, but no association was observed between anxiolytics and psychosocial functioning.
The robust associations observed between pain severity and functional impairment in this research are consistent with literature documenting the considerable individual and societal cost of chronic pain internationally (e.g., Blyth et al., 2001; Duckworth and Iezzi, 2005; Harstall and Ospina, 2003; Lynch and Watson, 2006). As the single largest factor contributing to impairment in this study, these data strongly suggest that pain management should be considered a central component of interventions targeting survivors of traumatic injury.
Regarding effective pain management, these data indicate that PTSD symptomology may be an important consideration in determining treatment modality for individuals with chronic pain. Medication use, across all categories and within both functional domains, failed to demonstrate a relationship with functioning in pain patients with comorbid PTSD. Existing models of PTSD and pain suggest factors that may contribute to the absence of treatment effects in this particular subsample. McLean et al. (2005) propose a biopsychosocial model whereby neurobiological and psychosocial systems interact to heighten pain processing and perpetuate PTSD symptomology. Others outline behavioral and psychological factors common to both chronic pain and PTSD (e.g., attentional biases, alterations in pain perception, avoidant coping styles) which contribute to stable, mutually reinforcing pathologies (Asmundson et al., 2002; Sharp and Harvey, 2001). To date, the potential complications of this comorbidity have not been addressed in research examining pharmacological interventions for either chronic pain or PTSD (e.g., Brady et al., 2000; Hale et al., 2007; Marshall et al., 2001; Webster et al., 2006). Outcome research targeting the complexities of this comorbidity is needed given the personal and public health costs associated with post-trauma pain and psychopathology.
Observed associations between medication use and functioning failed to provide evidence for therapeutic effects for any pharmacological agent. Opioid use held significant associations with increased physical and psychosocial impairment in patients without PTSD. Opioid use also was associated with greater psychosocial impairment among patients with high levels of pain. While surprising, these observations are consistent with epidemiological research reporting associations between opioid use and greater pain severity, higher rates of disability, lower rates of employment, and poorer quality of life (Eriksen et al., 2006). Side effect profiles associated with opioid use – tolerance, physical dependence, cognitive impairment – often are cited as factors contributing to potential decreases in functioning (Ballantyne and Shin, 2008; Eriksen et al., 2006). Evidence of paradoxical increases in pain sensitivity associated with opioid use, a condition known as opioid-induced hyperalgesia, also have been reported in both observational and experimental research (Angst and Clark, 2006). Although causal relationships cannot be attributed within these cross-sectional data, the present research adds to the growing body of literature questioning the utility of opioids in treating chronic, non-malignant pain on a large scale (Ballantyne and Shin, 2008). Given inconsistent findings regarding their utility over extended periods, potential for reductions in overall functioning, and the increased risk of abuse and dependency within psychiatric samples, health-care professionals may be well served to consider the relative costs and benefits of extended opioid therapy for patients with chronic pain subsequent to traumatic injury.
The relationship between SSRI use and psychosocial functioning also was marginally dependent on the presence or absence of diagnosable PTSD. Interestingly, SSRIs were not associated with improved physical or psychosocial functioning for any patient subsample. It is possible that the benefits associated with SSRIs observed in previous trauma samples (e.g., Brady et al., 2000; Marshall et al., 2001; Tucker et al., 2001) were negated by over-riding physical pain experienced by patients in the current study. Despite limited evidence for a therapeutic effect of these medications, reservations regarding the efficacy of SSRIs are moderated in that SSRIs are generally viewed as comparatively benign medications. Relative to opioid analgesics, SSRIs carry little to no abuse risk with generally tolerable side effect profiles. Further investigation may reveal only limited benefits associated with SSRI use among patients with comorbid pain and PTSD, but the low risk associated with their use should be an important consideration in determining treatment regiments for these patients.
Anxiolytic/sedative medications held a marginal interactive effect with PTSD with respect to physical functioning although post-hoc examination of this interaction revealed no pairwise group differences. Anxiolytics held no association with indices of psychosocial functioning. It is possible, as with the SSRIs, that the potential benefits conferred by these medications may have been lost secondary to the over-riding effects of chronic pain. It is also possible that the heterogeneous collection of medications included in this category masked the effects of any specific agent. Regardless, anxiolytic therapy generally has demonstrated limited efficacy with respect to the treatment of either PTSD (APA, 2004) or chronic pain (Sanders et al., 2005). Though sometimes prescribed for symptomatic benefit, the increased risk of abuse and dependence among PTSD samples argues for the judicious use of these medications.
Interpretation of these data should be made within the context of a number of methodological limitations. First, the cross-sectional nature of these data clearly precludes causal inferences regarding the observed relationships. Potentially relevant variables such as severity of physical injury, concurrent non-pain conditions, variations in medication adherence, litigation status, and general patient expectancies all are unmeasured, uncontrolled factors which may significantly influence functioning. Although the present study is constrained by limitations characteristic of observational research, the observed relationships are consistent with existing data and begin to explore a clinically relevant subset of patients that has been overlooked in the pharmacologic literature. These preliminary associations may be useful in developing specific hypotheses regarding the management of comorbid pain and PTSD.
The homogeneity of the present sample also generates concerns regarding generalization of these findings to more diverse patient populations. As with existing research exploring the efficacy of pharmacological interventions for PTSD and chronic pain (e.g., Brady et al., 2000; Connor et al., 1999; Jamison et al., 1998; Markenson et al., 2005), the present sample was predominantly middle-aged, Caucasian, and female. Ethnic and racial minorities were overrepresented among both non-completers and high-pain patients in the present study, and existing literature suggests that ethnicity may hold relationships both with chronic pain and PTSD (e.g., Edwards et al., 2001; Green et al., 2004; Norris, 1992). Inclusion of more diverse patient samples in future research would provide a means by which to evaluate the generalizability of the present results and to examine the impact of socio-cultural factors on the interface of chronic pain and PTSD.
Finally, the present data are limited to relationships with physical and psychosocial functioning and are unable to address the impact of medications on specific symptomology (e.g., pain severity, anhedonia, insomnia). It is possible, perhaps probable, that medication use is associated with amelioration of symptom level pathology that does not necessarily translate into more general improvements in overall functioning. Controversy regarding appropriate treatment outcome goals (e.g., reduction in symptom severity, increased occupational or interpersonal functioning, reducing ongoing use of health care services) continues in the public health literature and varies largely from discipline to discipline (Clark, 2008). In practice, these therapeutic objectives likely share some amount of overlap, but generalizations across level of treatment outcome should be made with care.
Despite evidence of a substantial comorbidity between chronic pain and PTSD following traumatic injury, very little is known regarding the effectiveness of pharmacological treatments within this patient population. The present data suggest that pain severity is one of the most salient factors with respect to overall impairment although PTSD appears to moderate the relationship between medication use and patient functioning in some cases. These data also suggest that the associations of PTSD and medication use with impairment may vary across domains of functioning. Given the current state of the literature, few recommendations can be made regarding preferred treatment modalities for individuals with comorbid pain and PTSD. Health care professionals are advised to be aware of the potential complications of this comorbidity and to consider the increased risk inherent to this particular patient group before proceeding with a course of treatment.
Acknowledgments
Omitted for anonymous review
Footnotes
Although employment and income evidenced associations with SIP physical and psychosocial scores, these relationships are expected given that employment status, and as a consequence income, are by definition indicators of functioning. The strong conceptual and practical overlap of these factors with primary outcome variables precluded their inclusion as covariates in the presented models (see Miller & Chapman, 2001 for a review on the appropriate use of covariates in ANCOVA).
Opioid prescriptions sum to greater than 108 due to multiple prescriptions within an individual patient (n = 14).
Group means and standard errors for this effect are as follows: PTSD-/Anx- (n = 84, M = 16.27, SE = 1.16); PTSD-/Anx+ (n = 13, M = 13.94, SE = 3.11); PTSD+/Anx- (n = 116, M = 14.54, SE = .90); PTSD+/Anx+ (n = 21, M = 19.53, SE = 2.38)
References
- American Psychiatric Association . The diagnostic and statistical manual of mental disorders. 4th Ed Washington, DC: 1994. [Google Scholar]
- Angst MS, Clark JD. Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- Asmundson GJG, Coons MJ, Taylor S, Katz J. PTSD and the experience of pain: Research and clinical implications of shared vulnerability and mutual maintenance models. Can J Psychiatry. 2002;47:930–937. doi: 10.1177/070674370204701004. [DOI] [PubMed] [Google Scholar]
- Ballantyne JC, Shin NS. Efficacy of opioids for chronic pain: A review of the evidence. Clin J Pain. 2008;24:469–478. doi: 10.1097/AJP.0b013e31816b2f26. [DOI] [PubMed] [Google Scholar]
- Bergner M, Bobbitt RA, Kressel S, Pollard WE, Gilson BS, Morris JR. The Sickness Impact Profile: Conceptual formulation and methodology for the development of a health status measure. Int J Health Serv. 1976;6:393–415. doi: 10.2190/RHE0-GGH4-410W-LA17. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Klauminzer G, Charney DS, Keane TM. Clinician-Administered PTSD Scale (CAPS) National Center for Post-traumatic Stress Disorder, Behavioral Science Division; Boston, MA: 1990. [Google Scholar]
- Blanchard EB, Hickling EJ. After the crash. American Psychological Association; Washington, DC: 1997. [Google Scholar]
- Blanchard EB, Hickling EJ, Taylor AE, Loos WR, Forneris CA, Jaccard J. Who develops PTSD from motor vehicle accidents? Behav Res Ther. 1996;34:1–10. doi: 10.1016/0005-7967(95)00058-6. [DOI] [PubMed] [Google Scholar]
- Blyth FM, March LM, Brnabic AJM, Jorm LR, Williamson M, Cousins MJ. Chronic pain in Australia: A prevalence study. Pain. 2001;89:127–134. doi: 10.1016/s0304-3959(00)00355-9. [DOI] [PubMed] [Google Scholar]
- Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR, Farfel GM. Efficacy and safety of sertraline treatment of posttraumatic stress disorder. JAMA. 2000;283:1837–1844. doi: 10.1001/jama.283.14.1837. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Marosszeky JE, Crooks J, Baguley IJ, Gurka JA. Interaction of Posttraumatic stress disorder and chronic pain following traumatic brain injury. J Head Trauma Rehabil. 1999;14:588–594. doi: 10.1097/00001199-199912000-00007. [DOI] [PubMed] [Google Scholar]
- Clark ME. Understanding appropriate long-term use of opioids – seventeen years and counting. Clin J Pain. 2008;24:467–468. doi: 10.1097/AJP.0b013e318174bb81. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Connor KM, Sutherland SM, Tupler LA, Malik ML, Davidson JR. Fluoxetine in post-traumatic stress disorder: Randomized, double-blind study. Br J Psychiatry. 1999;175:17–22. doi: 10.1192/bjp.175.1.17. [DOI] [PubMed] [Google Scholar]
- Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in the US: 1980 vs. 2000. Pain. 2004;109:514–519. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- DeBruin AF, DeWitte LP, Stevens F, Diederiks JPM. Sickness Impact Profile: The state of the art of a generic functional status measure. Soc Sci Med. 1992;35:1003–1014. doi: 10.1016/0277-9536(92)90240-q. [DOI] [PubMed] [Google Scholar]
- Deyo RA. Comparative validity of the Sickness Impact Profile and shorter scales for functional assessment in low-back pain. Spine. 1986;11:951–954. doi: 10.1097/00007632-198611000-00017. [DOI] [PubMed] [Google Scholar]
- Duckworth MP, Iezzi T. Chronic pain and posttraumatic stress symptoms in litigating motor vehicle accident victims. Clinical Journal of Pain. 2005;21:251–261. doi: 10.1097/00002508-200505000-00008. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, Kalso EA, Loeser JD, Miaskowski C, Nurmikko TJ, Portenoy RK, Rice ASC, Stacey BR, Treede RD, Turk DC, Wallace MS. Pharmacological management of neuropathic pain: Evidence-based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129:355–362. doi: 10.1016/j.pain.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Edwards CL, Fillingim RB, Keefe F. Race, ethnicity and pain. Pain. 2001;94:133–137. doi: 10.1016/S0304-3959(01)00408-0. [DOI] [PubMed] [Google Scholar]
- Eriksen J, Sjøgren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic on-cancer pain: An epidemiological study. Pain. 2006;125:172–179. doi: 10.1016/j.pain.2006.06.009. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Interview for DSM-IV Axis I Disorders: Patient Edition (SCID I/P) New York State Psychiatric Institute, Biometrics Department; New York: 1995. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forbes D, Creamer M, Biddle D. The validity of the PTSD checklist as a measure of symptomatic change in combat-related PTSD. Behav Res Ther. 2001;39:977–986. doi: 10.1016/s0005-7967(00)00084-x. [DOI] [PubMed] [Google Scholar]
- Friedland JF, Dawson DR. Function after motor vehicle accidents: A prospective study of mild head injury and posttraumatic stress. J Nerv Ment Dis. 2001;189:426–434. doi: 10.1097/00005053-200107000-00003. [DOI] [PubMed] [Google Scholar]
- Games PA, Howell JF. Pairwise multiple comparison procedures with unequal N's and/or variances: A Monte Carlo study. Journal of Educational and Behavioral Statistics. 1976;1:113–125. [Google Scholar]
- Geisser ME, Roth RS, Bachman JE, Eckert TA. The relationship between symptoms of post-traumatic stress disorder and pain, affective disturbance and disability among patients with accident and non-accident related pain. Pain. 1996;66:207–214. doi: 10.1016/0304-3959(96)03038-2. [DOI] [PubMed] [Google Scholar]
- Green CR, Ndao-Brumblay SK, Nagrant AM, Baker TA, Rothman E. Race, age, and gender influences among clusters of African American and white patients with chronic pain. J Pain. 2004;5:171–182. doi: 10.1016/j.jpain.2004.02.227. [DOI] [PubMed] [Google Scholar]
- Hale ME, Ahdieh H, Ma T, Rauck R. Efficacy and safety of OPANA ER (oxymorphone extended release) for relief of moderate to severe chronic low back pain in opioid-experienced patients: A 12-week, randomized, double-blind, placebo-controlled study. J Pain. 2007;8:175–184. doi: 10.1016/j.jpain.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Harstall C, Ospina M. How prevalent is chronic pain? Pain. 2003;11:1–4. [Google Scholar]
- Hickling EJ, Blanchard EB. Post-traumatic stress disorder and motor vehicle accidents. J Anxiety Disord. 1992;6:285–291. [Google Scholar]
- International Association for the Study of Pain Pain. 1986;(Suppl 3):S1–S225. [Google Scholar]
- Jamison RN, Raymond SA, Slawsby EA, Nedeljkovic SS, Katz NP. Opioid therapy for chronic noncancer back pain: A randomized prospective study. Spine. 1998;23:2591–2600. doi: 10.1097/00007632-199812010-00014. [DOI] [PubMed] [Google Scholar]
- Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain. 1985;23:345–356. doi: 10.1016/0304-3959(85)90004-1. [DOI] [PubMed] [Google Scholar]
- Lynch ME, Watson CPN. The pharmacotherapy of chronic pain: A review. Pain Res Manage. 2006;11:11–38. doi: 10.1155/2006/642568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markenson JA, Croft J, Zhang PG, Richards P. Treatment of persistent pain associated with osteoarthritis with controlled-release oxycodone tablets in a randomized controlled clinical trial. Clin J Pain. 2005;21:524–535. doi: 10.1097/01.ajp.0000146215.86038.38. [DOI] [PubMed] [Google Scholar]
- Marshall RD, Beebe KL, Oldham M, Zaninelli R. Efficacy and safety of paroxetine treatment for chronic PTSD: A fixed-dose, placebo-controlled study. Am J Psychiatry. 2001;158:1982–1988. doi: 10.1176/appi.ajp.158.12.1982. [DOI] [PubMed] [Google Scholar]
- McCleane G. Antidepressants as analgesics. CNS Drugs. 2008;22:139–156. doi: 10.2165/00023210-200822020-00005. [DOI] [PubMed] [Google Scholar]
- McLean SA, Clauw DJ, Abelson JL, Liberzon I. The development of persistent pain and psychological morbidity after motor vehicle collision: Integrating the potential role of stress response systems into a biopsychosocial model. Psychosom Med. 2005;67:783–790. doi: 10.1097/01.psy.0000181276.49204.bb. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Norris FH. Epidemiology of trauma: Frequency and impact of different potentially traumatic events on different demographic groups. J Consult Clin Psychol. 1992;60:409–418. doi: 10.1037//0022-006x.60.3.409. [DOI] [PubMed] [Google Scholar]
- Sanders SH, Harden RN, Vicente PJ. Evidence-based clinical practice guidelines for interdisciplinary rehabilitation of chronic nonmalignant pain syndrome patients. Pain Pract. 2005;5:303–315. doi: 10.1111/j.1533-2500.2005.00033.x. [DOI] [PubMed] [Google Scholar]
- Schwartz AC, Bradley R, Penza KM, Sexton M, Jay D, Haggard PJ, Garlow SJ, Ressler KJ. Pain medication use among patients with posttraumatic stress disorder. Psychosomatics. 2006;47:136–142. doi: 10.1176/appi.psy.47.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp TJ, Harvey AG. Chronic pain and posttraumatic stress disorder: Mutual maintenance? Clin Psychol Rev. 2001;21:857–877. doi: 10.1016/s0272-7358(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Sherman JJ, Turk DC, Okifuji A. Prevalence and impact of posttraumatic stress disorder-like symptoms on patients with fibromyalgia syndrome. Clinical Journal of Pain. 2000;16:127–134. doi: 10.1097/00002508-200006000-00006. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 4th ed. Allyn and Bacon; Boston, MA: 2001. [Google Scholar]
- Tucker P, Zaninelli R, Yehuda R, Ruggiero L, Dillingham K, Pitts CD. Paroxetine in the treatment of chronic posttraumatic stress disorder: Results of a placebo-controlled, flexible-dosage trial. J Clin Psychiatry. 2001;62:860–868. doi: 10.4088/jcp.v62n1105. [DOI] [PubMed] [Google Scholar]
- Webster LR, Butera PG, Moran LV, Wu N, Burns LH, Friedmann N. Oxytrex minimizes physical dependence while providing effective analgesia: A randomized controlled trial in low back pain. J Pain. 2006;7:937–946. doi: 10.1016/j.jpain.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Zatzick DF, Rivara FP, Nathens AB, Jurkovich GJ, Wang J, Fan M, Russo J, Salkever DS, Mackenzie EJ. A nationwide US study of post-traumatic stress after hospitalization for physical injury. Psychol Med. 2007;37:1469–1480. doi: 10.1017/S0033291707000943. [DOI] [PubMed] [Google Scholar]
- Zayfert C, Dums AR, Ferguson RJ, Hegel MT. Health functioning impairments associated with posttraumatic stress disorder, anxiety disorders, and depression. J Nerv Ment Dis. 2002;190:233–240. doi: 10.1097/00005053-200204000-00004. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association Practice guideline for the treatment of patients with acute stress disorder and posttraumatic stress disorder. 2004 http://www.psychiatryonline.com/pracGuide/loadGuidelinePdf.aspx?file=ASD_PTSD_05-15-06. [PubMed]