Abstract
Rationale
Sympathetic stimulation of the heart increases the force of contraction and rate of ventricular relaxation by triggering PKA-dependent phosphorylation of proteins that regulate intracellular calcium. We hypothesized that scaffolding of cAMP signaling complexes by AKAP5 is required for efficient sympathetic stimulation of calcium transients.
Objective
We examined the function of AKAP5 in the β-adrenergic signaling cascade.
Methods and Results
We used calcium imaging and electrophysiology to examine the sympathetic response of cardiomyocytes isolated from wild type and AKAP5 mutant animals. The β-adrenergic regulation of calcium transients and the phosphorylation of substrates involved in calcium handling were disrupted in AKAP5 knockout cardiomyocytes. The scaffolding protein, AKAP5 (also called AKAP150/79), targets adenylyl cyclase, PKA, and calcineurin to a caveolin 3-associated complex in ventricular myocytes that also binds a unique sub-population of Cav1.2 L-type calcium channels. Only the caveolin 3-associated Cav1.2 channels are phosphorylated by PKA in response to sympathetic stimulation in wild type heart. However, in the AKAP5 knockout heart the organization of this signaling complex is disrupted, adenylyl cyclase 5/6 no longer associates with caveolin 3 in the T-tubules, and non-caveolin 3 associated calcium channels become phosphorylated after β-adrenergic stimulation although this does not lead to an enhanced calcium transient. The signaling domain created by AKAP5 is also essential for the PKA-dependent phosphorylation of ryanodine receptors and phospholambam.
Conclusions
These findings identify an AKAP5-organized signaling module that is associated with caveolin 3 and is essential for sympathetic stimulation of the calcium transient in adult heart cells.
Keywords: AKAP, Ca2+ channels, adenylyl cyclase, cAMP, PKA
INTRODUCTION
The function of the heart is to pump blood to the pulmonary and systemic circulation. To do this, atrial and ventricular myocytes contract in response to an action potential in a process known as excitation-contraction (EC) coupling. During EC coupling, brief openings of sarcolemmal voltage-gated L-type Ca2+ channels (LTCC) in the transverse tubules and surface sarcolemma create local elevations in [Ca2+]i that activate closely apposed ryanodine receptors (RyR) in the sarcoplasmic reticulum (SR) by the mechanism of Ca2+ -induced Ca2+ release (CICR) 1. This causes release of Ca2+ from the SR thereby inducing a global, cell-wide increase in [Ca2+]i that activates contractile proteins. LTCCs are rapidly inactivated by a Ca2+-dependent mechanism and SR Ca2+ release is also terminated, which allows the SR Ca2+ -ATPase (SERCA) to recover the released Ca2+ prior to the next heart beat. A major negative regulator of SERCA is phospholamban (PLN), which binds to and decreases the affinity of SERCA for Ca2+. Phosphorylation of PLN by Protein Kinase A (PKA) causes dissociation of PLN from SERCA, increasing the rate of Ca2+ reuptake into the SR 2. Unlike the action potential, the amplitude of the global [Ca2+]i transient, and hence the force of contraction during EC coupling, are not all-or-none. Instead, the force of cardiac contraction is graded by the amplitude of the global [Ca2+]i transient. This represents the mechanistic basis of a vital function of the heart: to match its pumping force to physiological needs.
Sympathetic stimulation of the heart through β-adrenergic receptors (β-AR) increases the force of contraction (inotropy) and accelerates the rate of relaxation (lusitropy) as part of the “fight or flight” response 3. Chronic activation of this pathway has been linked to an increased risk of heart failure and arrhythmias. In this pathway, activation of β-ARs results in the production of cAMP and the activation of PKA. Both β1-AR and β2-AR are expressed in heart and both are capable of Gαs-mediated activation of adenylyl cyclase. However, activation of the β2-AR appears to produce only a transient and highly localized burst of cAMP which may be limited by subsequent coupling to Gαi 4. The duration and spatial dispersion of cAMP is limited by PDE4D isoforms which have been reported to be recruited directly to β-ARs 5. The specificity of cAMP signaling is also thought to be modulated by the binding of PKA to A-kinase anchoring proteins (AKAPs), which target the kinase to specific intracellular locations and provide spatial and temporal control over cAMP signaling events. The importance of AKAPs has been demonstrated through use of peptides (e.g. Ht31) that compete for PKA binding and thereby disrupt interaction with all AKAPs 6. However, over 11 AKAPs have been identified in cardiomyocytes and elucidating the specific role of each AKAP remains a long-term objective7, 8. Several AKAPs have been identified that interact with Ca2+ regulating proteins in adult cardiomyocytes: for example, AKAP7α (AKAP15/18α) binds LTCCs 9, AKAP6 (mAKAP) binds RyR2 10, AKAP12 (gravin) associates with β-AR 11, and AKAP7δ (AKAP18δ) binds to SERCA-PLN 12. AKAP5 can interact with multiple proteins, including the LTCC (Cav1.2), β-AR, adenylyl cyclase, PKA, Protein Kinase C (PKC), and calcineurin (protein phosphatase 2B, PP2B) 13–15. In smooth muscle, AKAP5 interacts with PKC and LTCCs and is required for angiotensin II-induced hypertension 16. In neurons, AKAP5 localizes PP2B to the LTCC and this interaction is required for nuclear signaling 17. Although AKAP5 is expressed in heart cells, its function has not been demonstrated. To gain insight into the potential role of AKAP5 in cardiac signaling, we examined adult cardiomyocytes from mice with mutations in AKAP5 and discovered that AKAP5 is essential for sympathetic stimulation of the Ca2+ transient.
METHODS
The Akap5 −/− allele was generated by gene targeting using 129S1/SvImJ ES cells and standard techniques as previously described13. Mice were age-matched littermates of 3 to 6 months of age maintained on a C57Bl/6 background after >10 backcrosses to C57BL/6. Animals were handled in accordance with the guidelines of the University of Washington Institutional Animal Care and Use Committee. Adult cardiomyocytes were isolated and incubated as previously described18. Electrophysiology was performed using whole cell patch-clamp as described in online Supplemental Methods. Whole cell [Ca2+]i transients were measured using Fluo-4 loaded cardiomyocytes subjected to field stimulation at 1 Hz. Immunocytochemistry was performed using standard techniques as described in Supplementary Methods. Immunoprecipitation and western blotting were done using heart extracts homogenized in lysis buffer containing (in mmol/L): 10 Na2HPO4, 150 NaCl, 5 EGTA, 5 EDTA, 5 NaF, with 1% TritonX-100 and 0.5% Na Deoxycholate. The cAMP measurements were performed on isolated cardiomyocytes in laminin coated wells and the cAMP determined with direct cAMP EIA (Assay Design, Ann Arbor, MI, USA). Significant differences were determined by two-way ANOVA and post hoc Student’s unpaired t test, as appropriate. Pearson’s correlation coefficients19 were calculated using Imaris software (Bitplane AG, Zurich, Switzerland) on nine to 16 images per point.
RESULTS
Ca2+ transients in AKAP5 KO cardiomyocytes do not respond to β-AR stimulation
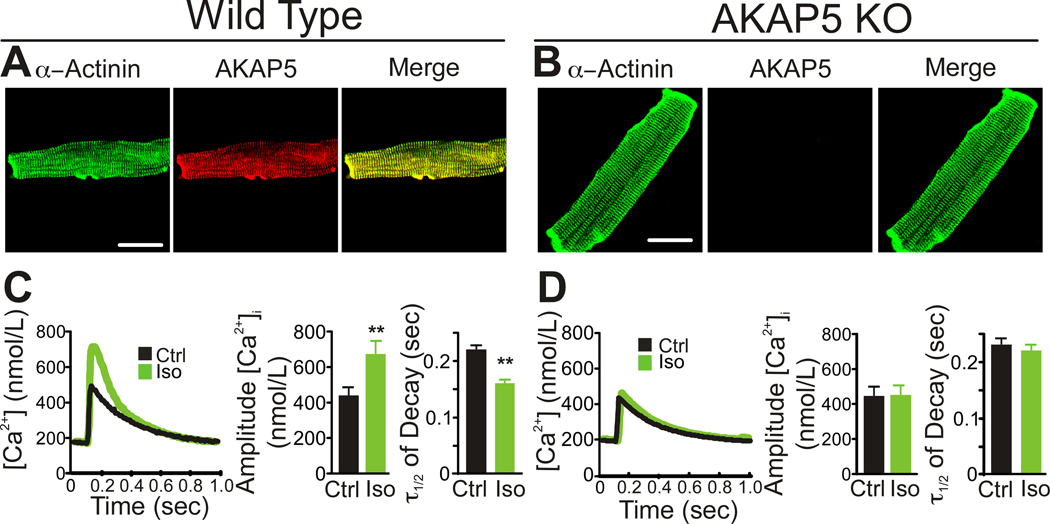
Immunocytochemistry revealed AKAP5 expression in adult ventricular cardiomyocytes predominantly localized near the T-tubules (Figure 1A) in wild type (WT) with no staining in AKAP5 knockout (KO) cardiomyocytes (Figure 1B). In order to measure calcium transients, adult cardiomyocytes were loaded with the calcium indicator dye, Fluo-4, and paced at 1 Hz in a perfusion chamber where the cells could be recorded by confocal microscopy. Treatment with 100 nmol/L isoproterenol (Iso) to activate the β-AR pathway increased the amplitude and reduced the decay time (τ1/2) of the [Ca2+]i transient in WT cells (Figure 1C). In contrast, β-AR activation did not increase the amplitude or the decay rate of the [Ca2+]i transient in AKAP5 KO cells (Figure 1D).
Figure 1. AKAP5 is required for β-adrenergic stimulation of [Ca2+]i transients.
A,B Staining of isolated mouse adult cardiomyocytes for α-actinin and AKAP5 in WT and KO. Scale bar = 15 µm. C–D, Isoproterenol, Iso, (100 nmol/L) stimulation of whole cell [Ca2+]i transients in cardiomyocytes isolated from WT and KO animals compared with no treatment (Ctrl). Cells were field stimulated at 1 Hz as described in Methods. Left panels are a representative [Ca2+]i trace, middle panels are bar graphs of the amplitude of the [Ca2+]i transient and right panels are the half life of [Ca2+]i transient decay. Calcium transients were measured with Fluo-4 and converted to calcium concentrations as described in Methods (mean ± s.e.m., n=9 animals, 3–4 cells analyzed per animal;**P<0.001).
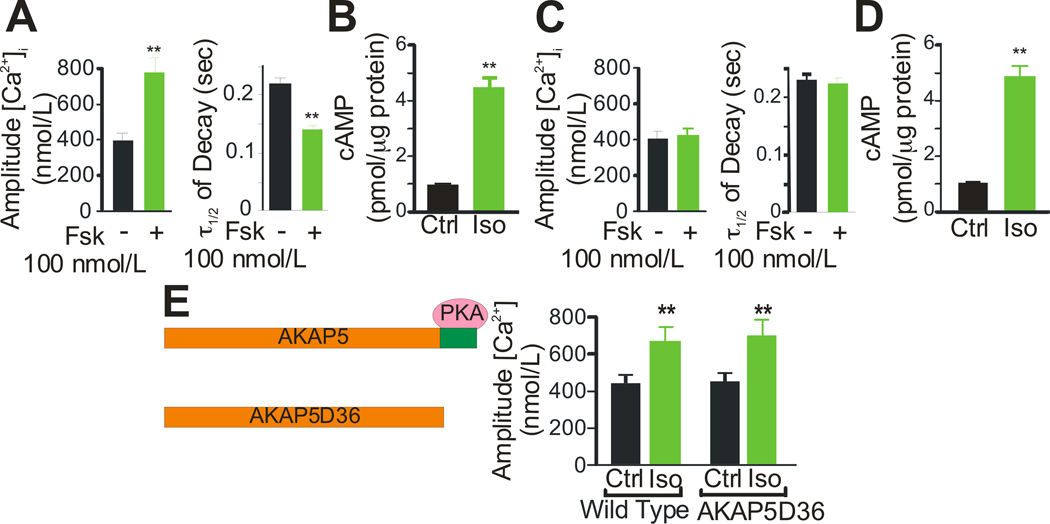
Because β-ARs signal through cAMP/PKA to phosphorylate Cav1.2, RyR2, and PLN 20, the lack of response in AKAP5 KO cardiomyocytes suggests that the cAMP/PKA pathway is disrupted. To determine where in the β-AR-signaling pathway AKAP5 is required, direct activation of AC was examined using forskolin (Fsk) to bypass the β-AR. Stimulation of AC with low concentrations of Fsk caused an increase in the amplitude and rate of decay of the [Ca2+]i transient in WT (Figure 2A) but not in KO cardiomyocytes (Figure 2C). To test whether cAMP was still being generated in KO cardiomyocytes we measured total cellular cAMP after stimulation with Iso and found equivalent increases in WT (Figure 2B) and KO cells (Figure 2D). We next tested the ability of higher concentrations of agonist or cAMP to overcome the deficits observed in AKAP5 KO myocytes. Stimulation of the β-AR with higher concentrations of Iso (100 umol/L) began to induce increased [Ca2+]i transients in KO cells but these higher doses were also toxic to the WT cells (Online Figure I). High doses of Fsk (10 µmol/L) could overcome the deficit in KO myocytes and no toxicity was observed in either WT or KO cells. High dose forskolin produced a 1.5 fold increase in intracellular cAMP compared with Iso as shown in Online Figure I. In contrast, when a cell permeable and phosphodiesterase (PDE)-resistant cAMP analog (8-CPT-cAMP) was used to elicit an increased [Ca2+]i transient, both KO and WT showed the same dose-response relationship and there was no difference in the onset of increased calcium transients after drug administration as shown in Online Figure I. These data demonstrate that AKAP5 is required for a sensitive response to either β-AR stimulation or cyclase activation and suggest that AKAP5 is helping to localize cAMP generation near PKA targets that modulate the [Ca2+]i transient. Exogenous cAMP analogs overcome the requirement for localized cAMP generation as expected.
Figure 2. Forskolin, cAMP, and the role of PKA binding to AKAP5.
A, C Forskolin, Fsk, (100 nmol/L) stimulation of [Ca2+]i transients in isolated cardiac myocytes from WT and KO animals (mean ± s.e.m., n=9 animals, 3–4 cells analyzed per animal;**P<0.001). B, D, Stimulation of cAMP production in isolated ventricular myocytes from WT and KO animals after a 90 sec treatment with 100 nmol/L Iso. E, AKAP5D36 lacks the last 36 amino acids and the binding site for PKA. Iso (100 nmol/L) stimulation of whole cell [Ca2+]i transients in both WT and D36 cardiac myocytes (mean ± s.e.m., n=4 animals;**P<0.001).
AKAP5 was first described as a PKA binding protein, but has now been reported to scaffold many additional signaling molecules, including Cav1.2, PP2B, PKC, β-ARs, and AC 6, 15. To directly test the requirement for PKA binding, we utilized another AKAP5 mutant mouse line, AKAP5 D36 21, that expresses a truncated AKAP5 that lacks the last 36 amino acids which contain the PKA binding site (Figure 2E) and one potential site of interaction with Cav1.217. This D36 mutant protein has been shown to express normally in the brain and continues to bind PP2B although PKA binding is completely eliminated22. In heart the D36 mutant protein continues to form a complex with both Cav1.2 and AC6 as does wild type AKAP5 (Online Figure IV B,C). Stimulation with Iso elicited a similar [Ca2+]i transient response in WT and D36 cardiomyocytes demonstrating that AKAP5-anchored PKA is not essential for β-AR stimulation of [Ca2+]i transients.
Cav1.2 responses to β-AR signaling
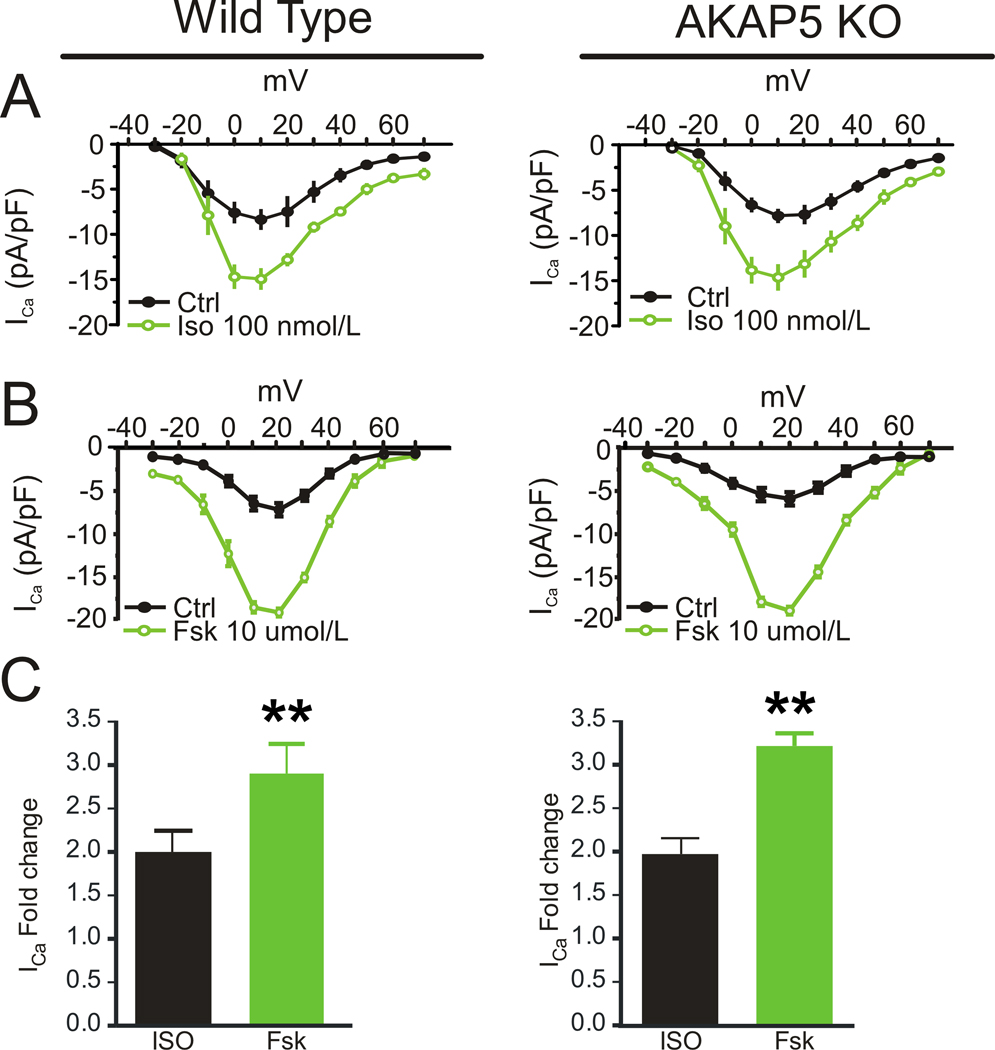
AKAP5 is localized near the T-tubules (Figure 1A) along with Cav1.2 LTCCs, which are juxtaposed within 15 nm of the RyR2 in the T-tubule/SR junctions of ventricular myocytes. Increased LTCC activity is a hallmark of β-AR activation in these cells. Thus, to determine the mechanisms by which loss of AKAP5 caused the elimination of β-AR-dependent modulation of EC coupling, we simultaneously recorded [Ca2+]i transients and LTCC currents in WT and KO cells. Surprisingly, β-AR stimulation with Iso (100 nmol/l) enhanced LTCC current in both WT and KO cells to a similar extent (Figure 3A). However, this increase in Ca2+ current did not trigger an increase in [Ca2+]i transients in the KO cells (Online Figure II) consistent with the results in Figure 1. When we analyze calcium transients in individual KO cells before and after Iso and calculate the ratio for each cell we can detect a 9% increase in peak calcium as depicted in Online Figure III A,B. The increase in calcium current in the KO appears to be offset in part by an Iso-stimulated increase in the activity of the Na+/Ca2+ exchanger (NCX) as shown in Online Figure III and this might explain why the KO cells do not become overloaded with Ca2+ in response to Iso.
Figure 3. Cav1.2 function and AKAP5.
A, Whole cell patch clamp recording of ICa as a function of voltage in WT (left panel) and KO (right panel) cardiomyocytes before and after stimulation by isoproterenol (100 nmol/L) (n=12 cells from 3 animals). B, Whole cell patch clamp recording of ICa as a function of voltage in WT (left panel) and KO (right panel) cardiomyocytes before and after stimulation with forskolin (Fsk, 10 umol/L) (n=10 cells from 3 animals). C, The fold change of the stimulated ICa measured at 10 mV for isoproterenol (Iso, 100 nmol/L) or forskolin (Fsk, 10 umol/L) (mean ± s.e.m., n=3 animals;**P<0.001)
When a high concentration of forskolin (10 umol/l) was used both WT and KO cells responded with current increases that were 1.5 fold higher than that seen with Iso (Figure 3B,C). Interestingly this treatment with high forskolin overcomes the defect in KO cells and produces an increased calcium transient of similar magnitude to WT. High forskolin also elevated cAMP about 1.5 fold greater than Iso treatment (Online Figure I).
Ca2+ sparks and phosphorylation of RyR2 and PLN
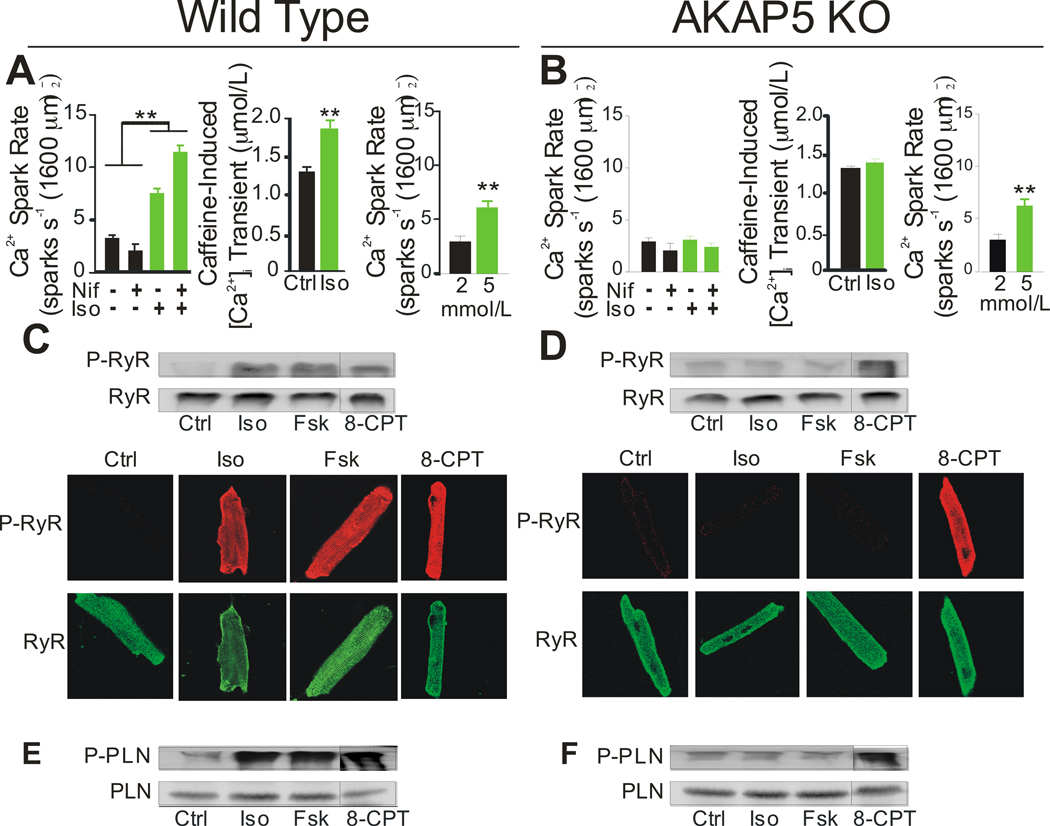
We investigated the effect of β-AR stimulation on calcium release from the SR directly by measuring Ca2+ sparks. Ca2+ sparks are spontaneous calcium release events caused by the opening of a cluster of RyR2 in the absence of LTCC opening 23. Iso treatment increased Ca2+ spark rate in WT but not in KO myocytes (Figure 4A, B, left panel). Blocking Cav1.2 with nifedipine had no significant effect on spark rate in either WT or KO indicating that the sparks were independent of spontaneous Cav1.2 openings. The amplitude of the Ca2+ sparks was also increased in WT but not KO cells after β-AR stimulation (Online Figure III D,E).
Figure 4. β-adrenergic stimulation of calcium sparks and phosphorylation of RyR2 and PLN.
A, B, Left panel: Iso (100 nmol/L) stimulation of [Ca2+]i sparks in WT and KO. Nifedipine (Nif, 25 nmol/L) was used to block L-type Ca2+ channels. Middle panel: SR [Ca2+] load as measured by caffeine-induced [Ca2+]i release under control and Iso (100 nmol/L) stimulation in WT and KO cells. Right panel: Cells perfused with different concentrations of external Ca2+ to measure increases in [Ca2+]i spark rate in WT and KO cells (4 animals, 8 cells/animal). C, D, Top panels: Western blots from isolated Wt and KO cardiomyocytes showing phosphorylated RyR2 at Ser2808 and total RyR2 after 3 min treatment with Iso (100 nmol/L), Fsk (100 nmol/L), or 8-CPT-cAMP (20 µM). Bottom panels: Immunocytochemistry of isolated cardiomyocytes to detect phosphorylated RyR2 at Ser2808 and total RyR2 after a 3 min treatment with Iso (100 nmol/L), Fsk (100 nmol/L), or 8-CPT-cAMP (20 µM). E, F, Western blots from isolated WT and KO cardiomyocytes to detect phosphorylated PLN at Ser16 and total PLN after a 3 min treatment with Iso (100 nmol/L), Fsk (100 nmol/L), or 8-CPT-cAMP (20 µM). Westerns and images are representative of 3 separate experiments. (**P<0.001)
β-AR stimulation increases spark frequency in isolated cardiomyocytes but the relative contribution of RyR2 phosphorylation and SR Ca2+ loading remains controversial 24, 25. Previous work has shown that an increase in the rate and amplitude of the Ca2+ sparks during β-AR activation is linked to increased SR Ca2+ load 18, 26. SR Ca2+ load was measured in WT and KO cells by caffeine-induced release before and after Iso stimulation. The SR Ca2+ load was similar under control conditions but β-AR activation increased SR Ca2+ load in WT but not KO cells (Figure 4A, B, middle panel). The failure of the KO cells to load more Ca2+ into the SR after β-AR stimulation could be due to a change in the regulation of SERCA in the KO cells. Perfusion of cells with high (5 mmol/L) external Ca2+ was used to increase SR load independently of the β-AR pathway and this resulted in increased Ca2+ spark rates in both WT (Figure 4A, right panel) and KO (Figure 4B, right panel) cells. We conclude that the defect in β-AR stimulated sparks is likely to be due to a loss of PKA-dependent regulation of calcium loading in the SR although RyR2 regulation may also contribute.
We next examined the PKA dependent phosphorylation of RyR2 and PLN under basal and β-AR-stimulated conditions. PKA phosphorylates Ser-2808 on the RyR2 10 and Ser-16 on PLN 27 in response to β-AR stimulation. In WT cells, there was a significant increase in the phosphorylation of Ser-2808 after 100 nmol/L Iso, 100 nmol/L Fsk or 20 uM 8-CPT-cAMP treatment as shown by western blot (Figure 4C, top panel) or immunocytochemistry (Figure 4C, bottom panel). However, in KO cells, Iso and Fsk treatment did not induce phosphorylation of Ser-2808 while 8-CPT-cAMP did (Figure 4D). PKA-dependent phosphorylation of PLN on Ser-16 attenuates PLN-dependent inhibition of the SERCA 2. Phosphorylation of PLN occurred in parallel with that observed for RyR2. Robust phosphorylation was observed after treatment with Iso, Fsk, or 8-CPT-cAMP in WT cells (Figure 4E) but the response to Iso and Fsk was lost in KO cells (Figure 4F). These observations raised an intriguing question: how could the phosphorylation and activation of RyR2 and PLN depend on AKAP5 when the activation of Cav1.2 did not?
AKAP5-dependent complexes in cardiomyocytes
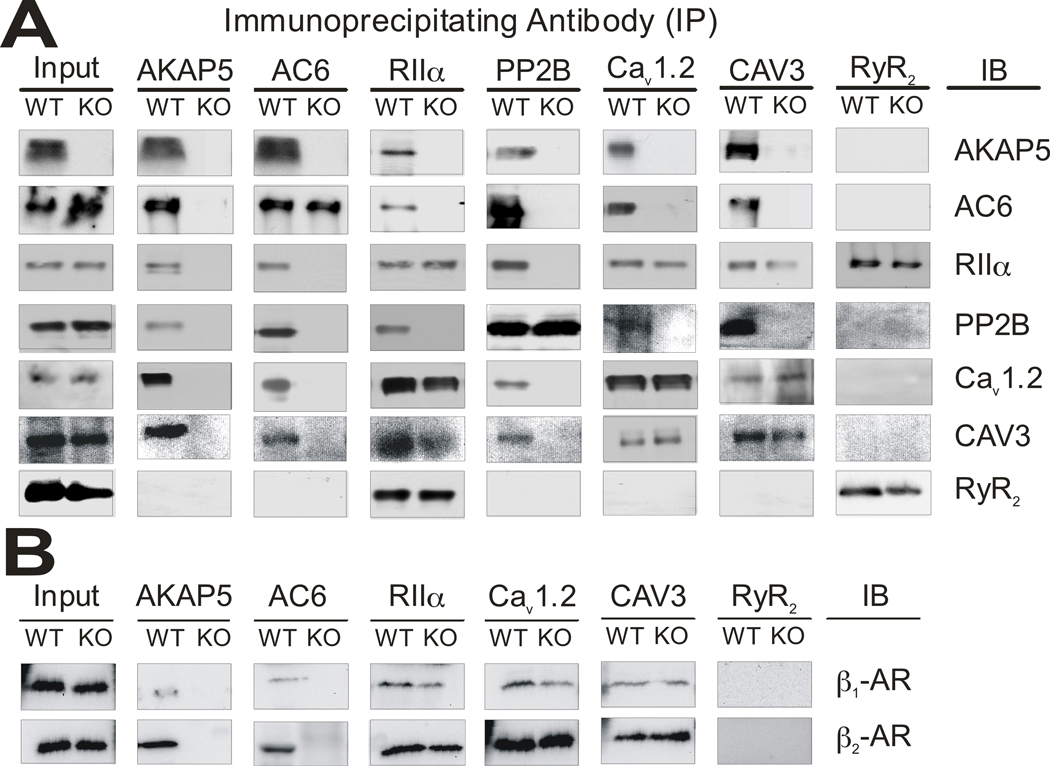
In order to address this question we first characterized the biochemical interactions between signaling molecules that are mediated by AKAP5. Cav1.2 is located in the sarcolemma and enriched in the T-tubules, while RyR2 and PLN are in the SR membrane 1. AKAP5 KO myocytes lack β-AR-dependent phosphorylation of RyR2 and PLN suggesting that AKAP5 is required for targeting cAMP signaling to the SR. We employed immunoprecipitation experiments with adult heart lysate to show that AKAP5 is in a complex that includes AC6, RIIα, PP2B, and Cav1.2 (Figure 5A). Since AC6 and Cav1.2 are present in the sarcolemma we interpret this result to indicate that AKAP5 is physically associated with the sarcolemma and T-tubule proteins but must be in close proximity with RyR and PLN in the SR. Both AC6 and AC5 are expressed in cardiac myocytes but the commercial antibody used to detect adenylyl cyclase in our immunoprecipitations was shown recently to react specifically with AC6 and not AC5 28; we have used an AC5-specific antibody to repeat these immunoprecipitations and find that both AC5 and AC6 associate with the AKAP5 complex (Online Figure IV A).
Figure 5. AKAP5 dependent signaling complexes.
Immunoprecipitations were done from heart extracts of WT and KO animals. Tissue was homogenized in buffer containing 1% TX-100 and 0.5% sodium deoxycholate and antibody-bound proteins were recovered using protein G magnetic beads as described in Supplemental Methods. A, Immunoprecipitations with antibodies against AKAP5, AC6, RIIα, PP2B, Cav1.2, CAV3, and RyR2 were run on western blots and probed with the antibodies listed under immunoblot (IB). B, Immunoprecipitations with antibodies against AKAP5, AC6, RIIα, Cav1.2, CAV3, and RyR2 were probed with antibodies for β1 and β2-AR.
The caveolin family of proteins form oligomers that can create 50 to 100 nm invaginations of the plasma membrane called caveolae that are enriched for cholesterol and sphingolipids 29. The major caveolin expressed in cardiomyocytes is CAV3 and its overall role in cardiac physiology has been studied previously by examining the cardiac phenotypes of CAV3 KO mice. The loss of CAV3 is associated with cardiomyopathy and cardiomyocyte hypertrophy as well as altered signaling leading to an increase in cAMP and an activation of the p42/44 MAPK cascade 30, 31. Localization of Cav1.2 to a caveolin 3 (CAV3) organized macromolecular signaling complex has been reported to be essential for regulation of the channels by β2-ARs 32, 33. We found that AKAP5 also associates with CAV3 in immunoprecipitation experiments (Figure 5A). β1 and β2-AR were both found associated with AKAP5 and CAV3 by immunoprecipitation and their association with CAV3 did not depend on AKAP5 since β-ARs remained in the complex in the AKAP5 KO (Figure 5B). It was previously reported that only the β2-AR is associated with CAV3 32 and consistent with this finding we see a greater fraction of the total cellular β2-AR in the CAV3 immunoprecipitation compared with β1-AR; however, a significant fraction of β1-AR was also found in the complex under our immunoprecipitation conditions (Figure 5B). The immunoprecipitation data suggest that the complex is linked by CAV3 and includes AKAP5, AC5/6, Cav1.2, PP2B, βAR, and PKA. Our immunoprecipitation data from AKAP5 KO heart shows that of the proteins examined only AC5/6 and PP2B appear to depend on AKAP5 for their association with CAV3. Previously it was reported that Cav1.2 interacts directly and with high affinity with PP2B 34 but at least under the conditions of our assay, AKAP5 is a major contributor to this association. Cav1.2, β-ARs, and PKA remain at similar levels in the complex with CAV3 in the absence of AKAP5. This does not exclude the possibility of additional protein-protein interactions between AKAP5 and either Cav1.2 or β-ARs as reported previously13, 35 but they do not seem to be essential for complex formation.
Functionally distinct populations of Cav1.2 in cardiomyocytes
The association of multiple signaling molecules bridged by CAV3 suggested the possibility that the Cav1.2 channels in the CAV3 complex with AKAP5 might constitute a unique population that responds preferentially to β-AR stimulation compared to Cav1.2 in other regions of the plasma membrane. In order to test this hypothesis we had to develop a more quantitative analysis of the Cav1.2 channels that associated with CAV3 and AKAP5 compared with those that did not. We used a CAV3 antibody to immunoprecipitate the complex because this antibody gave us quantitative precipitation of the CAV3 protein. Ventricular myocytes were exposed to either 100 nmol/l Iso or 10 umol/l forskolin for 90 sec and extracts were prepared and immuno-precipitated with antibody against CAV3. We then determined the amount of total Cav1.2 and P-Cav1.2 in the input homogenate, the CAV3 associated fraction, and the supernatant from the immunoprecipitation.
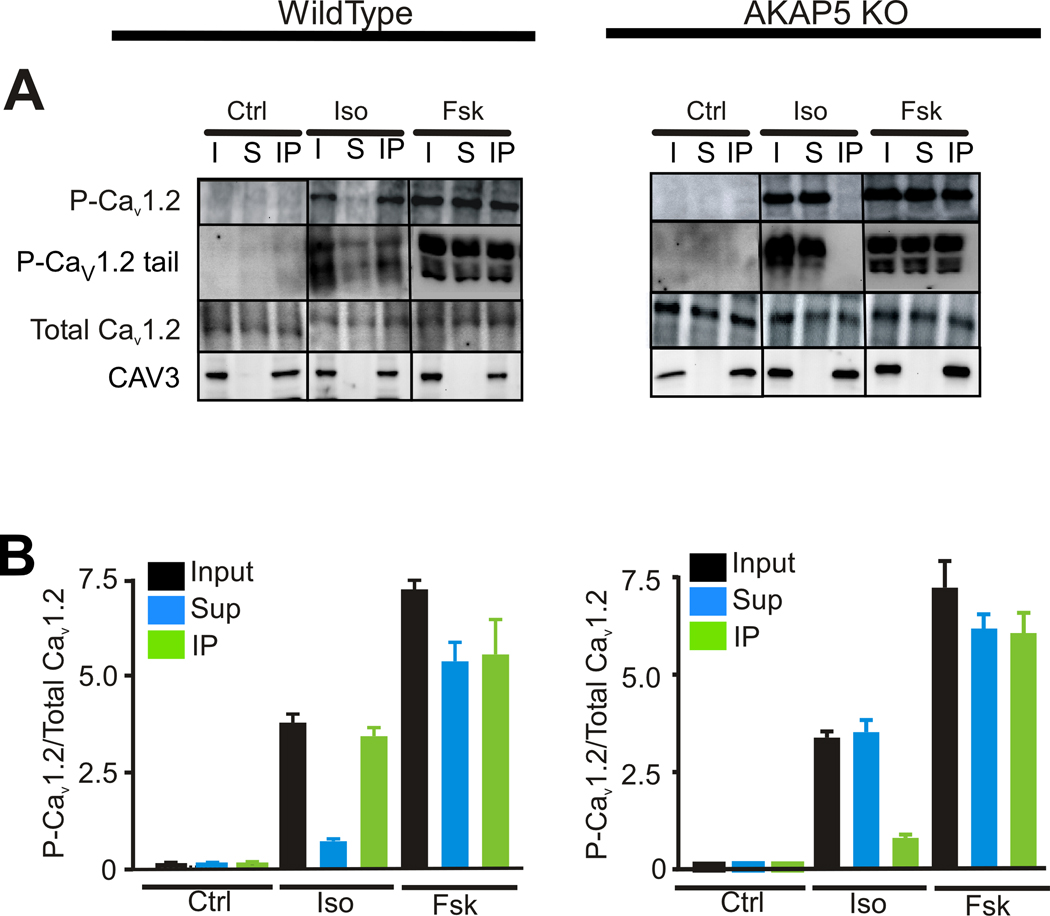
The pore forming α1 subunit of the LTCC, Cav1.2, is phosphorylated on Ser-1928 by PKA36. Phosphorylation at this site serves as an indicator that PKA has been activated in close proximity to the channel although recent evidence demonstrates that Ser-1928 is not essential for PKA stimulation of calcium current37, 38 suggesting that other sites on Cav1.2 or the β-subunits of the channel might be critical. Our resulted demonstrated that approximately 50% of the total Cav1.2 is associated with the CAV3 complex but surprisingly, only the CAV3-associated Cav1.2 was phosphorylated on Ser-1928 in response to Iso in WT mice (Figure 6A). Cav1.2 is partially cleaved in vivo leaving Ser-1928 in the distal C-terminal fragment but this fragment remains bound to the channel as a regulatory subunit39. The upper P-Cav1.2 band at ~240 kDa represents the uncleaved Cav1.2 and the lower doublet at 55–60 kDa is the phosphorylated C-terminal tail released after in vivo processing. The C-terminal tail migrated as several bands which may be due to differences in cleavage and processing or possibly additional phosphorylation sites that alter migration. When high forskolin was used we observed phosphorylation of Ser-1928 on Cav1.2 in both the IP and supernatant (S) fractions and an overall 2-fold increase in phosphorylation of Cav1.2 compared with Iso. In contrast, analysis of the KO revealed that Iso was targeting the non-CAV3 associated Cav1.2 for phosphorylation with much less phosphorylation of the CAV3-associated channels. High forskolin leads to phosphorylation of both the CAV3 associated and non-CAV3 associated fractions in both WT and KO cells. A quantitative analysis of these results averaged over 3 experiments is shown in Figure 6B.
Figure 6. Phosphorylation of Cav1.2 in cardiomyocytes after isoproterenol or high forskolin.
A, Isolated WT and KO cardiomyocytes were treated with isoproterenol (Iso, 100 nmol/L), or forskolin (Fsk, 10 umol/L) for 90 sec. Extracts were made and immunoprecipitated with an antibody against CAV3 as in Figure 5. An equal fraction of input (In), supernatant from the CAV3 immunoprecipitation (Sup) and the immunoprecipitated fraction (IP) were loaded for western blotting and probed with antibodies against Cav1.2 and P-Cav1.2 (Ser1928) and CAV3. Intact P-Cav1.2 (~240 KDa) and the P-Cav1.2 tail (~60 KDa) are shown. Total Cav1.2 at ~240 kDa and CAV3 at 18 KDa are shown. B, The P-Cav1.2/Cav1.2 ratio based on densitometry and averaged from 3 separate experiments is shown for WT cells (left panel) and KO cells (right panel) (mean ± s.e.m, N=3).
This surprising result distinguishes two populations of Cav1.2 channels in WT myocytes: a CAV3 and AKAP5 associated population that is highly sensitive to Iso and forskolin and a non-CAV3 associated population of channels that are only phosphorylated when cAMP levels are much higher after 10 umol/l forskolin treatment or after treatment with cell-permeable cAMP analogs. In order to determine whether this preferential phosphorylation of CAV3-associated Cav1.2 took place under in vivo conditions we treated WT and KO mice with either isoproterenol or the β-AR antagonist, propranolol for 5 min and then immediately isolated the ventricular portion of the heart for immunoprecipitation experiments similar to those shown in Figure 6. The results shown in Online Figure V confirm that the same differential phosphorylation of channels is occurring in vivo.
We have used specific β-AR antagonists together with Iso to examine whether Cav1.2 phosphorylation is dependent on β1 or β2-AR using cultured myocytes. In WT myocytes, Cav1.2 phosphorylation was inhibited by 44% using the β1-AR selective antagonist, and by 20% with the β2-AR specific antagonist. Both antagonists together blocked 95% of the Iso dependent increase in Cav1.2 phosphorylation suggesting that there may be some compensation when only one receptor is blocked (Online Figure VI). This is in good agreement with our previous results using immunocytochemistry to quantitate Cav1.2 phosphorylation in rat ventricular myocytes 36. In the AKAP5 KO myocytes, Cav1.2 phosphorylation was predominantly β1-AR specific in AKAP5 KO myocytes (Online Figure VI). The Iso dependent increase in LTCC current was completely lost in mice with a null mutation in the Adrb1 (β1-AR) gene as was the Iso-dependent stimulation of the calcium transient (Online Figure VI). We conclude that AKAP5 is required to facilitate phosphorylation of a sub-population of Cav1.2 channels that are associated with CAV3 and that this channel population is primarily responding through stimulation of the β1-AR. The role of β2-ARs in the regulation of calcium channel physiology remains unclear.
Subcellular localization of AKAP5 and CAV3 binding partners
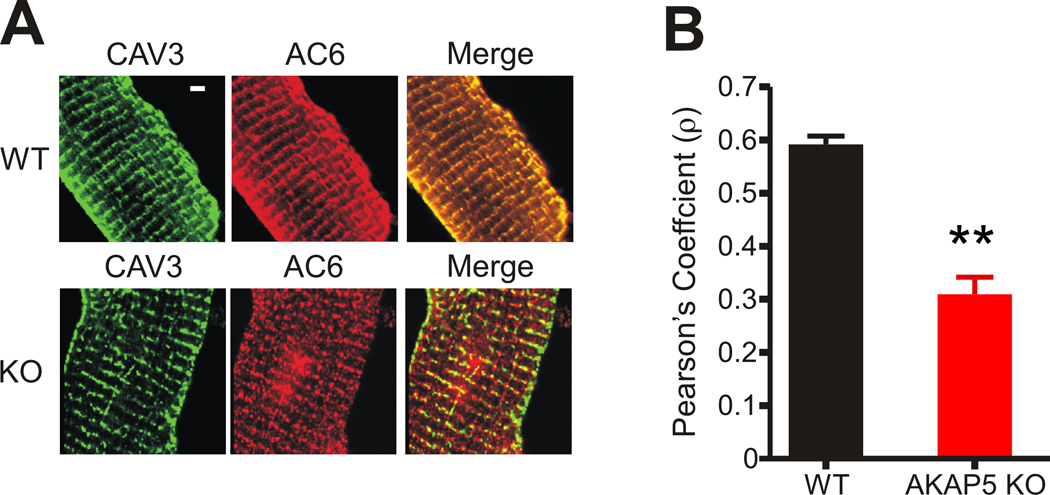
We next examined whether we could visualize the co-localization of AC6 with CAV3 by immunocytochemistry and whether co-localization would be disrupted in the AKAP5 KO as predicted from the immunoprecipitation experiments in Figure 5. In WT cells AC6 staining was localized to T-tubules with CAV3; however, in KO cells, AC6 showed a significantly reduced co-localization with CAV3 (Figure 7). We utilized the Pearson product-moment correlation coefficient as a quantitative measure of the co-localization of CAV3 and AC6 in cardiomyocytes from WT and KO heart and found a significant decrease in the association of AC6 with CAV3 (Figure 7B) as predicted from the immunoprecipitation experiments. The antibody specific for AC5 did not give a signal by immunocytochemistry but the immunoprecipitation data indicates that, like AC6, it is also released from the CAV3-associated signaling complex in the AKAP5 KO (Online Figure IV A). The association of PP2B with CAV3 also depends on AKAP5 based on the immunoprecipitation experiments but we could distinguish no changes in the localization of PP2B comparing WT and KO cardiomyocytes (Online Figure VII). This result might be expected if only a small fraction of the total PP2B in the cardiomyocyte is associated with AKAP5.
Figure 7. Immuno-localization of CAV3 and AC6 in WT and KO cardiomyocytes.
A, Immunocytochemistry of CAV3 (left panel) and AC6 (center panel) with the merged image (right panel) in WT and KO ventricular cardiomyocytes. Bar = 5 um.
B, Pearson's product-moment coefficient as a measure of co-localization of CAV3 and AC6. WT, n=30. KO, N=29 cells (mean ± s.e.m, ** = P<0.001).
DISCUSSION
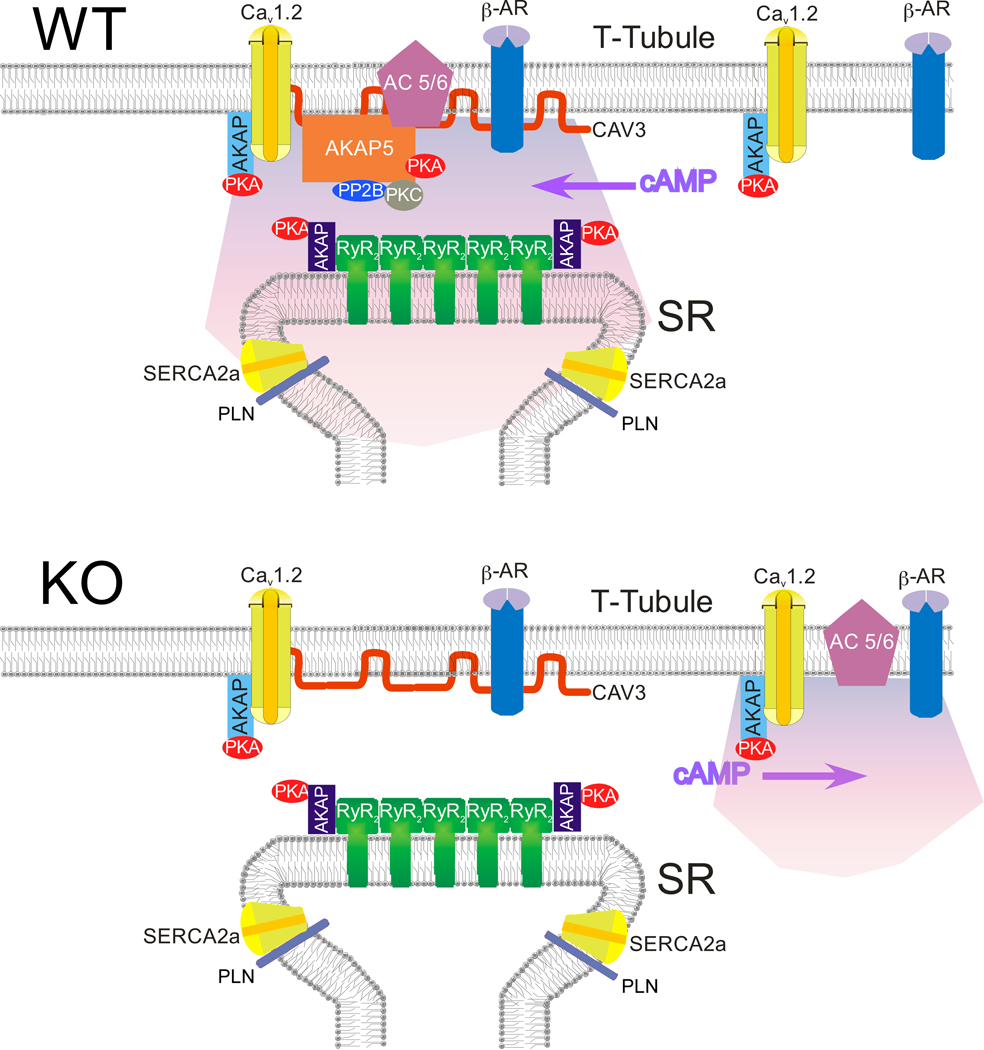
Our results show that AKAP5 plays an essential role in the sympathetic regulation of both the amplitude and rate of decay of calcium transients in cardiac cells. Co-immunoprecipitation experiments reveal a signaling complex containing AKAP5, AC5/6, PKA, PP2B, Cav1.2, and β-ARs that are tightly associated with CAV3 and we postulate that this complex generates a micro-domain of cAMP that is juxtaposed to the RyR2 containing junctional regions of the SR as depicted in the WT panel of Figure 8. When AKAP5 is missing in the KO, AC5 and AC6 are released from the CAV3 complex as judged by both co-immunoprecipitation and immunocytochemistry and the response of the calcium transient to β-AR stimulation is lost. More than 90% of the AKAP5 in cardiomyocytes is complexed with CAV3 based on our immunoprecipitation experiments; this leads us to suggest that the CAV3 complex is also responsible for generating the micro-domain of cAMP that is required to stimulate PKA-dependent phosphorylation of both the RyR2 and PLN.
Figure 8. Model of the hypothetical signaling complex at the T-Tubule/SR junction that responds to sympathetic stimulation.
We were surprised to find that AKAP5 KO cardiomyocytes had lost the ability to modulate calcium transients in response to Iso but still retained Iso-dependent increases in whole cell LTCC current (Figure 3). One explanation for this paradox is that the Cav1.2 channels that are conducting increased Ca2+ current in KO cells are not the same population of channels that we identified in the CAV3/AKAP5 complex. In support of this possibility, we identified two populations of Cav1.2 channels in the cardiomyocyte that can be distinguished biochemically and functionally. The Cav1.2 population associated with CAV3 and AKAP5 is phosphorylated by PKA in WT cells in response to β-AR stimulation and this event appears to be coupled to the β-AR-induced Ca2+ loading of the SR and an increased calcium transient. A second Cav1.2 population is depicted in the WT panel of Figure 8 that is not in the CAV3 complex and does not respond to β-AR stimulation in WT cells. However, in the KO myocytes this second non-CAV3 associated population is phosphorylated by β-AR stimulation. We believe this is because AC6 has been released from AKAP5 in the KO and that it now migrates closer to the non-CAV3 associated Cav1.2 channels as depicted in the KO panel of Figure 8. Co-localization experiments using immunocytochemistry confirmed this change in AC6 association with CAV3 as shown in Figure 7. It appears to be the non-CAV3 associated Cav1.2 channels that are responsible for the increased LTCC current when KO myocytes are treated with Iso. It is not clear whether these channels are in junctional complexes with the SR but since there is no concomitant phosphorylation of PLN to cause an increased calcium loading of the SR in the KO after Iso we hypothesize that the increased LTCC current alone is not sufficient to produce an enhanced calcium transient that is comparable to WT cells.
These findings compel modification of the generally accepted model for β-AR-mediated modulation of cardiac contraction. Our suggestion that the CAV3-associated Cav1.2 channels are functionally linked to β-AR regulation of SR calcium release conflicts with the observation that caveolae are typically visualized at the surface membrane of cardiac cells and not within the T-tubule network. However, mounting evidence exists for a substantial fraction of CAV3 that is not associated with the structurally distinct caveolae in adult cardiomyocytes and it has been suggested that CAV3 may form functional scaffolding domains that are independent of caveolae 40. The presence of CAV3 within the T-tubule structures where it co-localizes with RyR2 and a fraction of LTCCs 41 supports this idea and is consistent with our results. Co-immunoprecipitation experiments have also shown an interaction between CAV3 and both the RyR2 42 and junctophilin-2, a membrane spanning protein that contributes to the junctional membrane complex between the T-tubule membrane and the SR in heart 43. In our immunoprecipitations we did not see any interactions between RyR2 and either CAV3 or AKAP5 but if this complex is dependent on junctophilin or other intermediary proteins it may be very sensitive to detergent and homogenization conditions.
Our studies provide new insights into the compartmentalization of β-AR signaling complexes and their linkage to functionally distinct calcium channel populations in the heart. Many questions are raised by our results. Are the Cav1.2 channels associated with CAV3 in the T-tubules unique biochemically? A recent report suggests that the localization of Cav1.2 to CAV3 depends on association with specific channel β-subunits 44. Are the Cav1.2 channels that are not linked to CAV3 regulated by other hormonal signals that stimulate cAMP in the heart such as glucagon, GLP-1, adenosine, or PGE1? Cardiovascular stress results in chronic over-stimulation of the β-AR pathway, cardiac hypertrophy, and ultimately cardiac failure. Do changes or disruption of the AKAP5-signaling complex contribute to the pathophysiology of heart failure? Analysis of the in vivo physiological phenotypes of AKAP5 KO mice are complicated by the expression and functional role of AKAP5 in vascular smooth muscle where it regulates PKC dependent effects on vascular tone and hence, blood pressure16. AKAP5 is also expressed in neurons where it regulates a multitude of events underlying synaptic plasticity and behavior 13, 22, 45. Direct or indirect effects of the AKAP5 KO on the autonomic nervous system have not been examined. Analysis of the in vivo physiological phenotypes associated uniquely with the loss of AKAP5 function in the cardiomyocyte will require a cell type specific KO.
NOVELTY AND SIGNIFICANCE
What Is Known?
β-adrenergic stimulation of heart cells produces a highly compartmentalized increase in cAMP and PKA activity that culminates in increased contractility.
Scaffolding proteins like A Kinase Anchoring Proteins (AKAPs) are implicated in controlling this process and contribute to specificity and sub-cellular localization of responses.
PKA-dependent phosphorylation of both the L-type Ca2+ channel (LTCC) and phospholamban (PLN) are believed to regulate the amplitude and kinetics of the calcium transient evoked by an action potential during excitation-contraction coupling.
What New Information Does This Article Contribute?
AKAP5 (also known as AKAP150 in rodents and AKAP79 in humans) is essential for ventricular myocytes to increase the amplitude and decay rate of the cytosolic Ca2+ transient in response to β-adrenergic stimulation, yet the whole-cell LTCC current remains intact in AKAP5 knockout mycoytes.
The role of AKAP5 is to recruit adenylyl cyclase to a membrane-associated complex of signaling molecules which directs the PKA phosphorylation of PLN, RyR, and a sub-population of LTCCs.
The increase in LTCC current in AKAP5 knockout myocytes is due to phosphorylation of a different subpopulation of LTCCs than in wild-type myocytes.
Ventricular myocytes compartmentalize LTCCs such that only about 50% are responsive to physiological levels of β-adrenergic stimulation.
This study was designed to determine the role of AKAP5 in ventricular myocytes and resulted in the unexpected finding that AKAP5 is essential for the compartmentalized production of cAMP in the mouse heart. Our studies relied on targeted mutation of the AKAP5 gene in mice. Other AKAPs have been studied in the heart, including AKAP7 (AKAP15/18) which is thought to interact with LTCCs and PLN, AKAP9 (Yotiao) which interacts with and regulates a cardiac K+ channel, and AKAP6 (mAKAP) which interacts with the RyR. which interacts with the coordinate the β-adrenergic response pathway prior to this study. We also discovered that only a sub-population (about 50%) of the ventricular LTCCs are associated with AKAP5 and only this sub-population is efficiently phosphorylated by PKA when cells are treated with β-adrenergic receptor agonists. Acute β-adrenergic stimulation of the heart is beneficial and increases cardiac output; however, chronic over-stimulation is associated with heart failure and pathological changes in cardiac tissue. Our results implicate AKAP5 in normal cardiac signalling, suggesting a new target to investigate in the context of signaling changes that occur during heart failure.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs. P.S. Amieux and B.W. Jones for critical reading of the manuscript as well as members of the McKnight and Santana laboratories for helpful discussions. We thank G. Martin from the UW Keck center for assistance with confocal microscopy and J. Cabarrus for training in the isolation of cardiomyocytes. Antibody against phospho-RyR was provided by A.R. Marks (Columbia University), and antibody against adenylyl cyclase 5 was provided by S.F. Vatner (New Jersey Medical School).
SOURCES OF FUNDING:
C.B.N. is currently supported by HHMI/ UW Molecular Medicine Scholar Award. This work is supported by a NIH grants to GSM (GM032875), LFS (HL085686), and WAC (HL085372).
Non-standard Abbreviations and Acronyms
- LTCC
L-type Ca2+ channel
- RyR
ryanodine receptors
- SR
sarcoplasmic reticulum
- CICR
Ca2+ -induced Ca2+ release
- SERCA
sarcoplasmic reticulum Ca2+ -ATPase
- PLN
phospholamban
- β-AR
β-adrenergic receptors
- AKAP
A-kinase anchoring protein
- PKC
protein kinase C
- PP2B
calcineurin, protein phosphatase 2B
- WT
wild type
- KO
knockout
- Iso
isoproterenol
- AC
adenylyl cyclase
- Fsk
forskolin
- PDE
phosphodiesterase
- CAV3
caveolin 3
- EC
excitation-contraction
- NCX
Na+/Ca2+ exchanger
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES: none
REFERENCES
- 1.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 2.Chu G, Lester JW, Young KB, Luo W, Zhai J, Kranias EG. A single site (ser16) phosphorylation in phospholamban is sufficient in mediating its maximal cardiac responses to beta -agonists. J Biol Chem. 2000;275:38938–38943. doi: 10.1074/jbc.M004079200. [DOI] [PubMed] [Google Scholar]
- 3.Xiang Y, Kobilka BK. Myocyte adrenoceptor signaling pathways. Science. 2003;300:1530–1532. doi: 10.1126/science.1079206. [DOI] [PubMed] [Google Scholar]
- 4.Soto D, De Arcangelis V, Zhang J, Xiang Y. Dynamic protein kinase a activities induced by beta-adrenoceptors dictate signaling propagation for substrate phosphorylation and myocyte contraction. Circ Res. 2009;104:770–779. doi: 10.1161/CIRCRESAHA.108.187880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Arcangelis V, Liu R, Soto D, Xiang Y. Differential association of phosphodiesterase 4d isoforms with beta2-adrenoceptor in cardiac myocytes. J Biol Chem. 2009;284:33824–33832. doi: 10.1074/jbc.M109.020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McConnachie G, Langeberg LK, Scott JD. Akap signaling complexes: Getting to the heart of the matter. Trends Mol Med. 2006;12:317–323. doi: 10.1016/j.molmed.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Carnegie GK, Means CK, Scott JD. A-kinase anchoring proteins: From protein complexes to physiology and disease. IUBMB Life. 2009;61:394–406. doi: 10.1002/iub.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scholten A, van Veen TA, Vos MA, Heck AJ. Diversity of camp-dependent protein kinase isoforms and their anchoring proteins in mouse ventricular tissue. J Proteome Res. 2007;6:1705–1717. doi: 10.1021/pr060601a. [DOI] [PubMed] [Google Scholar]
- 9.Gray PC, Tibbs VC, Catterall WA, Murphy BJ. Identification of a 15-kda camp-dependent protein kinase-anchoring protein associated with skeletal muscle l-type calcium channels. J Biol Chem. 1997;272:6297–6302. doi: 10.1074/jbc.272.10.6297. [DOI] [PubMed] [Google Scholar]
- 10.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. Pka phosphorylation dissociates fkbp12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 11.Fan G, Shumay E, Wang H, Malbon CC. The scaffold protein gravin (camp-dependent protein kinase-anchoring protein 250) binds the beta 2-adrenergic receptor via the receptor cytoplasmic arg-329 to leu-413 domain and provides a mobile scaffold during desensitization. J Biol Chem. 2001;276:24005–24014. doi: 10.1074/jbc.M011199200. [DOI] [PubMed] [Google Scholar]
- 12.Lygren B, Carlson CR, Santamaria K, Lissandron V, McSorley T, Litzenberg J, Lorenz D, Wiesner B, Rosenthal W, Zaccolo M, Tasken K, Klussmann E. Akap complex regulates ca(2+) re-uptake into heart sarcoplasmic reticulum. EMBO Rep. 2007;8:1061–1067. doi: 10.1038/sj.embor.7401081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall DD, Davare MA, Shi M, Allen ML, Weisenhaus M, McKnight GS, Hell JW. Critical role of camp-dependent protein kinase anchoring to the l-type calcium channel cav1.2 via a-kinase anchor protein 150 in neurons. Biochemistry. 2007;46:1635–1646. doi: 10.1021/bi062217x. [DOI] [PubMed] [Google Scholar]
- 14.Wang HY, Tao J, Shumay E, Malbon CC. G-protein-coupled receptor-associated a-kinase anchoring proteins: Akap79 and akap250 (gravin) Eur J Cell Biol. 2006;85:643–650. doi: 10.1016/j.ejcb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Bauman AL, Soughayer J, Nguyen BT, Willoughby D, Carnegie GK, Wong W, Hoshi N, Langeberg LK, Cooper DM, Dessauer CW, Scott JD. Dynamic regulation of camp synthesis through anchored pka-adenylyl cyclase v/vi complexes. Mol Cell. 2006;23:925–931. doi: 10.1016/j.molcel.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navedo MF, Nieves-Cintron M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS, Santana LF. Akap150 is required for stuttering persistent ca2+ sparklets and angiotensin ii-induced hypertension. Circ Res. 2008;102:e1–e11. doi: 10.1161/CIRCRESAHA.107.167809. [DOI] [PubMed] [Google Scholar]
- 17.Oliveria SF, Dell'Acqua ML, Sather WA. Akap79/150 anchoring of calcineurin controls neuronal l-type ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santana LF, Kranias EG, Lederer WJ. Calcium sparks and excitation-contraction coupling in phospholamban-deficient mouse ventricular myocytes. J Physiol. 1997;503(Pt 1):21–29. doi: 10.1111/j.1469-7793.1997.021bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 20.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Allen M, Halt AR, Weisenhaus M, Dallapiazza RF, Hall DD, Usachev YM, McKnight GS, Hell JW. Age-dependent requirement of akap150-anchored pka and glur2-lacking ampa receptors in ltp. Embo J. 2007;26:4879–4890. doi: 10.1038/sj.emboj.7601884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisenhaus M, Allen ML, Yang L, Lu Y, Nichols CB, Su T, Hell JW, McKnight GS. Mutations in akap5 disrupt dendritic signaling complexes and lead to electrophysiological and behavioral phenotypes in mice. PLoS ONE. 2010 doi: 10.1371/journal.pone.0010325. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: Elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 24.Bers DM. Cardiac ryanodine receptor phosphorylation: Target sites and functional consequences. Biochem J. 2006;396:e1–e3. doi: 10.1042/BJ20060377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehnart S, Marks AR. Regulation of ryanodine receptors in the heart. Circ Res. 2007;101:746–749. doi: 10.1161/CIRCRESAHA.107.162479. [DOI] [PubMed] [Google Scholar]
- 26.Santana LF, Gomez AM, Kranias EG, Lederer WJ. Amount of calcium in the sarcoplasmic reticulum: Influence on excitation-contraction coupling in heart muscle. Heart Vessels. 1997;(Suppl 12):44–49. [PubMed] [Google Scholar]
- 27.MacLennan DH, Kranias EG. Phospholamban: A crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 28.Hu CL, Chandra R, Ge H, Pain J, Yan L, Babu GJ, Depre C, Iwatsubo K, Ishikawa Y, Sadoshima J, Vatner SF, Vatner DE. Adenylyl cyclase type 5 protein expression during cardiac development and stress. Am J Physiol Heart Circ Physiol. 2009 doi: 10.1152/ajpheart.00050.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–391. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodman SE, Park DS, Cohen AW, Cheung MW, Chandra M, Shirani J, Tang B, Jelicks LA, Kitsis RN, Christ GJ, Factor SM, Tanowitz HB, Lisanti MP. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 mapk cascade. J Biol Chem. 2002;277:38988–38997. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- 31.Augustus AS, Buchanan J, Addya S, Rengo G, Pestell RG, Fortina P, Koch WJ, Bensadoun A, Abel ED, Lisanti MP. Substrate uptake and metabolism are preserved in hypertrophic caveolin-3 knockout hearts. Am J Physiol Heart Circ Physiol. 2008;295:H657–H666. doi: 10.1152/ajpheart.00387.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac l-type ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proc Natl Acad Sci U S A. 2006;103:7500–7505. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calaghan S, Kozera L, White E. Compartmentalisation of camp-dependent signalling by caveolae in the adult cardiac myocyte. J Mol Cell Cardiol. 2008;45:88–92. doi: 10.1016/j.yjmcc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Tandan S, Wang Y, Wang TT, Jiang N, Hall DD, Hell JW, Luo X, Rothermel BA, Hill JA. Physical and functional interaction between calcineurin and the cardiac l-type Ca2+ channel. Circ Res. 2009;105:51–60. doi: 10.1161/CIRCRESAHA.109.199828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fraser ID, Cong M, Kim J, Rollins EN, Daaka Y, Lefkowitz RJ, Scott JD. Assembly of an a kinase-anchoring protein-beta(2)-adrenergic receptor complex facilitates receptor phosphorylation and signaling. Curr Biol. 2000;10:409–412. doi: 10.1016/s0960-9822(00)00419-x. [DOI] [PubMed] [Google Scholar]
- 36.Hulme JT, Westenbroek RE, Scheuer T, Catterall WA. Phosphorylation of serine 1928 in the distal c-terminal domain of cardiac cav1.2 channels during beta1-adrenergic regulation. Proc Natl Acad Sci U S A. 2006;103:16574–16579. doi: 10.1073/pnas.0607294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganesan AN, Maack C, Johns DC, Sidor A, O'Rourke B. Beta-adrenergic stimulation of l-type ca2+ channels in cardiac myocytes requires the distal carboxyl terminus of alpha1c but not serine 1928. Circ Res. 2006;98:e11–e18. doi: 10.1161/01.RES.0000202692.23001.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lemke T, Welling A, Christel CJ, Blaich A, Bernhard D, Lenhardt P, Hofmann F, Moosmang S. Unchanged beta-adrenergic stimulation of cardiac l-type calcium channels in ca v 1.2 phosphorylation site s1928a mutant mice. J Biol Chem. 2008;283:34738–34744. doi: 10.1074/jbc.M804981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hulme JT, Yarov-Yarovoy V, Lin TW, Scheuer T, Catterall WA. Autoinhibitory control of the cav1.2 channel by its proteolytically processed distal c-terminal domain. J Physiol. 2006;576:87–102. doi: 10.1113/jphysiol.2006.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Insel PA, Head BP, Patel HH, Roth DM, Bundey RA, Swaney JS. Compartmentation of g-protein-coupled receptors and their signalling components in lipid rafts and caveolae. Biochem Soc Trans. 2005;33:1131–1134. doi: 10.1042/BST20051131. [DOI] [PubMed] [Google Scholar]
- 41.Scriven DR, Klimek A, Asghari P, Bellve K, Moore ED. Caveolin-3 is adjacent to a group of extradyadic ryanodine receptors. Biophys J. 2005;89:1893–1901. doi: 10.1529/biophysj.105.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Head BP, Patel HH, Roth DM, Lai NC, Niesman IR, Farquhar MG, Insel PA. G-protein-coupled receptor signaling components localize in both sarcolemmal and intracellular caveolin-3-associated microdomains in adult cardiac myocytes. J Biol Chem. 2005;280:31036–31044. doi: 10.1074/jbc.M502540200. [DOI] [PubMed] [Google Scholar]
- 43.Minamisawa S, Oshikawa J, Takeshima H, Hoshijima M, Wang Y, Chien KR, Ishikawa Y, Matsuoka R. Junctophilin type 2 is associated with caveolin-3 and is down-regulated in the hypertrophic and dilated cardiomyopathies. Biochem Biophys Res Commun. 2004;325:852–856. doi: 10.1016/j.bbrc.2004.10.107. [DOI] [PubMed] [Google Scholar]
- 44.Balijepalli RC, Foell JD, Wang J, Best JM, Kamp TJ. Targeting of cav1.2 channels to caveolae requires cavb subunit association with caveolin-3 in the cardiomyocytes. Circulation. 2008;118:S 339. [Google Scholar]
- 45.Tunquist BJ, Hoshi N, Guire ES, Zhang F, Mullendorff K, Langeberg LK, Raber J, Scott JD. Loss of akap150 perturbs distinct neuronal processes in mice. Proc Natl Acad Sci U S A. 2008;105:12557–12562. doi: 10.1073/pnas.0805922105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.