Abstract
A potential role for TH17 cells has been suggested in a number of conditions including neurodevelopmental disorders such as autism spectrum disorders (ASD). In the current study, we investigated cellular release of IL-17 and IL-23 following an in-vitro immunological challenge of peripheral blood mononuclear cells (PBMC) from children with ASD compared to age-matched typically developing controls. Following stimulation, the concentration of IL-23, but not IL-17, was significantly reduced (p=0.021) in PBMC from ASD compared to controls. Decreased cellular IL-23 production in ASD warrants further research to determine its role on the generation and survival of TH17 cells, a cell subset important in neuroinflammatory conditions that may include ASD.
Keywords: Autism spectrum disorders, cytokines, inflammation, IL-17, IL-23, lymphocytes, TH17 cells
1. Introduction
Autism spectrum disorders (ASD) are neurodevelopmental disorders characterized by severe impairments in social interaction and communication, and restricted, stereotyped interests (APA, 1994). Symptoms of ASD generally manifest within the first three years of life and persist through adulthood in most cases. Evidence of immune dysregulation has been observed in some individuals with ASD, including increased levels of pro-inflammatory cytokines in brain tissues, CSF and plasma, and increased production of pro-inflammatory cytokines by peripheral blood mononuclear cell (PBMC) cultures when compared to typically developing controls (Ashwood et al., 2008; Ashwood and Wakefield, 2006; Enstrom et al., 2009a; Jyonouchi et al., 2001; Molloy et al., 2006; Vargas et al., 2005; Zimmerman et al., 2005).
Recently, TH17 cells (Bettelli et al., 2007) were described as a subset of T cells unique for their production of interleukin (IL)-17, a cytokine demonstrated to be important for defense against extracellular bacteria (Raffatellu et al., 2008; Tato and O’Shea, 2006), fungi (Taylor et al., 2007), and specific parasites (Reece et al., 2008). In addition, these cells have been implicated in the pathology of a number of inflammatory conditions including multiple sclerosis (MS), rheumatoid arthritis, Crohn’s disease, and psoriasis (Graber et al., 2008; Tzartos et al., 2008; Vaknin-Dembinsky et al., 2008). As a survival and proliferative factor for TH17 cells, IL-23 promotes sustained production of IL-17 by TH17 cells (Aggarwal et al., 2003). In animal models, anti-IL-23 therapy can ameliorate experimental autoimmune encephalomyelitis (EAE) (Chen et al., 2006), suggesting that dysregulation of TH17 cells may contribute to disorders with suspected autoimmune/neuroinflammatory mechanisms, such as ASD.
Dysregulated immune responses in some individuals with ASD could lead to the aberrant production of IL-23 from immune cells. We recently reported that plasma IL-23 levels were decreased in ASD children (Enstrom et al., 2008). To help elucidate whether there exists a potential dysregulation of IL-23 and IL-17 production in ASD, we examined the effect of immune stimulation of PBMC isolated from children with ASD and typically developing controls in the same age range.
2. Materials and Methods
Ninety-four subjects were recruited as part of the Childhood Autism Risks from Genetics and Environment (CHARGE) study (Hertz-Picciotto et al., 2006). For the experiments involving phytohemagglutinin (PHA) stimulation, 34 children with ASD (median age 3.83 (interquartile range 3.17-4.25), 29 males) and 26 typically developing (TD) controls (3.71(3.00-4.50), 21 males) were included. Experiments involving phorbol myristate acetate (PMA) stimulation included 18 ASD children ( 4.25(3.08-4.07), 14 males) and 16 TD controls (3.60(2.08-4.02), 13 males). ASD was diagnosed using gold standard assessments (DSM-IV criteria, Autism Diagnostic Observation Schedules (ADOS) and the Autism Diagnostic Interview-Revised (ADI-R)). Cognitive and adaptive assessments were performed on all participants using the Mullen Scales of Early Learning (MSEL) and the Vineland Adaptive Behavior Scales (VABS). Parents of all participants completed the Aberrant Behavior Checklist (ABC) to assess inappropriate and maladaptive behaviors. All developmental and behavioral testing was conducted by qualified clinicians and methodology is discussed in detail elsewhere (Hertz-Picciotto et al., 2006). TD controls were included if they did not have a sibling with ASD, had no apparent autism traits based on assessment with the Social Communication Questionnaire (SCQ), and scored within two standard deviations of the mean on MSEL and VABS assessments. No participants had a history of recent infections and/or fever.
Peripheral blood was collected in acid-citrate-dextrose Vacutainers (BD Biosciences, San Jose, CA). PBMC were separated by gradient centrifugation on Histopaque (Sigma), washed twice with Hank’s Balanced Salt Solution (HBSS) Sigma (St. Louis Missouri). The number of viable PBMC was determined by Trypan Blue exclusion (Sigma) and PBMC concentrations were adjusted to 1 × 106 cells/ml in a solution of 0.1% T-Stim (BD Biosciences) in X-Vivo media (Cambrex, Walkersville, MD) in 12-well tissue culture plate (Corning, Corning, NY). PBMC were either cultured in media alone, or stimulated with PHA (10 μg/mL; Sigma) for 24 hours at 37°C in 5% CO2. Following culture, plates were centrifuged before supernatants were harvested and stored at −80°C until the date of assay.
IL-23 and IL-17 concentrations were measured in the media and PHA stimulated cell culture supernatant of each subject by enzyme-linked immunosorbent assay (ELISA). The concentration of IL-23 was measured with the IL-23 ELISA Ready-SET-Go! Kit with pre-coated plates (eBiocience, San Diego, CA). The assay kit allowed for IL-23 detection between 15 pg/ml-2000 pg/ml. IL-17 concentration was measured with the IL-17 ELISA Ready-SET-Go! Kit with pre-coated plates (eBiocience). The kit allowed detection between 4 pg/ml-500 pg/ml. Samples and standards were run in duplicate, and the assay was performed according to the manufacturer’s recommended protocols. Plates were read on a Wallac Victor3 multilabel-plate reader (PerkinElmer, Boston, MA) at 450nm. Sample concentrations were determined by a standard curve correlating a known standard concentration and OD.
Flow cytometric analysis of TH17 cells in PBMC was determined in cell cultures that were either unstimulated (media alone) or treated with PMA (50 ng/ml) and Ionomycin (1 μM final concentration) for 24 hours, using methods previously described for intracellular staining (Ashwood and Wakefield 2006; Enstrom et al., 2009a). PBMC were stained for cell surface markers CD3, CD8 and CD4 (BD Biosciences, CA) before intracellular staining for anti-human IL-17 (eBioscience, San Diego, CA). The cells were analyzed on a LSR II flow cytometer (BD Immunocytometry Systems) and with FlowJo software (BD Immunocytometry Systems).
Statistical analysis to compare the induced cytokine production between groups was conducted with the Mann-Whitney U tests.. Evaluation of induced cytokine levels and clinical assessment and behavioral scores among children with ASD was determined using Spearman correlations. The statistical comparisons were made with SAS software (SAS Institute Inc., Cary, NC). All analyses were two-tailed, and p<0.05 were considered statistically significant.
3. Results
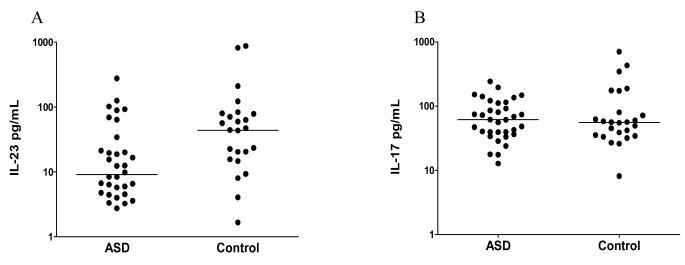
In the absence of PHA stimulation the levels of IL-23 and IL-17 were undetectable in cell culture supernatants from ASD and TD children. Following stimulation, IL-23 levels were significantly induced in cell culture supernatants from TD controls (median 43.69 pg/ml (interquartile range 13.31 pg/ml-78.34 pg/ml)) and were significantly higher than in PHA-stimulated cell culture supernatants from ASD children (9.09 pg/ml (4.50 pg/ml-24.52 pg/ml), p=0.021, Fig.1). Following stimulation IL-17 levels were equally induced in cell culture supernatants from TD controls (55.55 (34.01-103.30)) and ASD children (61.39 (38.52-112.00)) and were not statistically different (p=0.704) (Fig. 1). No differences in total PBMC numbers or T cell, B cell or monocyte numbers were observed between groups (data not shown).
Figure 1.
A) Median IL-23 levels in children with ASD and typically developing children (controls). There are significant differences in the induced cytokine levels of IL-23 produced following stimulation in cell cultures from ASD children compared with controls (p=0.021). B) Median IL-17 levels in ASD children and age-matched TD children. Levels of IL-17 produced by PBMC in-vitro are not significantly different between the two groups (p=0.70). P-values were determined with two-tailed Mann-Whitney U test.
There were no differences in the frequency of TH17 cells in ASD (0.58% (0.11-3.36%)) compared with TD (0.45% (0.07-1.52%), p=0.53) in the unstimulated cultures or after PMA stimulation in ASD (1.1% (0.3-5.1%) compared with TD (0.96% (0.39-2.91%), p=0.18). Similarly, there were no differences in the mean fluorescent intensity of IL-17 staining in unstimulated (27.19 (17.27-40.56) vs., 25.68 (22.72-40.01), ASD vs., TD controls, p=0.85) or stimulated (18.65 (11.97-37.24) vs., 25.62 (12.84-39.19), p=0.54) cell cultures.
We then examined whether there were associations between induced IL-23 or IL-17 levels and clinical variables among ASD participants. ADI-R, MSEL, VABS and ABC scores did not show association with IL-17 or IL-23 levels. Interestingly, we found a negative correlation (r = −0.44, p=0.03) between stimulated IL-23 levels and social interactions as measured with the ADOS, suggesting that more impaired social behaviors were associated with decreased PHA-mediated release of IL-23. Assessment of impaired communication using the ADOS also showed a trend for a negative correlation with PHA-stimulated IL-23 release but did not reach statistical significant (r = −0.32, p=0.13).
4. Discussion
The major finding of this study was that the production of IL-23 but not IL-17 following stimulation was significantly lower in children with ASD compared with TD controls. We previously reported that plasma levels of IL-23 but not IL-17 were decreased in children with ASD compared with TD controls (Enstrom et al., 2008). In addition, we found a negative correlation between stimulated IL-23 levels and ADOS scores of social interaction in ASD children. It is currently unclear how decreased IL-23 levels could affect social interactions during childhood in ASD and these data should be treated with caution until further investigations are performed. However, it should be noted, that several other studies have shown that altered levels of immune factors are associated with increased severity of social interaction impairments including transforming growth factor-beta1 (TGFβ1) (Ashwood et al., 2008), macrophage inhibitory factor (Grigorenko et al., 2008), platelet-endothelial adhesion molecule (Tsuchiya et al., 2007) and immunoglobulin-G4 (Enstrom et al., 2009b) and may suggest that dysfunctional immune responses and/or activity may affect social behaviors in children with ASD.
Decreased IL-23 release following immune challenge, observed in this study, may suggest that hypoactivity of immune cells is associated with increased risk for ASD. Previously, partial/incomplete or hypoactive T cell responses has been demonstrated in autism individuals following activation (Stubbs and Crawford, 1977; Warren et al.,1986; Plioplys et al., 1994; Denney et al.,1996), Neurogenesis is influenced by the interaction between T cells and CNS-microglia cells, whereby too little or too much immune activity can impair neurogenesis, which could ultimately alter behavior and cognition (Ziv et al., 2006; Ziv and Schwartz, 2008). Moreover, in a mouse model, deprivation of mature T-cells leads to spatial learning/memory impairments as assessed using the Morris Water Maze; and this impairment is improved by T-cell restoration (Kipnis et al., 2004). Interestingly, aberrant behaviors in ASD children can improve during episodes of fever but return after the child recovers (Curran et al., 2007). It is possible that these behavioral improvements could involve the activation of T-lymphocyte subsets.
Interestingly, TGF-β1 which can act independently of IL-23 to stimulate the production of TH17 cells (Yang et al., 2008) is also decreased in ASD and associated with worsening behaviors (Ashwood et al., 2008). Further experiments charting the time-course for induction of both TGFβ1 and IL-23 cytokines should be performed, to determine if TH-17 cells from ASD children lose their ability to produce IL-17 over time in the absence of appropriate maintenance signals. As this study focuses on children already diagnosed with ASD, this initial report cannot not rule out increased TH17 cell activity prior to diagnosis or a possible causative role of TH17 cells in the pathology of ASD but rather raises additional questions.
Our preliminary findings lead us to hypothesize that decreased IL-23 may be implicated in the pathophysiology of ASD. The biological impact of decreased IL-23 and its association with impaired social behaviors in ASD children is intriguing and warrants further consideration.
Acknowledgements
This study was funded by the NIEHS Children’s Center grant ( P01 ES011269), US EPA STAR program grant (R833292 and R829388), NIEHS CHARGE study (R01ES015359), Cure Autism Now Foundation, Peter Emch Foundation, The Boler Company Foundation, HEDCO foundation and a generous gift from the Johnson Family. We thank Isaac Pessah for his careful review and suggestions in the completion of this manuscript. We would like to thank the staff of both the UC Davis M.I.N.D. Institute and the CHARGE study for their technical support. The commitment of the families who took part in these studies, at both the M.I.N.D Institute and as part of the CHARGE study, is also gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Enstrom A, Krakowiak P, Hertz-Picciotto I, Hansen RL, Croen LA, Ozonoff S, Pessah IN, de Water JV. Decreased transforming growth factor beta1 in autism: A potential link between immune dysregulation and impairment in clinical behavioral outcomes. J Neuroimmunol. 2008;204:149–153. doi: 10.1016/j.jneuroim.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Wakefield AJ. Immune activation of peripheral blood and mucosal CD3+ lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms. J Neuroimmunol. 2006;173:126–134. doi: 10.1016/j.jneuroim.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, Hunter CA, Kastelein RA, Cua DJ. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran LK, Newschaffer CJ, Lee LC, Crawford SO, Johnston MV, Zimmerman AW. Behaviors associated with fever in children with autism spectrum disorders. Pediatrics. 2007;120:e1386–1392. doi: 10.1542/peds.2007-0360. [DOI] [PubMed] [Google Scholar]
- Denney DR, Frei BW, Gaffney GR. Lymphocyte subsets and interleukin-2 receptors in autistic children. J Autism Dev Disord. 1996;26(1):87–97. doi: 10.1007/BF02276236. [DOI] [PubMed] [Google Scholar]
- Enstrom A, Onore C, Hertz-Picciotto I, Hansen R, Croen L, Van de Water J, Ashwood P. Detection of IL-17 and IL-23 in Plasma Samples of Children with Autism. American Journal of Biochemistry and Biotechnology. 2008;4:114–120. doi: 10.3844/ajbbsp.2008.114.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstrom AM, Lit L, Onore CE, Gregg JP, Hansen RL, Pessah IN, Hertz-Picciotto I, Van de Water JA, Sharp FR, Ashwood P. Altered gene expression and function of peripheral blood natural killer cells in children with autism. Brain Behav Immun. 2009a;23:124–133. doi: 10.1016/j.bbi.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstrom A, Krakowiak P, Onore C, Pessah IN, Hertz-Picciotto I, Hansen RL, Van de Water JA, Ashwood P. Increased IgG4 levels in children with autism disorder. Brain Behav Immun. 2009b;23:389–395. doi: 10.1016/j.bbi.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber JJ, Allie SR, Mullen KM, Jones MV, Wang T, Krishnan C, Kaplin AI, Nath A, Kerr DA, Calabresi PA. Interleukin-17 in transverse myelitis and multiple sclerosis. J Neuroimmunol. 2008;196:124–132. doi: 10.1016/j.jneuroim.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Grigorenko EL, Han SS, Yrigollen CM, Leng L, Mizue Y, Anderson GM, Mulder EJ, de Bildt A, Minderaa RB, Volkmar FR, Chang JT, Bucala R. Macrophage migration inhibitory factor and autism spectrum disorders. Pediatrics. 2008;122:e438–445. doi: 10.1542/peds.2007-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: An epidemiological investigation of genetic and environmental factors contributing to autism. Environmental Health Perspectives. 2006;114:1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyonouchi H, Sun S, Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J Neuroimmunol. 2001;120:170–179. doi: 10.1016/s0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U S A. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy CA, Morrow AL, Meinzen-Derr J, Schleifer K, Dienger K, Manning-Courtney P, Altaye M, Wills-Karp M. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2006;172:198–205. doi: 10.1016/j.jneuroim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Plioplys AV, Greaves A, Kazemi K, Silverman E. Lymphocyte function in autism and Rett syndrome. Neuropsychobiology. 1994;29(1):12–16. doi: 10.1159/000119056. [DOI] [PubMed] [Google Scholar]
- Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, Kolls JK, Dandekar S, Baumler AJ. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece JJ, Siracusa MC, Southard TL, Brayton CF, Urban JF, Jr., Scott AL. Hookworm-induced persistent changes to the immunological environment of the lung. Infect Immun. 2008;76:3511–3524. doi: 10.1128/IAI.00192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs EG, Ash E, Williams CP. Autism and congenital cytomegalovirus. J. Autism Dev. Disord. 1984;14:183–189. doi: 10.1007/BF02409660. [DOI] [PubMed] [Google Scholar]
- Tato CM, O’Shea JJ. Immunology: what does it mean to be just 17? Nature. 2006;441:166–168. doi: 10.1038/441166a. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya KJ, Hashimoto K, Iwata Y, Tsujii M, Sekine Y, Sugihara G, Matsuzaki H, Suda S, Kawai M, Nakamura K, Minabe Y, Yagi A, Iyo M, Takei N, Mori N. Decreased serum levels of platelet-endothelial adhesion molecule (PECAM-1) in subjects with high-functioning autism: a negative correlation with head circumference at birth. Biol Psychiatry. 2007;62:1056–1058. doi: 10.1016/j.biopsych.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaknin-Dembinsky A, Murugaiyan G, Hafler DA, Astier AL, Weiner HL. Increased IL-23 secretion and altered chemokine production by dendritic cells upon CD46 activation in patients with multiple sclerosis. J Neuroimmunol. 2008;195:140–145. doi: 10.1016/j.jneuroim.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RP, Margaretten NC, Pace NC, Foster A. Immune abnormalities in patients with autism. J. Autism Dev. Disord. 1986;16:189–197. doi: 10.1007/BF01531729. [DOI] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman AW, Jyonouchi H, Comi AM, Connors SL, Milstien S, Varsou A, Heyes MP. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr Neurol. 2005;33:195–201. doi: 10.1016/j.pediatrneurol.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Schwartz M. Immune-based regulation of adult neurogenesis: implications for learning and memory. Brain Behav Immun. 2008;22:167–176. doi: 10.1016/j.bbi.2007.08.006. [DOI] [PubMed] [Google Scholar]