Abstract
Amyloid precursor protein (APP) has been implicated in squamous cell carcinoma. In this study we show that forced expression of the transcription factor activating protein 2α (AP-2α) results in significantly increased steady state levels of APP mRNA in human keratinocytes. Sequence analysis of the 5′ end of the human APP gene revealed five putative binding sites for AP-2, suggesting that APP is a direct target for transactivation by AP-2. AP-2 protein bound at least 3 of these putative promoter elements in vitro as determined by electrophoretic mobility shift assay. Chromatin immunoprecipitation (ChIP) analysis showed that these binding sites were occupied by AP-2 in cells, thus indicating the relevance to AP-2 binding in vivo. We then analyzed APP and AP-2 mRNA and protein expression in squamous cell carcinoma tumor samples. Analysis of RNA extracted from human tissue showed a significant positive correlation between AP-2α and APP mRNA expression. Immunohistochemical staining of tumor samples also demonstrated a positive correlation which was substantiated through western blot studies. Taken together, these findings demonstrate a role for the transcription factor AP-2α in the regulation of APP gene expression in human keratinocytes.
Keywords: Alzheimer’s Disease, Head and Neck, Skin, Amyloid precursor protein, Adenovirus, Chromatin Immunoprecipitation
Introduction
Amyloid precursor protein (APP) is a type 1 membrane glycoprotein (Kang, Lemaire et al. 1987; Ko, Lin et al. 2004). Expressed as multiple isoforms (Ling, Morgan et al. 2003), this transmembrane protein can be cleaved through proteolytic pathways resulting in a soluble form of APP (sAPP). APP is best known for its role in the pathogenesis of Alzheimer’s disease (Kouznetsova, Klingner et al. 2006; Rovelet-Lecrux, Hannequin et al. 2006); however, APP’s role in squamous cell carcinoma is not unexpected given that keratinocytes and neural cells both arise from the ectodermal embryonic cell layer. Recently, APP mRNA and protein levels were found to be significantly higher in oral squamous cell carcinoma samples compared to matched, non-cancerous tissue (Ko, Lin et al. 2004). This finding is consistent with APP’s multiple roles in keratinocyte cell growth and apoptosis. Kummer et al. demonstrated an increase in APP mRNA expression in keratinocytes during cutaneous wound repair (Kummer, Wehner et al. 2002). Likewise, sAPP has a growth promoting function on epithelial cells (Okamoto, Takeda et al. 1996; De Strooper and Annaert 2000; Hoffmann and Schulz 2005). APP also appears to play a role in apoptosis (Kogel, Schomburg et al. 2003).
The AP-2 family of proteins consists of five transcription factors (α, β, γ, δ, ε) (Tellez and Bar-Eli 2003). These proteins share a common structure which includes DNA binding and transactivating domains (Imagawa, Chiu et al. 1987; Williams, Admon et al. 1988). A specific member of this family, AP-2α, has been postulated to play an important role in the activation of known oncogenes and carcinogenesis (Leask, Byrne et al. 1991; Oyama, Takahashi et al. 2002). Analysis of a region upstream to the rat APP gene showed a number of potential AP-2 binding sites (Chernak 1993). These potential regulatory sites were present in corresponding human sequences. Further work demonstrated that AP-2 could increase activity of an APP promoter-reporter construct (Bourbonniere, Shekarabi et al. 1997). However, the increase in promoter activity was hypothesized to result from an indirect mechanism, not through direct interaction of the AP-2 protein and the APP promoter. Additional work in the human neocortex showed an absence of AP-2 binding in the APP promoter region despite the presence of potential binding sites (Lukiw, Rogaev et al. 1994).
The aim of this study was to determine if AP-2α regulates the expression of APP in cultured cells and human tissue. Furthermore, we sought to determine if AP-2α mediated changes in APP expression were the result of direct binding of AP-2α to the APP promoter. The current study provides evidence that AP-2 binds directly to the APP promoter region in human keratinocytes, leading to an increase in APP mRNA by transcriptional activation of the APP gene. Furthermore, correlations between APP and AP-2 mRNA and protein expression were confirmed by in vitro models, human cell lines and human squamous cell carcinoma tissue samples.
Materials and Methods
Cell Line, Culture Conditions and Transfection
An immortalized cell line (HaCaT) derived from normal, human keratinocytes was generously provided to us by Dr. Norbert Nusenig (Heidelburg, Germany) and used. Cells were cultured in Dulbecco’s Modified Eagle Medium media containing 10% fetal bovine serum with penicillin and streptomycin. They were treated for 24 hours with 100 multiplicity of infection (MOI) of a replication deficient adenoviruses containing the coding region for either activator protein 2α (AP-2α) or activator protein 2ε (AP-2ε). Ad-BglII was used as an empty vector control.
Microarray Analysis
Total cellular RNA was extracted from HaCaT cells infected with 100 MOI of the AP-2α adenovirus with the Qiagen RNA extraction kit (Qiagen, Valencia, CA). The microarray hybridization was done by the Arizona Cancer Center Genomics Shared Service and has been previously described (Oshiro, Watts et al. 2003). Labeled cDNAs were competitively hybridized to a 5,760 gene cDNA microarray, and genes up regulated ≥ 2-fold by forced over-expression of AP-2α were identified as AP-2 targets. From this list of genes APP was chosen for validation and further study.
Real Time RT-Polymerase Chain Reaction
HaCaT cells were infected with 100 MOI of AP-2α or AP-2ε for 24 hours. Total cellular RNA was extracted and reverse transcribed using the Applied Biosystems High Capacity cDNA Archive Kit (Foster City, CA). Quantitative real time PCR was then performed on an Applied Biosystems 7000 Sequence detection system (Foster City, CA). Measured levels of 18s rRNA were used as an internal control. The primers directed towards the AP-2α cDNA were 5′-ACCCCAACGAAGTCTTCTGTTC-3′ and 5′-ATTTTTAGACTTCGCCCTCCG-3′ while the primers for AP-2ε were 5′-AGTCCGTGATCAAGAAAGTGCC-3′ and 5′-TTGAGCTGAGCAGTGAAAGCC-3′. The following primers were designed to detect all amyloid precursor protein (APP) isoforms: 5′-TCCTTCCCGTGAATGGAGAGT-3′ and 5′-AGAACCTGGTCGAGTGGTCAG-3′. RNA from human squamous cell carcinoma tissue was extracted using Qiagen RNeasy Mini Kits (Valencia, CA) and real time PCR was performed using the previously described primer sets.
Electrophoretic Gel Mobility Shift Analysis
An electrophoretic mobility shift assay (EMSA) was performed using five double stranded DNA oligodeoxynucleotides and the Gel Shift Assay System from Promega (Madison, WI). Four DNA oligodeoxynucleotides were designed from the APP promoter region while a fifth was from the first intron of the APP gene. The DNA sequences (DS) and their corresponding locations were: DS1 – 5′-GCCACTGGC-3′ (−4377 to -4369), DS2 – 5′-GCCTCTTTGGC-3′ (−1390 to -1380), DS3 -5′- GCCGTCGGC-3′ (−167 to −159), DS4 – 5′-GCCAAGGGC-3′ (−123 to −115), DS5 – 5′-GCCTGGACGGC-3′ (155 - 166). The DNA was incubated with recombinant human AP-2α protein that had been synthesized using the TNT Quick Coupled System (Promega, Madison, WI). Prior to use in the EMSA, the protein purity was confirmed using western blotting. The combined DNA and protein was then electrophoresed on a nondenaturing polyacrylamide gel. The gel was placed on a phosphor screen (Molecular Dynamics, Sunnyvale, CA) then scanned using a Typhoon Scanner (GE Healthcare, Piscataway, NJ).
Chromatin Immunoprecipitation
HaCaT cells were cross-linked using 1% formaldehyde then collected in PBS and protease inhibitors. The cells were then pelleted, resuspended in soniciation buffer (50 mM Tris-Cl pH 8.1, 10 mM EDTA, and 1% SDS + protease inhibitors) and vortexed. Sonication conditions were determined empirically for each cell used in this study to achieve an optimal fragment length between 600-300 bp. Crosslinked DNA/histones were then diluted 1/10 using IP dilution buffer (0.01% SDS, 1.1% Trition-X 100, 1.2 mM EDTA, 16.7 mM Tris-Cl pH 8.1,167 mM NaCl) plus protease inhibitors. Samples were then pre-cleared using Protein G agarose (Upstate Biotech, Charlottesville, VA) then immunoprecipitated with anti-AP-2a (Upstate Biotech, Charlottesville, VA) or control mouse IgG. Chromatin/antibody complexes were collected using Protein G agarose followed by washing and elution. DNA was then purified from input chromatin and immunoprecipitation elutions by reversing crosslinks using 200 mM NaCl followed by the Qiagen DNeasy Kit (Qiagen, Valencia, CA). Purified DNA was then quantified (Biophotometer, Eppendorf, Hamburg, Germany). The amount of control region and APP promoter target in these samples was determined by SYBR green quantitative real time PCR. APP-control-forward: 5′- CAGGGCAGGAAATGGCATTA-3′, APP-control-reverse: 5′-TGCCTGCATGTTCTCTGGTTC-3′, APP1-forward: 5′- GGATGATTCAAGCTCACGGG-3′, APP1-reverse: 5′- CGTGAACAGTGGGAGGGAGA-3′.
Immunohistochemistry
Human squamous cell carcinoma tissue samples were obtained from surgical specimens in compliance with internal regulations governing the use of human tissue samples. A portion of the tumor was placed in formalin, fixed, dehydrated through graded alcohols, cleared in xylenes, infiltrated with paraffin wax, sliced to 4 μm sections and then mounted onto a glass slide. The immunoperoxidase reaction for APP and AP-2 was performed using the Envision + System/HRP method (Dako Company, Carpinteria, CA) using either an AP-2α/γ fusion antibody (Upstate Biotechnology, Lake Placid, NY) or an APP clone 22C11 antibody (Chemicon International, Temecula, CA). Sections were deparaffinized in xylenes, rehydrated in graded alcohols and rinsed in distilled water. Antigen unmasking was accomplished using citrate buffer for AP-2 and APP. Endogenous peroxidase activity was quenched using 3% H2O2. Sections were covered with the AP-2 or APP antibodies in primary antiserum at a 1:1000 dilution. The sections were then covered in Envision + System Labeled Polymer − HRP using anti-mouse antibody, rinsed then incubated with Liquid DAB + Substrate Chromagen System. A counter stain of Surgipath Company Hematoxylin (Harris Formula) was applied. Negative control slides were prepared by substituting mouse IgG. All rinses were performed with DakoCytomation Wash Buffer (Dako Company, Carpinteria, CA). Staining intensity was graded as absent, weak, moderate or strong by a pathologist (SW).
Western Blotting Analysis
Tissue samples were sectioned, minced then emulsified in 0.5 ml of phosphate buffered water (pH=7.1) on ice three. The protein was heated in Laemmli Sample Buffer (Sigma, St. Louis, MO), run on a 12% tris-HCL Bio-Rad Ready Gel (Hercules, CA), transferred to nitrocellulose paper and probed using an AP-2α/γ fusion antibody specific to AP-2α (Upstate Biotechnology, Lake Placid, NY) or an APP clone 22C11 antibody (Chemicon International, Temecula, CA) at 1:1000. p21, the loading control, was probed with antibody at 1:10,000. The blots were probed with horseradish peroxidase-linked goat anti mouse secondary antibody (Chemicon International, Temecula, CA) at 1:2000 for AP-2 and APP and 1:10,000 for p21 and signal was detected using the ECL Plus Western Blotting Detection System (GE Medical - Amersham Biosciences, Piscataway, NJ).
Results
Microarray Analysis Revealed APP as an AP-2 Target Gene
Microarray analysis of 5,760 different genes was performed on cDNA from total cellular RNA harvested from HaCaT cells infected with a replication deficient AP-2α over expressing adenovirus. Microarray analyses showed up-regulation of a number of genes as a result of forced AP-2α over-expression. Table 1 contains a list of those genes which had the highest fold increase in RNA expression. The 3.9 fold increase of the RNA encoding APP represented one of the highest fold increases.
Table 1.
Identification of AP-2α target genes in human keratinocytes. Microarray results revealed a number of genes that had increased expression levels upon infection with the replication deficient AP-2α expressing adenovirus. The amyloid precursor protein (APP) showed a 3.9 fold increase in expression level.
| Gene | Fold Increase | Accession Number |
|---|---|---|
| Amyloid Precursor Protein (APP) | 3.9 | NM_000484 |
| Fatty Acid Binding Protein 6 | 4.2 | NM_001445 |
| Glutathione S-transferase M3 (brain) | 2.4 | NM_000849 |
| Carbonic Anhydrase IX | 2.4 | NM_001216 |
| NADPH Menadione Oxidoreductase 1 | 2.4 | NM_000903 |
| Interferon, Gamma-Induced Protein 30 | 2.2 | NM_006332 |
| Glutathione Peroxidase (GPX1) | 2.2 | M21304 |
| Carcinoma Cell-Derived Alu RNA Transcript | 2.0 | M87940 |
APP mRNA Expression is Tightly Correlated with AP-2α mRNA Expression In Vitro
Real time RT-PCR was performed on normal, human, immortalized keratinocyte cells (HaCaT) infected with a replication deficient adenovirus containing the coding sequence for AP-2α or AP-2ε. HaCaT cells infected with the empty vector Ad-BglII were used as a control. The experiment was performed in triplicate. AP-2 over-expression was confirmed by real time RT-PCR (data not shown). Forced AP-2 over-expression resulted in 3.2 and 6.1 fold increases in APP mRNA for cells infected with AP-2α or AP-2ε respectively (Figure 1).
Figure 1.
AP-2 activates the expression of APP in human keratinocytes. HaCaT cells were transfected with 100 MOI of a replication deficient adenovirus containing either the AP-2α or AP-2ε coding sequences. Total cellular RNA was extracted, reverse transcribed and quantified using real time PCR. Infection with the AP-2α and AP-2ε containing viruses resulted in 3.2 and 6.1 fold increases in APP mRNA expression respectively.
AP-2α Binds to Sequences from the APP Promoter in vitro
To determine whether AP-2 interacts with putative DNA binding elements in the APP upstream regulatory region we performed a transcriptional element search system (TESS) analysis of the APP 5′ flanking region (Schug and Overton 1997). Four putative AP-2 binding sites were identified within the 5′ end of the APP gene (DS1-4) and a fifth sequence was identified within the first intron (DS5) (Figure 2). EMSA was performed using recombinant, human AP-2α protein incubated with each of the five radiolabeled DNA sequences corresponding to these portions of the APP promoter. Three of the five sites showed appreciable binding by the recombinant AP-2 protein (Figure 3).
Figure 2.
Schematic diagram of the APP gene indicating locations of putative regulatory elements. The five DNA sequences in the APP promoter and first intron were targeted during the electrophoresis mobility shift assay and are labeled as DS1-5. The rightward facing arrow represents the transcription start site with the shaded box representing the first exon. The ChIP control amplicon is located between DS1 and DS2 while the ChIP promoter amplicon is located near DS3 and DS4.
Figure 3.
AP-2α protein binds in vitro to sequences in regulatory regions of the APP gene. Lane assignments correspond to sequences shown in Figure 2. Lane 1 represents the segment furthest upstream while lane 5 is the furthest downstream segment. Lanes 1 – 4 are sites within the APP promoter while lane 5 is located within the first intron. Strong binding occurred in lanes 2, 4 and 5. Lanes 1 and 3 also showed association between the DNA sequences and the nuclear extracted protein.
AP-2α Binds to the APP Promoter In Vivo
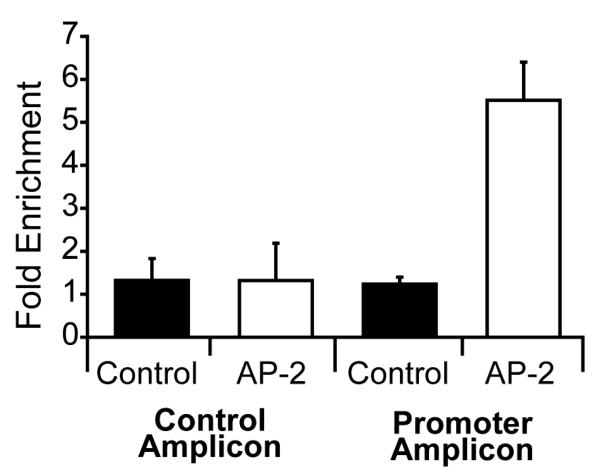
To determine which of the multiple putative AP-2 binding sites within the APP promoter might regulate APP expression in living HaCaT cells, we analyzed the putative AP-2 binding sites using chromatin immunoprecipitation. Real time PCR primers were designed to flank the AP-2 sites identified by EMSA analysis, as well as a neighboring region of the APP promoter lacking any putative AP-2 binding sites for use as a control amplicon (Figure 2). We then quantified the amount of AP-2 binding at these sites. The APP proximal promoter amplicon adjacent to EMSA sites DS3 and DS4 amplified sooner in AP-2α immunoprecipitations from AP-2α over-expressing HaCaT cells (Figure 4) indicating that AP-2α is bound to this region of the APP promoter in living cells. Amplification of the control amplicon was similar in chromatin immunoprecipitated with antibodies against either AP-2α or mouse IgG in both uninfected and AP-2α over expressing HaCaT cells (Figure 4). A pattern of amplification similar to that of the control amplicon was observed in regions corresponding to EMSA sites DS1, DS2 and DS5 (data not shown). The average fold enrichment of these target amplicons in AP-2α immunoprecipitations compared to IgG pulldowns was calculated for three independent experiments for the control and APP promoter amplicons (Figure 4). No significant difference in control amplicon enrichment was observed between control and AP-2α over-expressing HaCaT cells. In contrast, the APP proximal promoter amplicon was significantly enriched 5.5 fold in AP-2α immunoprecipitations from HaCaT cells over-expressing AP-2α (two tail Student’s t-test, p<0.05).
Figure 4.
AP-2-2α protein binds in vivo to sequences in regulatory regions of the APP gene ChIP results were analyzed for the ChIP control region (control amplicon) and promoter region (promoter amplicon). The control region was void of AP-2 binding sites. Fold enrichment of AP-2 associated DNA (AP-2) was measured for each amplicon and compared to total cellular input for each group (control). Fold enrichment of AP-2 for the control region showed no statistically significant difference compared to the total cellular input while AP-2 enrichment was approximately 4.5 fold (p<0.05) in the promoter amplicon compared to total cellular input.
APP Expression in Human Squamous Cell Carcinoma is Correlated with AP-2α Protein Expression
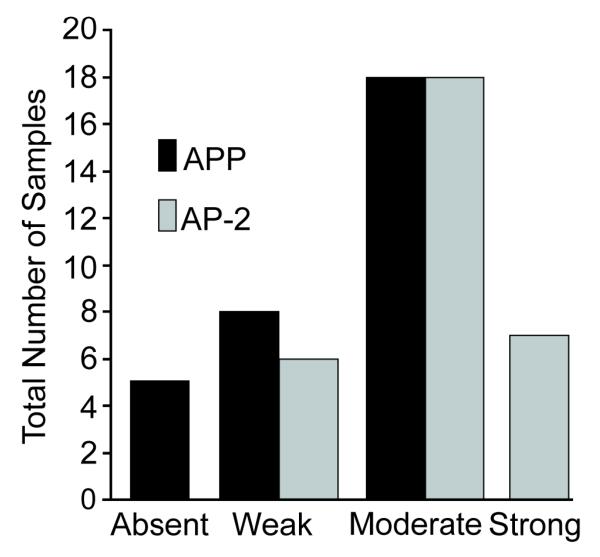
Immunohistochemistry staining for APP and AP-2 proteins was performed on 31 cases of squamous cell carcinoma of the head and neck (Figure 5). APP staining was localized to the cytoplasm of tumor cells while AP-2 staining was confined to the nucleus, in keeping with the known roles and locations of APP and AP-2. Staining intensity was graded as absent, weak, moderate, or strong. The majority of cases exhibited moderate staining intensity for both APP and AP-2 (Figure 6).
Figure 5.
AP-2α and APP proteins are both detectable in human oral squamous cell carcinomas. The tissue was sectioned and stained as described in materials and methods section. Each slide was stained with an antibody directed towards either the APP or AP-2 proteins. AP-2 staining appeared to be confined to the nucleus. Slides were then examined and scored for staining intensity. A – haematoxylin and eosin staining at 100× magnification; B – AP-2 staining at 100× magnification; C – AP-2 staining at 200× magnification; D – APP staining at 100× magnification; E – APP staining at 200× magnification.
Figure 6.
Semi-quantitative analysis of staining patterns in 31 specimens suggests a correlation between AP-2α and APP expression at the immunohistochemical level. Staining intensity was categorized as absent, weak, moderate or strong and listed on the x-axis. The total numbers of APP and AP-2 samples in each category were compared graphically. Staining intensity for APP and AP-2 demonstrated a positive correlation which was further substantiated with the RNA and western blot results performed on the same tissue samples.
Western blots were performed on total cellular protein extracted from human squamous cell carcinoma tissue samples. Echoing the immunohistochemical staining results, higher levels of AP-2 expression correlated with increased levels of APP expression (Figure 7).
Figure 7.
Western blotting revealed a tighter correlation between AP-2α and APP expression at the protein level. Western blots were performed on total cellular protein extracted from the squamous cell carcinoma samples. Western blots were performed using the same antibodies used for the immunohistochemistry. p21was used as a loading control for the APP and AP-2α proteins. There is a positive correlation between APP and AP-2 protein expression levels with higher levels of APP expression correlating with increased levels of AP-2 protein expression.
APP mRNA Expression is Tightly Correlated with AP-2α mRNA Expression In Vivo
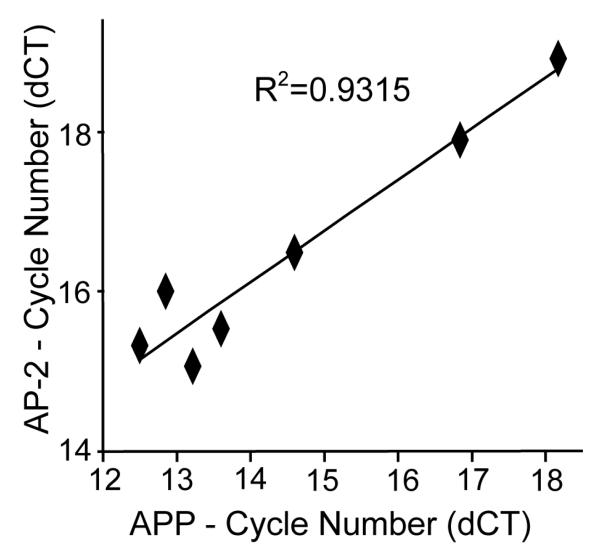
To further extend the association between APP and AP-2 expression, real time RT-PCR was performed on a subset of the squamous cell carcinoma samples that had been analyzed by immunohistochemistry. Increased expression of the APP gene was associated with increased expression of AP-2α with a chi square test showing R2=0.93 (Figure 8).
Figure 8.
AP-2α and APP expression are tightly correlated at the mRNA level in human squamous cell carcinoma explants. RNA was extracted from human squamous cell carcinoma samples, reverse transcribed and then quantified using real time PCR. There was a positive correlation between APP and AP-2α RNA expression levels with R2 = 0.9315. The x-axis shows APP mRNA PCR cycle number corrected for the 18s loading control while the y-axis shows AP-2 mRNA PCR cycle number corrected for 18s.
Discussion
APP and AP-2 are involved in the pathogenesis of a number of different cancers. For example, AP-2 has been shown to be involved with the pathogenesis of melanoma (Leask, Byrne et al. 1991) and cancers derived from the ectodermal cell layer (Bar-Eli 2001; Tellez and Bar-Eli 2003). In addition, APP is over-expressed in oral squamous cell carcinoma (Ko, Lin et al. 2004), and AP-2 has been implicated in the development of squamous cell carcinoma (Popa, Dahler et al. 2004).
Our findings suggest a coordinated regulation of AP-2 and APP in squamous cell carcinoma. Expression microarray analysis of AP-2 over expressing HaCaT cells showed a 4 fold increase in APP mRNA. Real time RT-PCR showed a 3.2 fold increase in APP mRNA. These findings are consistent with, and support, previous published data showing that AP-2 can increase APP promoter activity (Bourbonniere, Shekarabi et al. 1997). Moreover, AP-2 bound to sequences in the 5′ end of the APP gene. Our findings therefore define a role of AP-2 in APP gene expression.
The mechanism by which AP-2 can increase APP expression had previously been explored. Despite the presence of AP-2 putative binding sites within the 5′ upstream regulatory region of the APP gene (Chernak 1993), Bourbonniere et al hypothesized that AP-2 was playing an indirect role in increasing APP promoter activity. In Bourbonniere’s work, AP-2 binding was analyzed at position −222 to −195 at the 5′ end of the APP gene by electromobility shift assay. Despite the presence of canonical AP-2 binding sites, no gel mobility shift was observed upon incubating the APP DNA sequences with AP-2. Similar findings were obtained by Lukiw et al who performed an EMSA on a 258 base pair sequence spanning −203 at the 5′ end of the APP gene to +55 within the gene.
Our findings clearly demonstrate, using multiple experimental models, a direct relationship between AP-2 protein and the 5′ end of the APP gene. These apparently contradictory findings are easily explained given that the areas analyzed for our EMSA are in different regions from those previously analyzed by Bourbonniere et al. Our work shows that not all the sites containing a possible AP-2 binding sequence were associated with AP-2 binding. The ChIP data, in conjunction with the data from the real time RT-PCR and the EMSA assays, clearly demonstrate a direct relationship between AP-2 and APP.
In order to further explore this relationship we investigated the correlation between APP and AP-2 expression in a series of human squamous cell carcinoma samples. We identified a positive correlation (chi square test, R2 = 0.93) between APP and AP-2 mRNA expression using real time RT-PCR. These findings agree with those of Ko et al (Ko, Lin et al. 2004).
Immunohistochemical staining for APP and AP-2 of the same tumor samples used for mRNA extraction provided further evidence of this association. Staining results showed a positive relationship between AP-2 and APP staining. Protein expression, as measured by western blotting and immunohistochemistry staining intensity, showed that the association seen at the RNA level is also seen on the protein level. AP-2 protein expression was highest in those samples that had the highest APP expression.
It should be noted that some tumor samples did not have detectable levels of AP-2. APP protein expression in the absence of AP-2 protein, as seen in our western blot, may be due to alternative regulation of APP. APP expression is likely influenced by multiple proteins and signaling pathways (Theuns and Van Broeckhoven 2000; Lahiri and Ge 2004). The findings from the tumor samples indicate that the interaction between AP-2 and APP, as demonstrated more directly in the cell culture models, plays an important role in APP regulation in human tumor cells.
In conclusion, we have identified the human APP gene as a direct transcriptional target of AP-2 and shown that expression of APP and AP-2 are tightly correlated in squamous cell carcinoma samples. The interplay between APP and AP-2 provides a novel avenue in squamous cell carcinoma research and offers a new finding in the battle against this disease.
Acknowledgements
The authors thank Drs. Bernard Futscher and George Watts of the University of Arizona Cancer Center – Microarray Genomics Core, and Dr. Norbert Fusenig for the gift of HaCaT cells. This work was supported by NIH grants 5P20CA103672 and 5P01CA66081. MJP and MJH received salary support from NIH grants 5T32DC000040 and 5T32CA078586 respectively.
Footnotes
Disclosure/Conflict of Interest The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bar-Eli M. Gene regulation in melanoma progression by the AP-2 transcription factor. Pigment Cell Res. 2001;14(2):78–85. doi: 10.1034/j.1600-0749.2001.140202.x. [DOI] [PubMed] [Google Scholar]
- Bourbonniere M, Shekarabi M, et al. Enhanced expression of amyloid precursor protein in response to dibutyryl cyclic AMP is not mediated by the transcription factor AP-2. J Neurochem. 1997;68(3):909–16. doi: 10.1046/j.1471-4159.1997.68030909.x. [DOI] [PubMed] [Google Scholar]
- Chernak JM. Structural features of the 5′ upstream regulatory region of the gene encoding rat amyloid precursor protein. Gene. 1993;133(2):255–60. doi: 10.1016/0378-1119(93)90648-m. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W. Proteolytic processing and cell biological functions of the amyloid precursor protein. J Cell Sci. 2000;113(Pt 11):1857–70. doi: 10.1242/jcs.113.11.1857. [DOI] [PubMed] [Google Scholar]
- Hoffmann MJ, Schulz WA. Causes and consequences of DNA hypomethylation in human cancer. Biochem Cell Biol. 2005;83(3):296–321. doi: 10.1139/o05-036. [DOI] [PubMed] [Google Scholar]
- Imagawa M, Chiu R, et al. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987;51(2):251–60. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Kang J, Lemaire HG, et al. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325(6106):733–6. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Ko SY, Lin SC, et al. Increased expression of amyloid precursor protein in oral squamous cell carcinoma. Int J Cancer. 2004;111(5):727–32. doi: 10.1002/ijc.20328. [DOI] [PubMed] [Google Scholar]
- Kogel D, Schomburg R, et al. The amyloid precursor protein protects PC12 cells against endoplasmic reticulum stress-induced apoptosis. J Neurochem. 2003;87(1):248–56. doi: 10.1046/j.1471-4159.2003.02000.x. [DOI] [PubMed] [Google Scholar]
- Kouznetsova E, Klingner M, et al. Developmental and amyloid plaque-related changes in cerebral cortical capillaries in transgenic Tg2576 Alzheimer mice. Int J Dev Neurosci. 2006;24(2-3):187–193. doi: 10.1016/j.ijdevneu.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Kummer C, Wehner S, et al. Expression and potential function of beta-amyloid precursor proteins during cutaneous wound repair. Exp Cell Res. 2002;280(2):222–32. doi: 10.1006/excr.2002.5631. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Ge YW. Role of the APP promoter in Alzheimer’s disease: cell type-specific expression of the beta-amyloid precursor protein. Ann N Y Acad Sci. 2004;1030:310–6. doi: 10.1196/annals.1329.039. [DOI] [PubMed] [Google Scholar]
- Leask A, Byrne C, et al. Transcription factor AP2 and its role in epidermal-specific gene expression. Proc Natl Acad Sci U S A. 1991;88(18):7948–52. doi: 10.1073/pnas.88.18.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y, Morgan K, et al. Amyloid precursor protein (APP) and the biology of proteolytic processing: relevance to Alzheimer’s disease. Int J Biochem Cell Biol. 2003;35(11):1505–35. doi: 10.1016/s1357-2725(03)00133-x. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Rogaev EI, et al. Protein-DNA interactions in the promoter region of the amyloid precursor protein (APP) gene in human neocortex. Brain Res Mol Brain Res. 1994;22(1-4):121–31. doi: 10.1016/0169-328x(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Takeda S, et al. Intrinsic signaling function of APP as a novel target of three V642 mutations linked to familial Alzheimer’s disease. Embo J. 1996;15(15):3769–77. [PMC free article] [PubMed] [Google Scholar]
- Oshiro MM, Watts GS, et al. Mutant p53 and aberrant cytosine methylation cooperate to silence gene expression. Oncogene. 2003;22(23):3624–34. doi: 10.1038/sj.onc.1206545. [DOI] [PubMed] [Google Scholar]
- Oyama N, Takahashi H, et al. Different properties of three isoforms (alpha, beta, and gamma) of transcription factor AP-2 in the expression of human keratinocyte genes. Arch Dermatol Res. 2002;294(6):273–80. doi: 10.1007/s00403-002-0327-x. [DOI] [PubMed] [Google Scholar]
- Popa C, Dahler AL, et al. AP-2 transcription factor family member expression, activity, and regulation in human epidermal keratinocytes in vitro. Differentiation. 2004;72(5):185–97. doi: 10.1111/j.1432-0436.2004.07205001.x. [DOI] [PubMed] [Google Scholar]
- Rovelet-Lecrux A, Hannequin D, et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet. 2006;38(1):24–6. doi: 10.1038/ng1718. [DOI] [PubMed] [Google Scholar]
- Schug J, Overton G. Technical Report CBIL-TR-1997-1001-v0.0. 1997 from URL: http://www.cbil.upenn.edu/tess.
- Tellez C, Bar-Eli M. Role and regulation of the thrombin receptor (PAR-1) in human melanoma. Oncogene. 2003;22(20):3130–7. doi: 10.1038/sj.onc.1206453. [DOI] [PubMed] [Google Scholar]
- Theuns J, Van Broeckhoven C. Transcriptional regulation of Alzheimer’s disease genes: implications for susceptibility. Hum Mol Genet. 2000;9(16):2383–94. doi: 10.1093/hmg/9.16.2383. [DOI] [PubMed] [Google Scholar]
- Williams T, Admon A, et al. Cloning and expression of AP-2, a cell-type-specific transcription factor that activates inducible enhancer elements. Genes Dev. 1988;2(12A):1557–69. doi: 10.1101/gad.2.12a.1557. [DOI] [PubMed] [Google Scholar]