Abstract
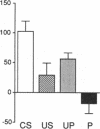
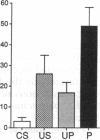
A semi-intact preparation was used to study the effects of classical conditioning on the type of siphon response elicited by a conditioned stimulus to the mantle of Aplysia. Five pairings of the conditioned stimulus with an unconditioned stimulus to nerves from the tail transformed the constricting alpha response of the siphon into a conditioned flaring response resembling the unconditioned response to stimulation of the tail nerves. Although some pseudoconditioning occurred, an associative component was indicated by the significantly greater incidence of flaring responses after paired training than after unpaired presentations of the conditioned and unconditioned stimulus or the unconditioned stimulus alone. Previously described cellular plasticity in the underlying neural circuits suggests a testable model based on cell-wide rather than synapse-specific mechanisms, which can account for specific conditioned responses. In this model, effective stimulus-response associations are produced by a concatenation of stimulus-specific facilitation of sensory neurons (a mechanism for alpha conditioning) and response-specific facilitation of motor neurons (a mechanism for pseudoconditioning).
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkon D. L. Calcium-mediated reduction of ionic currents: a biophysical memory trace. Science. 1984 Nov 30;226(4678):1037–1045. doi: 10.1126/science.6093258. [DOI] [PubMed] [Google Scholar]
- Billy A. J., Walters E. T. Long-term expansion and sensitization of mechanosensory receptive fields in Aplysia support an activity-dependent model of whole-cell sensory plasticity. J Neurosci. 1989 Apr;9(4):1254–1262. doi: 10.1523/JNEUROSCI.09-04-01254.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brons J. F., Woody C. D. Long-term changes in excitability of cortical neurons after Pavlovian conditioning and extinction. J Neurophysiol. 1980 Sep;44(3):605–615. doi: 10.1152/jn.1980.44.3.605. [DOI] [PubMed] [Google Scholar]
- Carew T. J., Hawkins R. D., Abrams T. W., Kandel E. R. A test of Hebb's postulate at identified synapses which mediate classical conditioning in Aplysia. J Neurosci. 1984 May;4(5):1217–1224. doi: 10.1523/JNEUROSCI.04-05-01217.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew T. J., Hawkins R. D., Kandel E. R. Differential classical conditioning of a defensive withdrawal reflex in Aplysia californica. Science. 1983 Jan 28;219(4583):397–400. doi: 10.1126/science.6681571. [DOI] [PubMed] [Google Scholar]
- Carew T. J., Kandel E. R. Inking in Aplysia californica. III. Two different synaptic conductance mechanisms for triggering central program for inking. J Neurophysiol. 1977 May;40(3):721–734. doi: 10.1152/jn.1977.40.3.721. [DOI] [PubMed] [Google Scholar]
- Carew T. J., Walters E. T., Kandel E. R. Classical conditioning in a simple withdrawal reflex in Aplysia californica. J Neurosci. 1981 Dec;1(12):1426–1437. doi: 10.1523/JNEUROSCI.01-12-01426.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. J., Gelperin A. Rapid taste-aversion learning by an isolated molluscan central nervous system. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6204–6206. doi: 10.1073/pnas.77.10.6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G. A., Kandel E. R. Branch-specific heterosynaptic facilitation in Aplysia siphon sensory cells. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2577–2581. doi: 10.1073/pnas.81.8.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T. J., Alkon D. L. Associative behavioral modification in hermissenda: cellular correlates. Science. 1980 Jul 18;209(4454):412–414. doi: 10.1126/science.209.4454.412. [DOI] [PubMed] [Google Scholar]
- Disterhoft J. F., Coulter D. A., Alkon D. L. Conditioning-specific membrane changes of rabbit hippocampal neurons measured in vitro. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2733–2737. doi: 10.1073/pnas.83.8.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson M. T., Walters E. T. Differential expression of pseudoconditioning and sensitization by siphon responses in Aplysia: novel response selection after training. J Neurosci. 1988 Aug;8(8):3000–3010. doi: 10.1523/JNEUROSCI.08-08-03000.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost W. N., Clark G. A., Kandel E. R. Parallel processing of short-term memory for sensitization in Aplysia. J Neurobiol. 1988 Jun;19(4):297–334. doi: 10.1002/neu.480190402. [DOI] [PubMed] [Google Scholar]
- Groves P. M., Thompson R. F. Habituation: a dual-process theory. Psychol Rev. 1970 Sep;77(5):419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Hawkins R. D., Abrams T. W., Carew T. J., Kandel E. R. A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science. 1983 Jan 28;219(4583):400–405. doi: 10.1126/science.6294833. [DOI] [PubMed] [Google Scholar]
- Hawkins R. D., Carew T. J., Kandel E. R. Effects of interstimulus interval and contingency on classical conditioning of the Aplysia siphon withdrawal reflex. J Neurosci. 1986 Jun;6(6):1695–1701. doi: 10.1523/JNEUROSCI.06-06-01695.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. D., Kandel E. R. Is there a cell-biological alphabet for simple forms of learning? Psychol Rev. 1984 Jul;91(3):375–391. [PubMed] [Google Scholar]
- Hawkins R. D., Lalevic N., Clark G. A., Kandel E. R. Classical conditioning of the Aplysia siphon-withdrawal reflex exhibits response specificity. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7620–7624. doi: 10.1073/pnas.86.19.7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacklet J. W., Rine J. Facilitation at neuromuscular junctions: contribution to habituation and dishabituation of the Aplysia gill withdrawal reflex. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1267–1271. doi: 10.1073/pnas.74.3.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso S. R., Ganong A. H., Brown T. H. Hebbian synapses in hippocampus. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5326–5330. doi: 10.1073/pnas.83.14.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Hochner B., Kandel E. R. Facilitatory transmitters and cAMP can modulate accommodation as well as transmitter release in Aplysia sensory neurons: Evidence for parallel processing in a single cell. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7994–7998. doi: 10.1073/pnas.83.20.7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederhendler I. I., Gart S., Alkon D. L. Classical conditioning of Hermissenda: origin of a new response. J Neurosci. 1986 May;6(5):1325–1331. doi: 10.1523/JNEUROSCI.06-05-01325.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowiak K. In vitro classical conditioning of a gill withdrawal reflex in Aplysia: neural correlates and possible neural mechanisms. J Neurobiol. 1986 Mar;17(2):83–101. doi: 10.1002/neu.480170204. [DOI] [PubMed] [Google Scholar]
- Mpitsos G. J., Davis W. J. Learning: classical and avoidance conditioning the mollusk Pleurobranchaea. Science. 1973 Apr 20;180(4083):317–320. doi: 10.1126/science.180.4083.317. [DOI] [PubMed] [Google Scholar]
- Sahley C. L., Ready D. F. Associative learning modifies two behaviors in the leech, Hirudo medicinalis. J Neurosci. 1988 Dec;8(12):4612–4620. doi: 10.1523/JNEUROSCI.08-12-04612.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritt S. H., Byrne J. H. Motor controls of opaline secretion in Aplysia californica. J Neurophysiol. 1980 Mar;43(3):581–594. doi: 10.1152/jn.1980.43.3.581. [DOI] [PubMed] [Google Scholar]
- Walters E. T., Byrne J. H. Associative conditioning of single sensory neurons suggests a cellular mechanism for learning. Science. 1983 Jan 28;219(4583):405–408. doi: 10.1126/science.6294834. [DOI] [PubMed] [Google Scholar]
- Walters E. T., Byrne J. H. Long-term enhancement produced by activity-dependent modulation of Aplysia sensory neurons. J Neurosci. 1985 Mar;5(3):662–672. doi: 10.1523/JNEUROSCI.05-03-00662.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters E. T., Erickson M. T. Directional control and the functional organization of defensive responses in Aplysia. J Comp Physiol A. 1986 Sep;159(3):339–351. doi: 10.1007/BF00603980. [DOI] [PubMed] [Google Scholar]
- Walters E. T. Multiple sensory neuronal correlates of site-specific sensitization in Aplysia. J Neurosci. 1987 Feb;7(2):408–417. doi: 10.1523/JNEUROSCI.07-02-00408.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters E. T. Site-specific sensitization of defensive reflexes in Aplysia: a simple model of long-term hyperalgesia. J Neurosci. 1987 Feb;7(2):400–407. doi: 10.1523/JNEUROSCI.07-02-00400.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]