Abstract
N-chloro-2,2,6,6-tetramethyl-4-piperidinol laurate (Cl-TMPL) was prepared by reacting 2,2,6,6-tetramethyl-4-piperidinol hydrochloride (TMP·HCl) with lauroyl chloride, followed by chlorination with sodium dichloroisocyanurate. The chemical structure of Cl-TMPL was characterized with FT-IR, NMR, DSC, and TGA analyses. The antimicrobial performance of Cl-TMPL against Gram-positive and Gram-negative bacteria was compared with 1-chloro-3-dodecyl-5,5-dimethylhydantoin (Cl-DDMH), a amide N-halamine, and chloro-2,4-diamino-6-dodecyl-1,3,5-triazine (Cl-DADT), a melamine (imino) N-halamine. The three classes of N-halamines were used as additives for polyurethane (PU). Visible light transparency data indicated that up to 6% of Cl-DDMH or Cl-DADT could be compatibly mixed with PU, but Cl-TMPL had low compatibility with PU at higher than 2% of Cl-TMPL. With the same additive content, Cl-DDMH and Cl-DADT provided more powerful antimicrobial and biofilm-controlling effects than Cl-TMPL. In stability studies, however, PU samples with Cl-TMPL released the lowest amount of active chlorine into the immersing solution, suggesting the highest stability of the antimicrobial and biofilm-controlling efficacy.
Keywords: Antimicrobial additive, Amide, Melamine, Amine, N-halamine, Antimicrobial activity, Structure/property relationship
Introduction
There has been a rapidly growing need for controlling microbial colonization and biofilm formation on solid surfaces in a wide range of industrial, environmental, institutional and hygienic applications. Microbes on the contaminated materials can not only transfer infectious agents, but also reduce heat transfer in industrial cooling towers, corrode pipes, block filters, and cause other serious problems.1, 2 As a result, the development of antimicrobial and biofilm-controlling polymers has attracted substantial academic and industrial interests. A number of polymers with anti-biofilm effects have been reported, and some of these studies have achieved encouraging results.3-15
The research interest in this group focuses on N-halamine-based polymers for antimicrobial and biofilm-controlling applications. N-halamines are compounds containing one or more nitrogenhalogen covalent bonds.11 The antimicrobial action of N-halamines is related to the transfer of positive halogens from the N-halamines through the charged microbial cell walls14 to appropriate receptors in the cells (either directly or indirectly). This process can effectively destroy or inhibit the enzymatic or metabolic cell processes, resulting in the expiration of the organisms.11 Compared with inorganic halogens (e.g., chlorine or bromine), N-halamines are much more stable, less corrosive, and have a much less tendency to generate halogenated hydrocarbons. Thanks to these attractive properties, monomeric N-halamines have been widely used as food and water disinfectants.11
Much effort has been devoted to the development of polymeric N-halamines for a broad range of antimicrobial applications. Most of the current approaches involve chemical reactions (e.g., direct functional group transformation,7,10 homo- and/or co-polymerization,5 grafting,15,16 etc.) to introduce N-halamine structures into the target polymers to achieve antimicrobial effects. On the other hand, however, the chemical reaction approaches may not be suitable for a range of polymeric materials: in some cases, the materials are chemically inert, making the chemical reactions difficult to perform; in other situations, the chemical reactions may deteriorate the physical properties of the materials and/or complicate the fabrication processes of the materials.
Our previous studies demonstrated that amine N-halamine compounds could be used as antimicrobial additives to provide non-leaching anti-biofilm activities.3 Building on these results, our continuing studies further developed a series of amide-based N-halamine additives10 and melamine (imino)-based N-halamine additives17 for antimicrobial treatments of various polymers. These investigations revealed that the additive approach could be a simple and practical alternative of the chemical reaction strategy in introducing antimicrobial functions into conventional polymer materials. All three classes of N-halamine additives are potential candidates to achieve this goal. Nonetheless, due to the structural differences of these N-halamines, their performances as antimicrobial additives differ significantly. Apparently, the wider success of the antimicrobial additive approach depends on not only a broader selective option of available additives, but also a deeper understanding of the structure-property relationships of the amine-, amide-, and melamine-based N-halamines.
In this study, we evaluated the performances of the three classes of additives with the same alkyl chain but different N-halamine structures (see Figure 1 for chemical structure): 1-chloro-3- dodecyl-5,5-dimethylhydantoin (Cl-DDMH) is a amide N-halamine with a C-12 alkyl chain; chloro-2,4-diamino-6-dodecyl-1,3,5-triazine (Cl-DADT) is a melamine N-halamine with a C-12 alkyl chain; and N-chloro-2,2,6,6-tetramethyl-4-piperidinol laurate (Cl-TMPL) is an amine N-halamine with a C-12 alkyl chain. Cl-DDMH and Cl-DADT have already been synthesized in our previous studies.10,17 However, the preparation and characterization of Cl-TMPL have not been reported in the public literature. Thus, in order to make a meaningful comparison of the three classes of N-halamines, the first part of the current study investigated the synthesis of Cl-TMPL by reacting 2,2,6,6-tetramethyl-4-piperidinol hydrochloride with lauroyl chloride, followed by chlorination with sodium dichloroisocyanurate. The structure of Cl-TMPL was confirmed with FT-IR, 1H-NMR, UV-VIS, DSC and TGA analyses. In the second part, the antimicrobial activity, biofilm-controlling efficacy, and stability of Cl-DDMH, Cl-DADT, and Cl-TMPL as antimicrobial additives in polyurethane, one of the most versatile polymers in industrial, environmental, institutional and hygienic applications, were evaluated.
Figure 1.
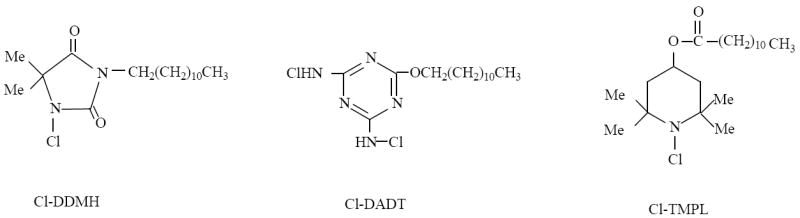
Structures of Cl-DDMH, Cl-DADT, and Cl-TMPL
Experimental Section
Materials
Cl-DDMH and Cl-DADT were prepared following procedures we reported previously.10,17 Lauroyl chloride, sodium dichloroisocyanurate (DCC-Na), and 2,2,6,6-tetramethyl-4-piperidinol (TMP) were purchased from Aldrich and used as received. Other chemicals were analytical grade and used without further purification. Polyether-type thermoplastic polyurethane was provided by Lubrizol Inc. (Estane®5714). The microorganisms, Staphylococcus aureus (S. aureus ATCC 6538, Gram-positive) and Escherichia coli (E. coli ATCC 15597, Gram-negative) were obtained from American Type Culture Collection (ATCC).
Instruments
Fourier transform infrared (FT-IR) spectra of the samples were recorded on a Thermo Nicolet 6700 FT-IR spectrometer (Woburn, MA). 1H-NMR studies were carried out using a Varian Unity-300 spectrometer (Palo Alto, CA) at ambient temperature. UV-VIS measurements were made on a Beckman DU 520 general purpose UV-Vis spectrophotometer (Beckman Instruments Inc., CA). Thermal properties of the samples were characterized with DSC-Q200 and TA Q50 (TA Instruments, DE) under N2 atmosphere at a heating rate of 10 °C/min. The surface morphologies of the samples were observed with a scanning electron microscope (SEM) S-3200N (Hitachi, Japan).
Preparation of 2,2,6,6-tetramethyl-4-piperidinol laurate (TMPL)
Since 2,2,6,6-tetramethyl-4-piperidinol (TMP) has a hydroxyl group and a secondary amine group, both of which can react with lauroyl chloride, the first step of our approach was to react TMP with HCl to produce 2,2,6,6-tetramethyl-4-piperidinol hydrochloride (TMP·HCl) so as to transform the amine group into a salt that cannot be acylated. In the second step, TMP·HCl reacted with lauroyl chloride through a conventional acylation reaction to produce 2,2,6,6-tetramethyl-4-piperidinol laurate (TMPL), which was further transformed into Cl-TMPL by chlorination with DCC-Na.
Thus, TMP·HCl was prepared from TMP with hydrochloric acid. Briefly, 0.04 mol TMP was dissolved in 100 mL chloroform, and a solution containing 0.05 mol hydrochloric acid in 10 mL chloroform was slowly dropped into the system. After stirring for 1 h at ambient temperature, the precipitates were collected by filtration. The resulting powder was washed with dry chloroform. The isolated product was recrystallized from chloroform and dried over CaCl2 in a vacuum oven at ambient temperature to obtain yellowish powders. Yield: 7.63 g (98.5 %).
2,2,6,6-tetramethyl-4-piperidinol laurate (TMPL) was synthesized from TMP·HCl by acylation with lauroyl chloride. In a typical run, 0.02 mol TMP·HCl was dissolved in 50 mL dry DMSO in the presence of 0.025 mol dry NaHCO3 fine powders. A solution containing 0.022 mol lauroyl chloride in 10 mL dry DMSO was gradually dropped into the system, while stirring at ambient temperature. After 8 h stirring, the whole system was slowly heated to 80 °C and reacted for another 4 h. After cooling to ambient temperature, the mixture was adjusted to pH 7 by 0.01 M NaOH aqueous solutions. The precipitates were collected by filtration, washed with copious deionized water, and dried over CaCl2 in a vacuum oven at ambient temperature. The resulting product was recrystallized twice from acetone to obtain white powders. Yield: 6.53 g (86.8 %).
Synthesis of N-chloro-2,2,6,6-tetramethyl-4-piperidinol laurate (Cl-TMPL)
Cl-TMPL was prepared by the chlorination of TMPL with DCC-Na. In this study, 0.02 mol DCC-Na dissolved in 50 mL distilled water was slowly dropped into 50 mL chloroform containing 0.02 mol of TMPL.18 The mixture was stirred vigorously at ambient temperature for 1 h. The insoluble solids were filtered off, and the chloroform layer was separated and dried with magnesium sulfate for 24 h. Magnesium sulfate was filtrated off and chloroform was removed by evaporation. The solids were recrystallized from acetone twice and dried in a vacuum oven at ambient temperature for 48 h to obtain Cl-TMPL as white powders. Yield: 7.20 g (96.3 %).
The active chlorine content of Cl-TMPL was determined by iodimetric titration.3,10,17 Briefly, around 0.05 g Cl-TMPL was dissolved in 40 mL absolute ethanol containing 1.0 wt% acetic acid. One gram of potassium iodide was added, and the mixture was stirred for 1 h at ambient temperature under N2 atmosphere. The released iodine was titrated with 0.01 N sodium thiosulfate aqueous solution using 5 mL 0.1% starch aqueous solution as indicator. The same procedure was applied to the same amount of unchlorinated TMPL to serve as controls. Percentage chlorine content was calculated according to the following equation:
| (1) |
where VCl and V0 were the volumes (mL) of sodium thiosulfate solutions consumed in the titration of the chlorinated and un-chlorinated TMPL, respectively, and WCl (g) was the weight of Cl-TMPL.Each test was repeated three times and the average was recorded.
Antimicrobial activities of Cl-DDMH, Cl-DADT, and Cl-TMPL
The antimicrobial activities of each of the three N-halamine additives, Cl-DDMH, Cl-DADT, and Cl-TMPL, were tested with Stapylococcus aureus (S. aureus ATCC 6538, Gram-positive) and Escherichia coli (E. coli ATCC 15597, Gram-negative).3,10 The bacteria were grown in broth solutions [Tryptic Soy broth for S. aureus, and Luria-Bertani (LB) broth for E. coli] at 37 °C overnight according to ATCC’s recommendation The cells were harvested in a centrifuge, washed twice with sterile phosphate buffered saline (PBS), resuspended in PBS, and then diluted to densities of 108-109 CFU/mL (the number of cfu/ml was evaluated by direct agar plating and growth at 37 °C for 24 h). A known amount of Cl-DDMH, Cl-DADT, or Cl-TMPL was individually dispersed in 10 mL sterile distilled water. The mixture was vortexed for 3 min and then sonicated for 30 min to disperse Cl-DDMH, Cl-DADT, or Cl-TMPL into the suspensions. Afterwards, 100 μL of the bacteria solution was added into the suspension, and the mixture was vortexed for 60 s. After a certain period of contact time (5 min, 15 min, 30 min, 60 min, 120 min, 240 min, and 480 min), 100 μL of the suspension was taken out of the mixture and transferred into 900 μL of sterile sodium thiosulfate aqueous solution (0.03 wt%) to quench the active chlorine. Our screening studies have shown that without the presence of Cl-DDMH, Cl-DADT, or Cl-TMPL, the sonication/quenching treatments do not affect the growth of the bacteria. The resulting solutions were vortexed for 60 s and then serially diluted, and each dilution was placed onto Tryptic soy agar (for S. aureus) or LB agar (for E. coli) plates. The same procedure was also applied to the correspondent unchlorinated DDMH, DADT, and TMPL to serve as controls. Bacterial colonies on the plates were counted after incubation at 37 °C for 24 h. Each test was repeated three times, and the longest minimum contact time of the three tests for a total kill of the microbes (the weakest antimicrobial efficacy observed) was reported.
Preparation of polyurethane films containing the new N-halamine antimicrobial additives
A known amount of Cl-DDMH, Cl-DADT, or Cl-TMPL was added into 10 wt% polyurethane (PU) THF solution with constant stirring until a clear solution was formed. The solution was poured onto glass slides. After evaporation of most of the solvent in a fume hood at ambient temperature for 3 days, the slides were further dried in a vacuum oven at ambient temperature for 2 days. PU films were obtained with a thickness of 100±10 μm (measured with a digital caliper). The same procedure was also applied to pure PU to serve as controls.
Antimicrobial activities of PU films containing the N-halamine antimicrobial additives
In the antimicrobial tests, 10 μL S. aureus or E. coli PBS suspension (108-109 CFU/mL) was placed onto the surface of a PU film (4×4 cm) containing a certain amount of Cl-DDMH, Cl-DADT, or Cl-TMPL3,10. The film was “sandwiched” with another identical film. A sterile weight (100 g) was added onto the films to ensure sufficient contact. After a certain period of contact time (5 min, 15 min, 30 min, 60 min, 120 min, 240 min, and 480 min), the whole “sandwich” was transferred into 10 mL sterile sodium thiosulfate aqueous solution (0.03 wt%) to quench the active chlorines. The resulting solution was vortexed for 1 min to separate the two films and sonicated for 5 min to remove the adherent cells into the solution. Our screening studies with pure PU films have shown that such a sonication/quenching treatment does not affect the growth of the bacteria. An aliquot of the solution was serially diluted, and 100 μL of each dilution was plated onto agar plates (Tryptic soy agar for S. aureus, and LB agar for E. coli). Bacterial colonies on the plates were counted after incubation at 37 °C for 24 h. The same procedure was applied to pure PU films to serve as controls. Each test was repeated three times, and the longest minimum contact time of the three tests for a total kill of the microbes (the weakest antimicrobial efficacy observed) was reported.
Anti-biofilm function of PU films containing the new N-halamine antimicrobial additives
An overnight culture of S. aureus was centrifuged, washed with PBS, and then diluted with sterile PBS to densities of 107-108 CFU/mL. An antimicrobial additive-containing PU film (1×1 cm) was immersed in 5 mL of the microbial suspension, which was shaken gently at 37 °C for 30 min to permit bacterial adhesion 10,15. The film was taken out of the suspension and washed gently with 10 mL non-flowing PBS to remove the loosely attached bacteria. The film was then transferred into 10 mL Tryptic soy broth solutions. The whole system was put in a water bath and gently shaken at 37 °C. After 24 h of incubation, the film was rinsed gently with 0.1 M sodium cacodylate buffer (SCB) to remove loosely attached bacteria, and then fixed with 3 % glutaraldehyde in SCB at 4 °C for 24 h. After washing gently with SCB, the film was dehydrated through an alcohol gradient,2 mounted onto sample holders, sputter coated with gold-palladium, and observed under a Hitachi S-3200N scanning electron microscope to evaluate the anti-colonization/anti-biofilm functions of the N-halamine-containing PU films. The same procedure was also applied to pure PU films as controls.
Stability study
To investigate the stability of the chorines in the N-halamines, a series of PU films (ca. 1×1 cm) containing a predetermined amount of Cl-DDMH, Cl-DADT, or Cl-TMPL were immersed individually in 10 mL deionized water under constant shaking (50 rpm) at ambient temperature. After a certain period of time, the film was taken out and the water was iodometrically titrated to determine the level of active chlorines released from the films into the immersing solutions.
Results and Discussion
Synthesis and characterization of Cl-TMPL
The major objective of the current study was to investigate the effects of chemical structures on antimicrobial and biofilm-controlling efficacies of the three classes of antimicrobial additives (with the same alkyl chain but different N-halamine structures; see Figure 1). As noted earlier, while Cl-DDMH10 and Cl-DADT17 have been prepared in our previous studies, no reports regarding the synthesis and characterization of Cl-TMPL could be found in the public literature. In order to make a meaningful comparison of the three classes of N-halamine additives, we decided to synthesize Cl-TMPL in the first part of this investigation. In our approach, Cl-TMPL was prepared through a three-step process, as shown in Figure 2.
Figure 2.
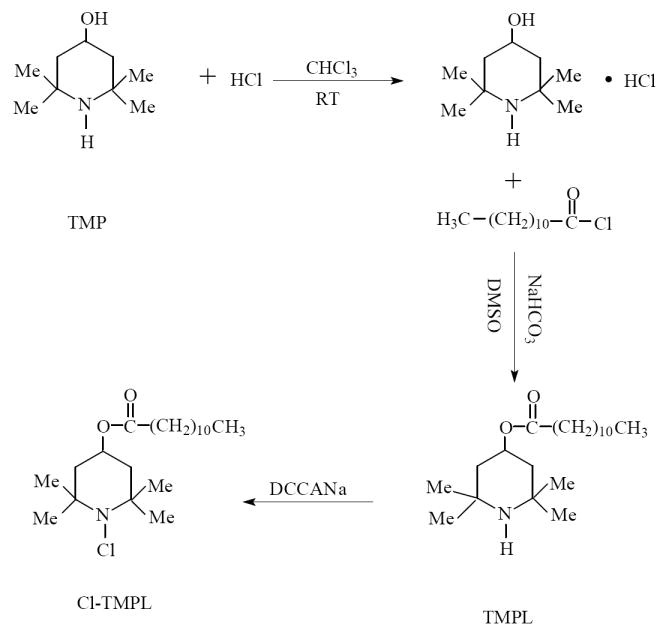
Preparation of N-chloro-2,2,6,6-tetramethyl-4-piperidinol laurate
FT-IR analysis was used to follow the reactions. As shown in Figure 3, in the spectrum of TMP, the bands at 3253 and 3153 cm-1 are attributable to hydroxyl groups. The 3400 cm-1 weak, broad peak is related to N-H stretching vibration. After acylation, the O-H bands around 3253 cm-1 and 3153 cm-1 disappear in the spectrum of TMPL. A new band at 1700 cm-1 can be observed, which must be caused by the ester carbonyl group. The C-H stretching vibrations of the long alkyl chain present around 2914 cm-1. Upon chlorination, the N-H structure was transferred into N-Cl; consequently, in the spectrum of Cl-TMPL, the N-H stretching vibration band disappears.3,19-24
Figure 3.
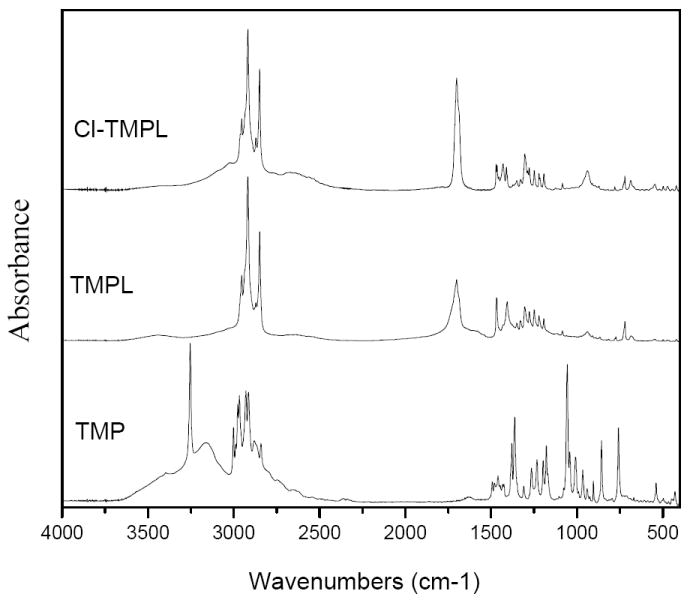
FT-IR spectra of TMP, TMPL, and Cl-TMPL
The chemical structures of the samples were also confirmed by 1H-NMR studies, as shown in Figure 4. In the spectrum of TMP, the peak at 4.5 ppm (H 1) is caused by the proton of the hydroxyl group, and the signal at 3.8 ppm is related to the neighboring C- H (H2) of the O-H group. The signals at 1.65-1.75 ppm and 1.02-1.12 ppm are attributable to the protons of the methylene groups (H3/4) of the piperidine ring and the methyl groups (H5/6) that are bonded to the piperidine ring, respectively. The peak at 0.85 ppm is assigned to the the proton of the N-H group (H7). After acylation reaction, the signal of O-H at 4.5 ppm disappears in the spectrum of TMPL, and the peak of the neighboring C-H (H2) shifts to 2.18 ppm, which overlaps with the C-H of the ester carboxyl group (H8). The new peaks around 0.82 ppm could be attributed to protons of the long alkyl chain methyl group (H10). After chlorination, the -NH group was transformed into -NCl group. As a result, the NH (H7) signal at 0.85 ppm can no longer be detected in the spectrum of Cl-TMPL, confirming the replacement of N-H structure with N-Cl group.3
Figure 4.
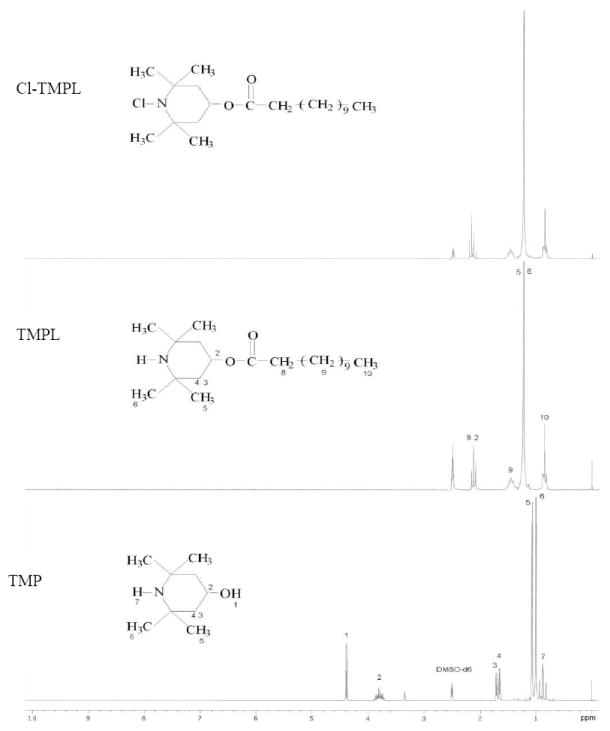
1H-NMR spectra of TMP, TMPL, and Cl-TMPL.
To provide further information about the reactions, the samples are characterized by thermal analyses. Figure 5 shows the DSC curves of TMP, TMPL, and Cl-TMPL. TMP shows a melting pointat 131 °C and an endothermal decomposition peak at 235 °C. After acylation reaction, the introduction of dodecyloxyl moiety breaks intermolecular hydrogen bonds; consequently, the melting point of TMPL decreases to 50 °C. The decomposition temperature of TMPL shifts to 189 °C, which may be caused by the thermal decomposition of less stable ester bonds. After chlorination, the melting point of Cl-TMPL decreases to 45 °C and a broad endothermal peak can be observed at 176 °C, which can be attributed to the decomposition of the N-Cl bond overlapped with the decomposition of the ester groups.
Figure 5.
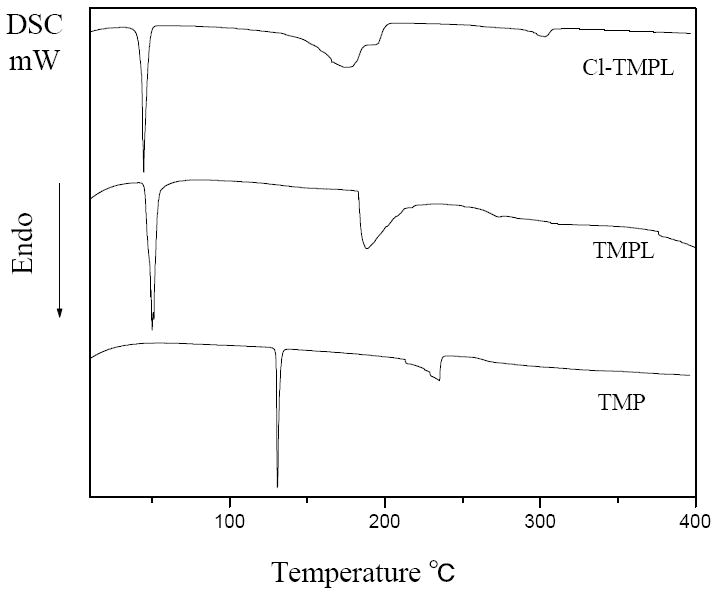
DSC curves of TMP, TMPL, and Cl-TMPL.
In TGA studies (Figure 6), TMP volatilizes and results in a fast weight loss and very low weight residue. TMPL shows a much slower weight loss due to the presence of the long alkyl chain, which prevents the volatilization of the sample. On the other hand, the chlorinated TMPL (Cl-TMPL) shows a lower thermal stability (faster weight loss and less weight residue) than the un-chlorinated TMPL, suggesting that the decomposition of the N-Cl bonds (which generates Cl and alkyl free radicals) in the Cl-TMPL may have accelerated the thermal decomposition and promoted weight loss of the sample.
Figure 6.
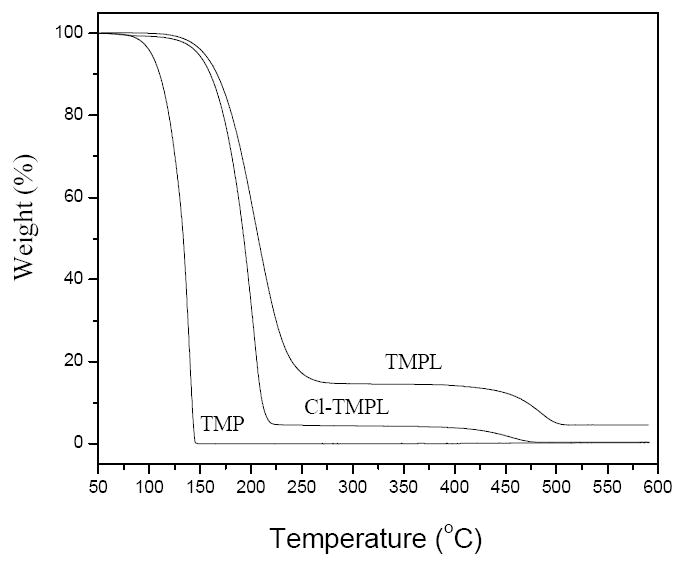
TGA curves of TMP, TMPL, and Cl-TMPL.
All these findings suggest that Cl-TMPL has been successfully synthesized, which can be used for the comparison studies of the three classes of N-halamine antimicrobial additives.
Antimicrobial activities of Cl-DDMH, Cl-DADT, and Cl-TMPL
The antimicrobial activities of Cl-DDMH, Cl-DADT, and Cl-TMPL were tested with S. aureus ATCC 6538 (Gram-positive) and E. coli ATCC 15597 (Gram-negative). The data is summarized in Table I. All the N-halamine additives provide antimicrobial functions against both species, but to different extents. Cl-DDMH has the most potent antimicrobial activity: with only 10 ppm of available chlorine, Cl-DDMH provides a total kill of 108-109 CFU/mL of the bacteria in 15 min; with 50 ppm of bound chlorine, the minimum contact time for a total kill of the same species reduces to 5 min. After that, further increasing Cl-DDMH content does not substantially enhance antimicrobial activity. On the other hand, Cl-TMPL shows the weakest antimicrobial effects: at lower than 200 ppm of available chlorine, Cl-TMPL can only provide a 2-3 log reduction of 108-109 CFU/mL of E. coli and S. aureus, and no total kill can be observed after as long as 8 h of contact. With 200 ppm of chlorine, a total kill of the microorganisms is achieved after 120 min of contact. When the chlorine content is increased to 1,000 ppm, the contact time for a total kill of the bacteria gradually decreases to 60 min. The antimicrobial activity of Cl-DADT is slightly lower than that of Cl-DDMH, but is much more potent than that of Cl-TMPL, as shown in Table I.
Table I.
Minimum Contact Time of Cl-DDMH, Cl-DADT, and Cl-TMPL to Provide a Total Kill of S. aureus and E. coli at Different Chlorine Content*
| Sample | Minimum contact time for a total kill at different chlorine content against S. aureus (min) | Minimum contact time for a total kill at different chlorine content against E. coli (min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10 ppm | 50 ppm | 200 ppm | 400 ppm | 1000 ppm | 10 ppm | 50 ppm | 200 ppm | 400 ppm | 1000 ppm | |
| Cl-DDMH | 15 | 5 | 5 | 5 | 5 | 15 | 5 | 5 | 5 | 5 |
| Cl-DADT | 120 | 15 | 5 | 5 | 5 | 60 | 30 | 5 | 5 | 5 |
| Cl-TMPL | ND** | ND | 120 | 120 | 60 | ND | ND | 120 | 120 | 60 |
Each test was repeated three times, and the longest minimum contact time of the three tests for a total kill of the microbes (the weakest antimicrobial efficacy observed) was reported.
ND: At this concentration, the sample could only provide a 2-3 log reduction of the test organisms; no total kill of the organisms could be observed within the 8-h testing period.
These differences can be caused by electronic effects. As pointed out by Worley and Williams,11 in N-halamine structures with a general formula of N(R1R2)-X (X= Cl or Br), X bears partial positive charge, and the strength of the N-X bond is affected by R1 and R2. In the case of the amine N-halamine Cl-TMPL, R1 and R2 are electron-donating -C-(CH3)2 groups, and these tend to destabilize any developing negative charge on N as X+ leaves the molecule. Thus, the N-X bond in Cl-TMPL has a high stability, leading to slow antimicrobial actions. On the other hand, in the structure of Cl-DDMH, the N-X group is connected to an electron-withdrawing carbonyl group, and this will stabilize the developing negative charge on N as X+ leaves the molecule, resulting in fast antimicrobial actions. Cl-DADT is an amine N-halamine; however, the electron-withdrawing, delocalized resonance structures of the triazine ring can substantially stabilize N-, and thus, considerably enhance antimicrobial activity.
It is interesting to note that Gram-positive bacteria and Gram-negative bacteria show similar sensitivity to a given class of N-halamines, as summarized in Table I. It has been reported that unlike that of Gram-positive bacteria, the cell wall of Gram-negative bacteria is overlaid with an outer membrane comprising lipopolysaccharide, which offers a supplementary barrier limiting or preventing penetration of a wide range of antimicrobial agents into the cell.25-27 Therefore, Gram-negative bacteria (e.g., E. coli) are normally more difficult to kill than Gram-positive bacteria (e.g., S. aureus). However, under our testing conditions, at the same available chlorine content, it takes the same contact time for Cl-DDMH to provide a total kill of either E. coli or S. aureus. The same trend is also observed in the testing of Cl-DADT or Cl-TMPL (see Table I). These results suggest that Cl+ may be too small for the lipopolysaccharide layer to block; thus, Cl+ can rapidly penetrate inside the charged Gram-positive or Gram-negative cells14 to provide antimicrobial effects.
Performances of Cl-DDMH, Cl-DADT and Cl-TMPL as antimicrobial additives
The performances of Cl-DDMH, Cl-DADT and Cl-TMPL as antimicrobial additives were evaluated with polyurethane (PU), one of the most versatile polymers in industrial, environmental, institutional and hygienic applications. To determine the suitability of the N-halamines as additives, various amounts of Cl-DDMH, Cl-DADT and Cl-TMPL were added into PU films, and the transparencies (λ=600 nm) of the samples were compared, as shown in Figure 7. Pure PU film has a transparency of 92.26 ± 2.6% (n=5). At 2% of additive content, the transparencies of the additive-containing films are very close to that of pure PU, suggesting good compatibility and little or no aggregation of Cl-DDMH, Cl-DADT or Cl-TMPL in PU at this additive concentration. With the further increase of additive contents to 4% and 6%, the transparencies of the films containing Cl-DDMH and Cl-DADT show only slight decrease, but those with Cl-TMPL reveals a substantial decrease, i.e., 71.6±2.3% with 4% of Cl-TMPL, and 58.3±0.6% with 6% of Cl-TMPL, indicating that Cl-TMPL has a low compatibility with PU at higher additive contents. Since the three additives have the same C-12 alkyl chain, this difference in compatibility with PU must be caused by the N-halamine-containing ring structures. That is, Cl-DDMH has a five-member ring with an imide carbonyl group and an amide carbonyl group, and CL-DADT has a triazine ring with three nitrogen atoms in the ring structure. Both rings can form strong dipole-dipole interactions and hydrogen bonds with the urethane groups and ether groups in PU, and this is expected to enhance intermolecular forces and thus improve compatibility of the additives with the PU polymer. On the other hand, the piperidinol ring of Cl-TMPL is less polar, and only the ester carbonyl group connecting the ring and alkyl chain can form hydrogen bonds/dipole-dipole interaction with PU polymer, which can be responsible for the relatively low compatibility between the additive and the polymer at higher Cl-TMPL contents.
Figure 7.
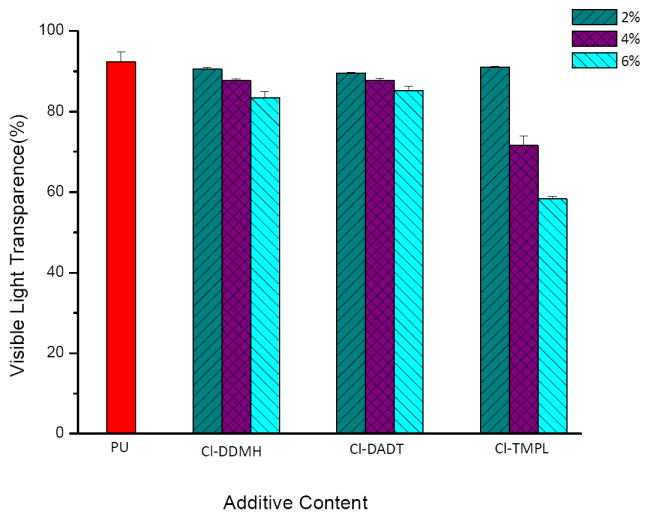
Visible light transparencies of pure PU film and PU films containing different amounts of Cl-DDMH, Cl-DADT, and Cl-TMPL.
The antimicrobial efficacies of PU films containing Cl-DDMH, Cl-DADT or Cl-TMPL were evaluated against S. aureus and E. coli. Pure PU films were used as controls, which did not show any detectable antimicrobial effects. On the other hand, all the additive-containing PU films demonstrated antimicrobial effects against the Gram-positive and Gram-negative bacteria, but to different extents, as shown in Table II. Among the three additives, Cl-DDMH again shows the most potent antimicrobial action when used as an additive in PU: at 2% of Cl-DDMH content, the film provides a total kill of 108-109 CFU/mL of S. aureus and E. coli after only 5 min of contact. Further increase in Cl-DDMH content to 6% has little effect on minimum contact time for a total kill, suggesting that with 2% of Cl-DDMH, the available chlorine level is already high enough; the rate- determining step in antimicrobial action is not chlorine level but other factors (e.g., contact between the polymer and the microbes, rate of the chorine transfer reaction between Cl-DDMH and the bacteria, bacterial response to the transferred chlorine, etc). Cl-TMPL has the lowest antimicrobial activity: with 2% or 4% of Cl-TMPL, the films could only provide a 2-3 log reduction of the test organisms; no total kill of the organisms could be observed within the 8-h testing period. When the Cl-TMPL content is increased to 6%, a total kill of S. aureus or E. coli is detected after 240 min of contact. The low antimicrobial efficacy can be attributable to the following factors: (1) Cl-TMPL is a less potent antimicrobial agent than Cl-DDMH and Cl-DADT (see Table I), and (2) Cl-TMPL has low compatibility with PU (see Figure 7): at 4% or 6% of additive content, Cl-TMPL aggregates and forms its own domain. Thus, not all the added Cl-TMPL can make contact with the microbes to provide antimicrobial effects, further reducing antimicrobial potency. Again, as an additive, Cl-DADT provides a slightly lower antimicrobial effect than that of Cl-DDMH, but its activity is much more potent than that of Cl-TMPL.
Table II.
Minimum Contact Time of Cl-DDMH, Cl-DADT, and Cl-TMPL as Additives in PU to Provide a Total Kill of S. aureus and E. coli*
| Sample | Minimum contact time for a total kill at different additive level against S. aureus (min) | Minimum contact time for a total kill at different additive level against E. coli (min) | ||||
|---|---|---|---|---|---|---|
| 2 % | 4 % | 6 % | 2 % | 4 % | 6 % | |
| Cl-DDMH | 5 | 5 | 5 | 5 | 5 | 5 |
| Cl-DADT | 30 | 15 | 5 | 30 | 30 | 5 |
| Cl-TMPL | ND** | ND | 240 | ND | ND | 240 |
Each test was repeated three times, and the longest minimum contact time of the three tests for a total kill of the microbes (the weakest antimicrobial efficacy observed) was reported.
ND: At this concentration, the sample could only provide a 2-3 log reduction of the test organisms; no total kill of the organisms could be observed within the 8 h of testing period.
Because all the additive-containing PU films are able to provide antimicrobial effects, it is highly possible that they can prevent the formation and development of biofilms, a serious problem in a broad range of applications.1,2 To confirm the biofilm-controlling activity, PU films containing different amounts of Cl-DDMH, Cl-DADT or Cl-TMPL were exposed to 107-108 CFU/mL of S. aureus for 30 min to allow bacteria initial adhesion. Afterwards, the resultant films were immersed in the corresponding broth solutions for 24 h to facilitate microbial colonization and biofilm formation. SEM results showed that PU films containing higher than 2% of Cl-DDMH or Cl-DADT completely prevented biofilm formation. In the case of PU films containing CL-TMPL, however, only at higher than 4% of additive content could biofilm-controlling effect be observed. As an example, Figure 8 presents the representative SEM results of pure PU films (the control) and PU films containing 4% of Cl-DDMH, Cl-DADT or Cl-TMPL after biofilm testing: a large amount of adherent S. aureus can be detected on the surface of the pure PU film (Figure 8, A). The bacteria cells aggregate together and form several layers, suggesting formation of biofilms. PU films containing Cl-DDMH or Cl-DADT show much cleaner surfaces, and almost no adherent bacteria can be observed (Figure 8, B and C). On the surface of PU films containing 4% of Cl-TMPL (Figure 8, D), although scattered adherent bacteria can be observed, the adherent level is much lower than that of the control, and no biofilms are formed. The biofilm-controlling function is clearly related to the antimicrobial effects of the additives: when bacteria came into contact with the films, most of them were inactivated during and/or after adherence/colonization, resulting in cleaner surfaces. It might also be possible that when bacteria were approaching to the films, they retreated rapidly when they sensed that the surface was inappropriate for adhesion.28
Figure 8.
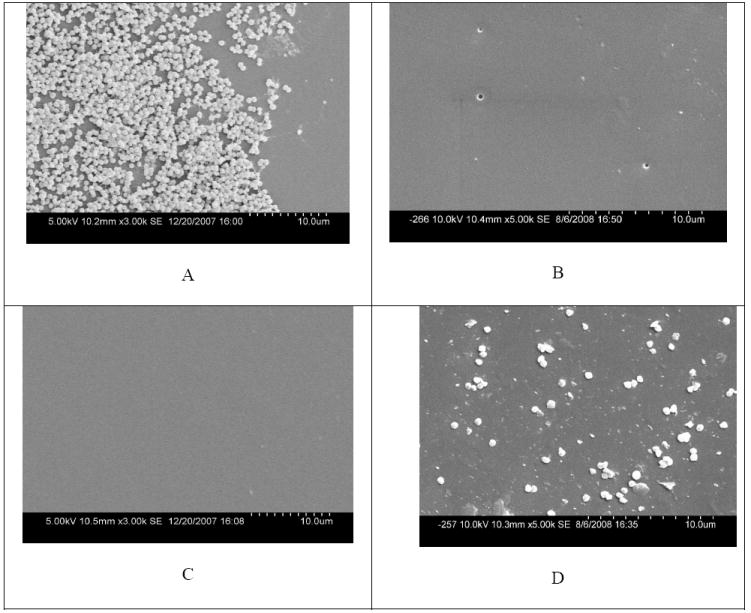
Representative SEM results of (A) pure PU film, (B) PU film containing 4 % Cl-DDMH, (C) PU film containing 4 % Cl-DADT, and (D) PU film containing 4 % Cl-TMPL after immersing in S. aureus for 24 h
In stability studies, a series of PU films (ca. 1×1 cm) containing 6% of Cl-DDMH, Cl-DADT, or Cl-TMPL were immersed individually in 10 mL deionized water under constant shaking at ambient temperature. After a certain period of time, the films were taken out, and the active chlorine levels in the immersing solutions were iodometrically titrated to determine the extent of chlorine release from the additive-containing films into the surrounding environments. As shown in Figure 9, PU film containing Cl-DDMH shows the fastest chlorine release: after 72 h, 0.49 μg/ml of active chlorine is released. Under the same condition, PU films with Cl-DADT release 0.39 μg/ml of chlorine, and those with Cl-TMPL release 0.28 μg/ml of chlorine. Again, this trend can be explained by the electronic effects, i.e., electron-withdrawing groups (in the cases of Cl-DDMH and Cl-DADT) can stabilize N-, resulting in fast chlorine release; electron-donating groups (in the case of Cl-TMPL) can destabilize N- and reduce chlorine release rate. Since chlorine release rate is directly related to the antimicrobial duration of the samples, in long-term applications, PU films with the same amount of each additives are expected to have the chlorine stability (and thus the antimicrobial duration) order of Cl-TMPL>Cl-DADT>Cl-DDMH. On the other hand, it should be noted that the active chlorine levels released from any of the additive-containing PU films are much lower than the current EPA Maximum Residual Disinfectant Level (MRDL) in drinking water of 4 ppm, pointing to excellent potentials of Cl-DDMH, Cl-DADT, or Cl-TMPL for a wide range of related applications.
Figure 9.
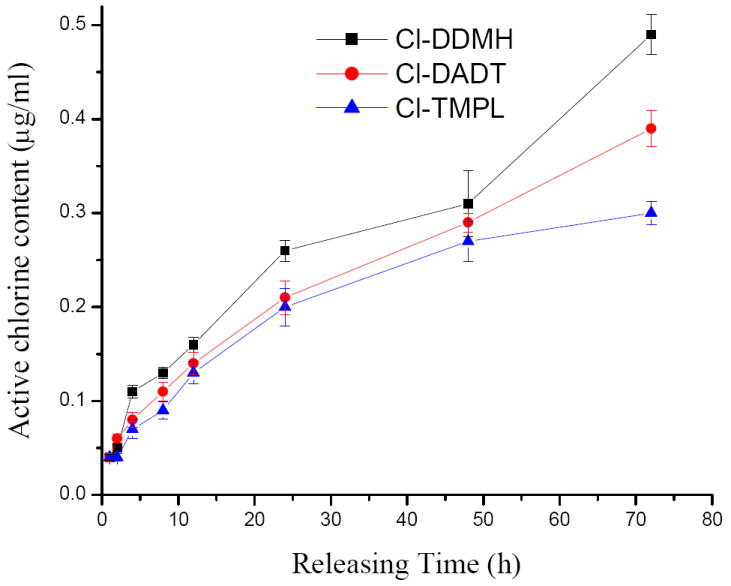
Active chlorine level in the immersing solution after different releasing periods from PU films containing 6% of Cl-DDMH, CL-DADT, or Cl-TMPL
Conclusion
An amine N-halamine antimicrobial additive, N-chloro-2,2,6,6-tetramethyl-4-piperidinol laurate (Cl-TMPL), was synthesized and characterized in this study. The antimicrobial efficacy of Cl-TMPL was compared with those of 1-chloro-3-dodecyl-5,5-dimethylhydantoin (Cl-DDMH), a amide N-halamine with the same C-12 alkyl chain, and chloro-2,4-diamino-6-dodecyl-1,3,5-triazine (Cl-DADT), a melamine (imino) N-halamine with the same C-12 alkyl chain. Due to the structural differences in the N-halamine moieties, Cl-DDMH and Cl-DADT showed much faster antimicrobial action than Cl-TMPL. All three classes of N-halamines could serve as antimicrobial and biofilm-controlling additives in PU, one of the most versatile polymers in a broad range of applications. Cl-DDMH and Cl-DADT had higher compatibility with PU, and provided more powerful antimicrobial and anti-biofilm effects than Cl-TMPL. On the other hand, PU samples with Cl-TMPL released the lowest amount of active chlorine into the surrounding environment, pointing to the highest stability of the antimicrobial and biofilm-controlling effects. These results shed lights on the selection of amide, melamine, or amine N-halamine additives in designing antimicrobial and biofilm-controlling PU and other related polymeric materials for a broad range of industrial, environmental, institutional, and hygienic applications.
Acknowledgments
This study was sponsored by NIH, NIDCR (Grant number R01DE018707).
Literature Cited
- 1.Stoodley LH, Costerton JW, Stoodley P. Nature Rev. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 2.An YH, Friedman RJ, editors. Handbook of bacterial adhesion principles, methods, and applications. New York: Humana press; 2000. [Google Scholar]
- 3.Chen ZB, Sun YY. Macromolecules. 2005;38:8116–8119. [Google Scholar]
- 4.Sun YY, Chen ZB, Braun M. Ind Eng Chem Res. 2005;44:7916–7920. [Google Scholar]
- 5.Sun YY, Sun G. Macromolecules. 2002;35:8909–8912. [Google Scholar]
- 6.Ren X, Zhu C, Kou L, Worley SD, Kocer HB, Broughton RM, Huang TS. J Bioact Compat Polym. 2010;25:392–405. [Google Scholar]
- 7.Sun X, Zhang L, Cao Z, Deng Y, Liu L, Fong H, Sun Y. ACS Appl Mater Interface. 2010;2:952–956. doi: 10.1021/am100018k. [DOI] [PubMed] [Google Scholar]
- 8.Ahearn DG, Grace DT, Jennings MJ, Borazjani RN, Boles KJ, Rose LJ, Simmons RB, Ahanotu EN. Curr Microbiol. 2000;41:120–125. doi: 10.1007/s002840010105. [DOI] [PubMed] [Google Scholar]
- 9.Sun YY, Sun G. Ind Eng Chem Res. 2004;43:5015–5020. [Google Scholar]
- 10.Chen ZB, Sun YY. Ind Eng Chem Res. 2006;45:2634–2640. doi: 10.1021/ie060088a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Worley SD, Williams DE. Crit Rev Env Contr. 1988;18:133–175. [Google Scholar]
- 12.Dagostin VS, Golçalves DL, Pacheco CB, Almeida WB, Thomé IP, Pich CT, Paula MMS, Silva L, Angioletto E, Fiori MA. Mater Sci Eng C. 2010;30:705–708. [Google Scholar]
- 13.Fiori MA, Paula MMS, Bernardin AM, Riella HG, Angioletto E. Mater Sci Eng C. 2009;29:1569–1573. [Google Scholar]
- 14.Wiese A, Munstermann M, Gutsmann T, Lindner B, Kawahara K, Zahringer U, Seydel U. J Membr Biol. 1998;162:127–138. doi: 10.1007/s002329900350. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Luo J, Sun Y. Biomaterials. 2007;28:1597–1609. doi: 10.1016/j.biomaterials.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun YY, Sun G. J Appl Polym Sci. 2003;88:1032–1039. [Google Scholar]
- 17.Sun X, Cao Z, Sun YY. Ind Eng Chem Res. 2009;48:607–612. [Google Scholar]
- 18.Zakrzewski J. Synth Commu. 1988;18:2135–2140. [Google Scholar]
- 19.Toshikazu Kurosaki KWLMO. J Polym Sci part A-1, Polym Chem. 1972;10:3295–3310. [Google Scholar]
- 20.Heasley VL, Kovacic P, Lange RM. J Org Chem. 1966;31:3050–3052. [Google Scholar]
- 21.Moore GE, Badger RM. J Am Chem Soc. 1952;74:6076–6080. [Google Scholar]
- 22.Pan J, Yang Z, Zhang T, Lau W, Lee CS. Polym Degrad Stab. 1994;44:85–91. [Google Scholar]
- 23.Lee CS, Lau WWY, Lee SY, Goh SH. J Polym Sci part A, Polym Chem. 1992;30:983–988. [Google Scholar]
- 24.Pan JQ, Lau WWY, Lee CS. J Polym Sci part A, Polym Chem. 1994;32:997–1000. [Google Scholar]
- 25.Denver SP. Int Biodet Biodeg. 1995;36:227–245. [Google Scholar]
- 26.Russell AD. Int Biodet Biodeg. 1995;36:247–265. [Google Scholar]
- 27.Denver SP, Stewart GSAB. Int Biodet Biodeg. 1998;41:261–268. [Google Scholar]
- 28.Williams JF, Worley SD. J Endourol. 2000;14:395–400. doi: 10.1089/end.2000.14.395. [DOI] [PubMed] [Google Scholar]


