Abstract
Unexpandable lung is the inability of the lung to expand to the chest wall allowing for normal visceral and parietal pleural apposition. It is the direct result of either pleural disease, endobronchial obstruction resulting in lobar collapse, or chronic atelectasis. Unexpandable lung occurring as a consequence of active or remote pleural disease may present as a post-thoracentesis hydropneumothorax or an effusion that cannot be completely drained because of the development of anterior chest pain. Pleural manometry is useful for identifying unexpandable lung during initial pleural drainage. Unexpandable lung occurring as a consequence of active or remote pleural disease may be separated into two distinct clinical entities termed trapped lung and lung entrapment. Trapped lung is a diagnosis proper and is caused by the formation of a fibrous visceral pleural peel (in the absence of malignancy or active pleural inflammation). The mechanical effect of the pleural peel constitutes the primary clinical problem. Lung entrapment may result from a visceral pleural peel secondary to active pleural inflammation, infection, or malignancy. In these cases, the underlying malignant or inflammatory condition is the primary clinical problem, which may or may not be complicated by unexpandable lung due to visceral pleural involvement. The recognition of trapped lung and lung entrapment as related, but distinct, clinical entities has direct consequences on clinical management. In our practice, pleural manometry is routinely performed during therapeutic thoracentesis and is useful for identification of unexpandable lung and has allowed us to understand the mechanisms surrounding a post-thoracentesis pneumothorax.
Introduction and context
Unexpandable lung is a mechanical complication resulting in the inability of the lung to expand to the chest wall. In clinical practice, an unexpandable lung may be due to either the presence of pleural disease, endobronchial obstruction resulting in lobar collapse, or chronic atelectasis [1]. Unexpandable lung due to pleural disease is clinically obvious when a pneumothorax develops following thoracentesis or when chest pain develops during or following pleural fluid removal. However, with the application of pleural manometry, all cases of unexpandable lung can be accurately diagnosed.
Recent advances
The term trapped lung has traditionally been used interchangeably to describe an unexpandable lung occurring from either active or remote pleural disease. Currently, we have adopted a more strict definition for a trapped lung. Trapped lung is the sequela of remote pleural space inflammation resulting in the development of a mature, fibrous membrane that impedes lung expansion during fluid removal (Figure 1). In other words, trapped lung can be considered to be defective pleural healing once inflammation in the pleural space has resolved. In contrast, lung entrapment is considered a complication of active pleural inflammation, malignancy, or hemothorax. Identifying active pleural inflammation or malignancy is critical in differentiating lung entrapment from trapped lung. Clinical conditions associated with the development of lung entrapment include coronary artery bypass graft surgery, post cardiac injury syndrome, empyema/complicated parapneumonic effusions, uremia, radiation therapy, and rheumatoid pleurisy [1-3].
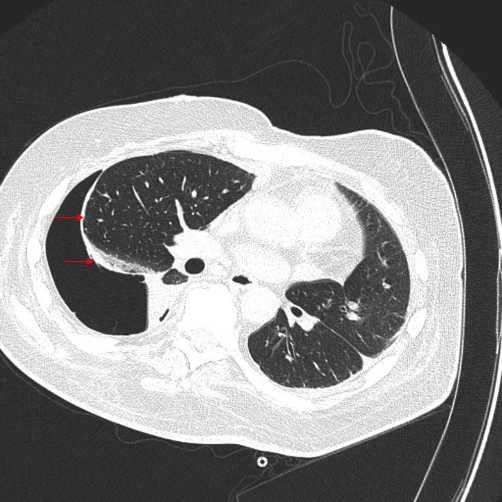
Figure 1. Computed tomography scan showing abnormal visceral pleural thickening.
This is an air-contrast chest computed tomography scan showing abnormal visceral pleural thickening (arrows) in the setting of lung entrapment from a resolving hemothorax.
In the setting of lung entrapment, chest radiography may reveal contra-lateral mediastinal shift. Pleural fluid analysis is typically exudative by both protein and lactate dehydrogenase (LDH) criteria; however, discordant protein and LDH exudates can occur as the inflammatory process is resolving. An increased total nucleated cell count with either a lymphocyte or neutrophil predominance is observed depending upon the etiology of the lung entrapment.
Treatment of lung entrapment is dictated by the underlying process that resulted in the unexpandable lung. Rapid evacuation of a hemothorax or prompt antibiotic therapy for pneumonia with parapneumonic effusion usually prevents the development of trapped lung following these conditions. If dysfunctional pleural space healing occurs, the end result is a trapped lung. However, in the setting of malignancy, the presence of lung entrapment will significantly decrease the success of pleurodesis [2].
Pleural manometry remains the method of choice for detecting unexpandable lung [3-9]. Standardization of pleural manometry was described by Doelken and colleagues [4]. The measurement of pleural liquid pressure can be performed either by using a simple water column manometer or with elaborate electronic, physiologic systems connected to a standard hemodynamic transducer [4]. A simple, over-damped water manometer can be easily employed by all physicians performing therapeutic thoracentesis.
Measuring pleural liquid pressure may improve patient safety and reduce the development of re-expansion pulmonary edema (RPE). RPE is a well-recognized complication of therapeutic thoracentesis and is characterized by the development of unilateral pulmonary edema in a lung re-inflated rapidly after a period of collapse from either a pleural effusion or pneumothorax. The clinical presentation of RPE ranges from relatively benign to life-threatening. Historical recommendations to prevent RPE suggest that fluid removal should not exceed 1-1.5 liters at a single sitting. Current recommendations to terminate thoracentesis include the development of excessively negative pleural pressures or the development of chest pain [3-9].
In addition to providing improved patient safety, pleural manometry is useful for the diagnosis of an unexpandable lung at the bedside and for predicting pleurodesis success. Pressure measured with a catheter residing in a pleural effusion is representative of the actual pressure at that level of the effusion. Changes in pressure during respiration represent pressure changes throughout the effusion. The pressure measured is reflective of the recoil pressures of the lung and chest wall and the vertical extent of the pleural effusion. The measured pressure changes are reflective of the changes of the recoil forces, and pleural space elastance (PEL) can be calculated (PEL = change in pleural liquid pressure in cm H2O per liter of fluid removed).
Three pressure/volume curves are typically encountered. The pressure/volume curve of a patient with an expandable lung is monophasic with a normal elastance, defined as a PEL ≤14.5 cm H2O/L [3,5,8-10]. Terminal negative pressure deflections are seen when minimal amounts of pleural fluid remain and are attributed to local deformation forces around the catheter. The pressure/volume curve of a patient with a trapped lung is monophasic with an elevated PEL >14.5 cm H2O/L. Finally, a biphasic pressure/volume curve demonstrates a normal PEL prior to an inflection point and a high PEL in the terminal portion of the pressure/volume curve. The presence of a biphasic pressure/volume curve is consistent with lung entrapment assuming that the clinical history and pleural fluid analysis supports a finding of malignancy, infection, or inflammation (Figure 2).
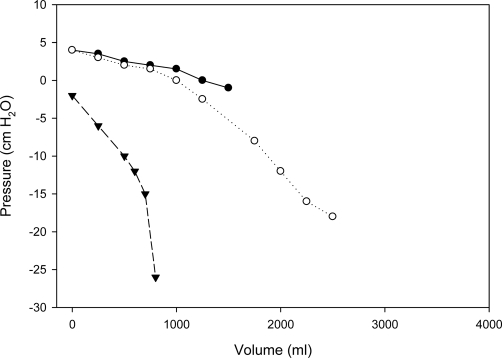
Figure 2. A normal elastance curve, a biphasic curve (malignancy), and a curve from a trapped lung (remote complicated parapneumonic effusion).
Three pressure/volume curves are shown. The curve denoted by the solid circles represents a monophasic pressure/volume curve with normal pleural elastance predicting normal lung expansion. This was a case of hepatic hydrothorax. The curve denoted by open circles is a biphasic pressure/volume curve from a patient with malignant lung entrapment. Note that the calculated pleural space elastance (PEL) prior to the inflection point is normal, while the calculated PEL after the inflection point is increased, predicting abnormal lung expansion. This was a case of a patient with a malignant pleural effusion. The curve denoted by the solid triangles represents a monophasic pressure/volume curve with increased PEL. This shows a trapped lung resulting from a remote parapneumonic effusion.
Implications for clinical practice
Unexpandable lung resulting from an endobronchial obstruction is managed by removing either the tumor or foreign body impeding normal lung expansion. In the setting of chronic atelectasis, the lung may re-expand over several days as long as the underlying lung parenchyma is devoid of significant fibrosis. Most patients with trapped lung are asymptomatic; however, it may be the cause of significant restriction and dyspnea. In this case, the only effective therapy would be surgical decortication only after other causes of dyspnea are excluded. In contrast, the focus of initial treatment for lung entrapment is dependent on the nature of the active process. For example, antibiotics and pleural drainage are required for treating a complicated parapneumonic effusion and pleural drainage is required for hemothorax evacuation. In contrast, lung entrapment in the setting of a malignant pleural effusion can only be successfully managed with a chronic, indwelling pleural catheter.
Finally, we have obtained better insights into the mechanism by which a pneumothorax occurs following ultrasound-guided thoracentesis and why chest tubes can be safely removed despite the presence of an air leak or pneumothorax. Pneumothorax following ultrasound-guided thoracentesis is a rare complication. The development of pneumothorax is commonly due to the drainage itself and is not related to direct puncture of the visceral pleural or to the introduction of air into the pleural space via the drainage system. The pathophysiology of drainage-related pneumothorax is the result of transient, pressure-dependent, parenchymal-pleural fistulas, which develop as a consequence of an existing unexpandable lung unable to conform to the shape of the chest wall with fluid removal [11]. This pathophysiologic mechanism is further supported by the safety data reported by Cerfolio and colleagues [12] on removing chest tubes in post-lobectomy patients despite the presence of a persistent air leak or a pneumothorax.
Abbreviations
- LDH
lactate dehydrogenase
- PEL
pleural space elastance
- RPE
re-expansion pulmonary edema
Competing Interests
The authors declare that they have no competing interests.
The electronic version of this article is the complete one and can be found at: http://f1000.com/reports/m/2/77
References
- 1.Doelken P, Sahn SA. Trapped lung. Sem Respir Crit Care Med. 2001;22:631–5. doi: 10.1055/s-2001-18799. [DOI] [PubMed] [Google Scholar]
- 2.Lan RS, Lo SK, Chuang ML, Yang CT, Tsao TC, Lee CH. Elastance of the pleural space: a predictor for the outcome of pleurodesis in patients with malignant pleural effusion. Ann Intern Med. 1997;126:768–74. doi: 10.7326/0003-4819-126-10-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 3.Huggins JT, Sahn SA, Heidecker J, Ravenel JG, Doelken P. Characterisitics of trapped lung: pleural fluid analysis, manometry, and air-contrasted CT. Chest. 2007;131:206–13. doi: 10.1378/chest.06-0430. [DOI] [PubMed] [Google Scholar]
- 4.Doelken P, Huggins JT, Pastis NJ, Sahn SA. Pleural manometry: technique and clinical implications. Chest. 2004;126:1764–9. doi: 10.1378/chest.126.6.1764. [DOI] [PubMed] [Google Scholar]
- 5.Huggins JT, Doelken P. Pleural manometry. Clin Chest Med. 2006;27:229–40. doi: 10.1016/j.ccm.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Villena V, López-Encuentra A, Pozo F, De-Pablo A, Martín-Escribano P. Measurement of pleural pressure during therapeutic thoracentesis. Am J Respir Crit Care Med. 2000;162:1534–8. doi: 10.1164/ajrccm.162.4.9907047. [DOI] [PubMed] [Google Scholar]
- 7.Light RW, Standbury DW, Brown SE. The relationship between pleural pressures and changes in pulmonary function after therapeutic thoracentesis. Am Rev Respir Dis. 1986;133:658–61. doi: 10.1164/arrd.1986.133.4.658. [DOI] [PubMed] [Google Scholar]
- 8.Feller-Kopman D, Parker MJ, Schwartzstein M. Assessment of pleural pressure in the evaluation of pleural effusions. Chest. 2009;135:201–9. doi: 10.1378/chest.08-1788. [DOI] [PubMed] [Google Scholar]
- 9.Feller-Kopman D. Therapeutic thoracentesis: the role of ultrasound and pleural manometry. Curr Opin Pulm Med. 2007;13:312–8. doi: 10.1097/MCP.0b013e3281214492. [DOI] [PubMed] [Google Scholar]
- 10.Feller-Kopman D, Walkey A, Berkowitz D, Ernst A. The relationship of pleural pressure to symptom development during therapeutic thoracentesis. Chest. 2006;129:1556–60. doi: 10.1378/chest.129.6.1556. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Steven Sahn 24 August 2010
- 11.Heidecker J, Huggins JT, Sahn SA, Doelken P. Pathophysiology of pneumothorax following ultrasound-guided thoracentesis. Chest. 2006;130:1173–84. doi: 10.1378/chest.130.4.1173. [DOI] [PubMed] [Google Scholar]
- 12.Cerfolio RJ, Minnich DJ, Bryant AS. The removal of chest tubes despite an air leak or a pneumothorax. Ann Thorac Surg. 2009;87:1690–6. doi: 10.1016/j.athoracsur.2009.01.077. [DOI] [PubMed] [Google Scholar]