Abstract
Genetic types of plasminogen were determined from a donor and a recipient before and after hepatic homotransplantation. Examination of the plasminogen types demonstrated that the liver is the principal site of synthesis of human plasminogen.
The glycoprotein plasminogen is the zymogen form of the proteolytic enzyme plasmin (E.C. 3.4.21.7). Plasmin functions in degradation of fibrinogen and the fibrin blood clot. The site of synthesis of the plasma protein plasminogen has been the subject of controversy. Genetic typing of proteins in a liver donor and a recipient before hepatic homo-transplantation and from the recipient after transplantation has established that the liver is the primary site of synthesis of haptoglobin, Gc-globulin, orosomucoid, transferrin, α1-antitrypsin, and the complement proteins, factor B, C3, C6, and C8 (1).
Initially, plasminogen was localized in the eosinophilic series of granulocytes in the bone marrow by immunofluorescence (2). In that experiment, no plasminogen was detected in surgical biopsy specimens of liver, spleen, lymph nodes, or lung. This was interpreted to mean that plasminogen is synthesized within developing and maturing eosinophils of the bone marrow. Other investigators observed no production of plasminogen by the isolated perfused rat liver (3). In addition, plasminogen was detected in human granulocytes and monocytes, depending upon the maturation stage (4). The zymogen appeared in promyelocytes, reached highest activity in metamyelocytes and in band cells, and then gradually decreased during maturation. No differences in plasminogen content among mature neutrophils, eosinophils, and basophils were detected. Plasminogen has also been detected in red blood cells (5), although at a low level.
In contrast, a study of patients with abnormal liver functions revealed that 75 percent had low levels of plasminogen (6). In a study with rats, streptokinase was injected intravenously to deplete the circulating plasminogen (7). After sudden depletion, concentration of plasmin in the renal vein was twice that in the renal artery, while no arteriovenous difference was detected for the liver. These studies did not resolve whether plasminogen was synthesized de novo in the kidney or activated there. Siefring and Castellino described de novo biosynthesis of plasminogen in the anephric rat (8). None of these investigators examined the production of plasminogen in vivo after homotransplantation, although experiments of this type would establish the principal site of synthesis of plasma proteins.
We have delineated a common genetic polymorphism in human plasminogen by treating serum or plasma samples with neuraminidase. Genetic differences in asialo plasminogens were detected by using isoelectric focusing in polyacrylamide gel and either immunofixation or caseinolytic overlay after urokinase activation (9). Briefly, serum or plasma samples (7 μl) were treated with 10 μ1 of Clostridium perfringens neuraminidase (10 U/ml, pH 6.8) (Sigma, type VI) containing 0.005M EDTA. The mixture was subjected to constant dialysis against a phosphate buffer for 5 hours at room temperature. Ampholytes (LKB Instruments) ranging in pH from 3.5 to 10 at a final concentration of 2 percent were incorporated into 5 percent acrylamide gels with 0.2M taurine. Gels were polymerized with riboflavin and light. Samples of the mixture (approximately 15 μl) were applied by moistening small rectangles of Whatman No. 1 filter paper and placing them on the gel surface near the anode. Isoelectric focusing was performed for 16 to 18 hours at 450 V. After isoelectric focusing, the gels were exposed to 1 ml of urokinase at 100 U/ml (Calbiochem) and overlaid with a casein-agarose gel. The overlaid plates were then incubated for 2 to 4 hours at 37°C in a moist chamber. Plates were fixed in 10 percent trichloroacetic acid for 30 to 60 seconds and washed overnight in distilled water.
Plasminogen patterns consisting of clear proteolyzed areas in a milky casein background were immediately discernible. Two common plasminogen alleles, PLGN*A and PLGN*B (10), have been observed in each major race. Several rare alleles with isoelectric points more acidic than PLGN A or more basic than PLGN B have also been observed. After a plasma sample is treated with neuraminidase, each allele product resolves into two major bands. Mixtures of plasma from a person with PLGN A and from one with PLGN B mimic the pattern produced by PLGN AB plasma. A similar polymorphism has been described for plasminogen in untreated plasma (11). However, there is ambiguity in typing plasminogen in untreated plasma.
Plasminogen types were determined in plasma samples from a recipient of kidney homotransplantation and from his mother, the donor, before transplantation and in two samples from the recipient after transplantation. Figure 1 shows the plasminogen type of the donor, PLGN BBr (rare), and of the recipient, PLGN ABr, before renal transplantation. The Br (B rare) pattern consists of three major bands, two of which are shared with the B variant. The recipient did not lose the two major A bands in the 2 weeks after the transplantation. Figure 2 shows the plasminogen type of a donor, PLGN A, and of the recipient, PLGN B, before hepatic transplantation. In plasma samples obtained from the recipient after the liver transplant, the plasminogen type shows a change to that of the donor.
Fig. 1.
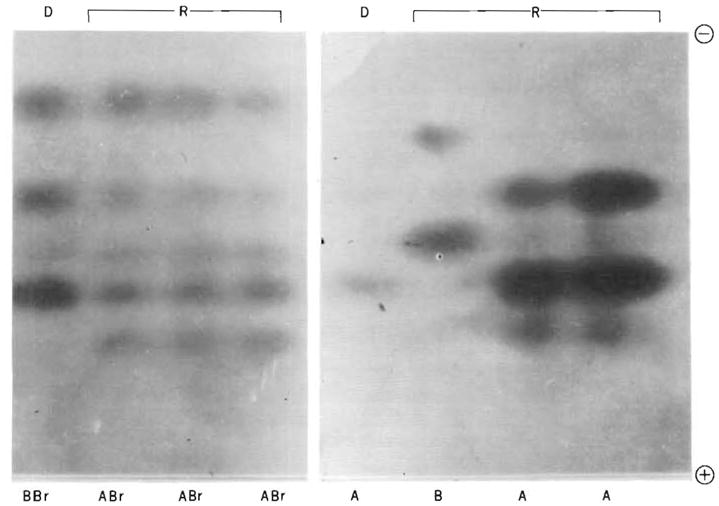
(left). Plasminogen types in a donor (D) and recipient (R) before and after renal homotransplantation, demonstrated by caseinolytic overlay. From left to right: donor, recipient before transplantation, recipient 1 week after transplantation, and recipient 2 weeks after transplantation. Fig. 2 (right). Plasminogen types in a donor and recipient before and after liver homotransplantation, demonstrated by caseinolytic overlay. From left to right: donor, recipient before transplantation, recipient 1 month after transplantation, and recipient 6 months after transplantation. The specimen from the donor was obtained immediately prior to death, and it appeared to show reduced plasminogen activity at that time.
We conclude that most, if not all, plasminogen in human plasma is synthesized in the liver. These experiments confirm that virtually all plasma proteins except for the immunoglobulins are synthesized by the liver in vivo (12).
Contributor Information
Donald Raum, Center for Blood Research, Boston, Massachusetts 02115.
Deborah Marcus, Center for Blood Research, Boston, Massachusetts 02115.
Chester A. Alper, Center for Blood Research, Boston, Massachusetts 02115
Raphael Levey, Department of Surgery, Children’s Hospital Medical Center, Boston, Massachusetts 02115.
Paul D. Taylor, Department of Surgery, University of Colorado Medical Center, Denver 80262
Thomas E. Starzl, Department of Surgery, University of Colorado Medical Center, Denver 80262
References and Notes
- 1.Starzl TE, Marchioro TL, Rowlands DT, Jr, Kirkpatrick CH, Wilson WEC, Rifkind D, Waddell WR. Ann Surg. 1964;160:411. doi: 10.1097/00000658-196409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kashiwagi N, Groth CG, Starzl TE. Proc Soc Exp Biol Med. 1968;128:247. doi: 10.3181/00379727-128-32988. [DOI] [PMC free article] [PubMed] [Google Scholar]; Alper CA, Johnson AM, Birtch AG, Moore FD. Science. 1969;163:286. doi: 10.1126/science.163.3864.286. [DOI] [PubMed] [Google Scholar]; Hobart MJ, Lachmann PJ, Calne RY. J Exp Med. 1977;146:629. doi: 10.1084/jem.146.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]; Alper CA, Raum D, Awdeh ZL, Petersen BH, Taylor PD, Starzl TE. Clin Immunol Immunpathol. doi: 10.1016/0090-1229(80)90169-5. in press. [DOI] [PubMed] [Google Scholar]
- 2.Barnhart MI, Riddle JM. Blood. 1963;21:306. [PubMed] [Google Scholar]
- 3.Mattii R, Ambrus JL, Sokal JE, Mink I. Proc Soc Exp Biol Med. 1964;116:69. doi: 10.3181/00379727-116-29161. [DOI] [PubMed] [Google Scholar]
- 4.Prokopowicz J. Biochim Biophys Acta. 1968;154:91. doi: 10.1016/0005-2795(68)90262-6. [DOI] [PubMed] [Google Scholar]; Prokopowicz J, Stormorken H. Scand J Haematol. 1968;5:129. doi: 10.1111/j.1600-0609.1968.tb01727.x. [DOI] [PubMed] [Google Scholar]
- 5.Sakuragawa N. Acta Haematol Jpn. 1966;29:910. [PubMed] [Google Scholar]
- 6.Davis RD, Picoff RC. Am J Clin Pathol. 1969;52:661. doi: 10.1093/ajcp/52.6.661. [DOI] [PubMed] [Google Scholar]
- 7.Highsmith RF, Kline DL. Am J Physiol. 1973;225:1032. doi: 10.1152/ajplegacy.1973.225.5.1032. [DOI] [PubMed] [Google Scholar]; Kline DL, Highsmith RF. Ser Haematol. 1973;7:513. [PubMed] [Google Scholar]
- 8.Siefring GE, Jr, Castellino FJ. J Appl Physiol. 1975;38:114. doi: 10.1152/jappl.1975.38.1.114. [DOI] [PubMed] [Google Scholar]
- 9.Raum D, Marcus D, Alper CA. Clin Res. 1979;27:458A. [Google Scholar]; Am J Hum Genet. in press. [Google Scholar]
- 10.The nomenclature for genetic polymorphism of human plasminogen outlined here is designed to conform to the recently formulated international system for human gene nomenclature ( Shows B, et al. Cytogenet Cell Genet. in press.).
- 11.Hobart MJ. Ann Hum Genet. 1979;42:419. doi: 10.1111/j.1469-1809.1979.tb00675.x. [DOI] [PubMed] [Google Scholar]
- 12.Witherspoon RP, Schanfield MS, Storb R, Thomas ED, Giblett ER. Transplantation. 1978;26:407. doi: 10.1097/00007890-197812000-00008. [DOI] [PubMed] [Google Scholar]; Miller LL, Bale WF. J Exp Med. 1954;99:125. doi: 10.1084/jem.99.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]; Miller LL, Bly CG, Bale WF. :133. doi: 10.1084/jem.99.2.133. ibid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.This work was supported by grants AI 14157, AI 13626, AM 16392, and AI 15033 from the National Institutes of Health, grant 6–183 from the National Foundation-March of Dimes, and the Charles E. Merrill Trust.


