Abstract
Coxiella burnetii, the etiological agent of human Q fever, occupies a unique niche inside the host cell, where it replicates in a modified acidic phagolysosome or parasitophorous vacuole (PV). The PV membrane is cholesterol-rich, and inhibition of host cholesterol metabolism negatively impacts PV biogenesis and pathogen replication. The precise source(s) of PV membrane cholesterol is unknown, as is whether the bacterium actively diverts and/or modifies host cell cholesterol or sterol precursors. C. burnetii lacks enzymes for de novo cholesterol biosynthesis; however, the organism encodes a eukaryote-like Δ24 sterol reductase homolog, CBU1206. Absent in other prokaryotes, this enzyme is predicted to reduce sterol double bonds at carbon 24 in the final step of cholesterol or ergosterol biosynthesis. In the present study, we examined the functional activity of CBU1206. Amino acid alignments revealed the greatest sequence identity (51.7%) with a Δ24 sterol reductase from the soil amoeba Naegleria gruberi. CBU1206 activity was examined by expressing the protein in a Saccharomyces cerevisiae erg4 mutant under the control of a galactose-inducible promoter. Erg4 is a yeast Δ24 sterol reductase responsible for the final reduction step in ergosterol synthesis. Like Erg4-green fluorescent protein (GFP), a CBU1206-GFP fusion protein localized to the yeast endoplasmic reticulum. Heterologous expression of CBU1206 rescued S. cerevisiae erg4 sensitivity to growth in the presence of brefeldin A and cycloheximide and resulted in new synthesis of ergosterol. These data indicate CBU1206 is an active sterol reductase and suggest the enzyme may act on host sterols during C. burnetii intracellular growth.
The intracellular Gram-negative bacterium Coxiella burnetii is the causative agent of the zoonosis Q fever. Inside the host cell, C. burnetii thrives in a unique parasitophorous vacuole (PV) that is considered “phagolysosome-like” due to its moderate acidity (pH ∼5), the presence of active hydrolytic enzymes, and labeling with lysosomal markers (14, 21). Proteins secreted by a specialized Dot/Icm type IV secretion system (T4SS) are thought to modify the PV to support pathogen replication (21). The C. burnetii PV promiscuously fuses with endosomes and lysosomes; however, it does not appear to intercept Golgi body-derived vesicles or to closely associate with the endoplasmic reticulum (ER) (4, 12).
The C. burnetii PV membrane is structurally strong and contains lipid raft proteins such as flotillin, characteristics that likely reflect its high cholesterol content (13). Cholesterol is a critical component of cellular membranes, where it provides structural stability and platforms for signaling proteins. Cholesterol is also a precursor for a variety of signaling molecules (8). Intracellular pathogens exploit cholesterol as sources of energy (6, 19) and membrane lipid (7) and interact with cholesterol to manipulate host cell trafficking (10, 25). Indeed, we have previously shown that pharmacological inhibition of host cell cholesterol biosynthesis or uptake blocks C. burnetii PV formation and growth (13), suggesting a critical role for sterols in C. burnetii pathogenesis.
Cholesterol synthesis occurs in the ER through a complex series of enzymatic reactions, with the final and required step being the reduction of the carbon-24 bond by a Δ24 sterol reductase (Fig. 1). Mutations in the human Δ24 sterol reductase DHCR24 result in desmosterolosis, where the absence of cholesterol results in severe developmental and neurological problems (24). Interestingly, analysis of the C. burnetii genome revealed two genes encoding putative sterol reductases: CBU1158 and CBU1206 (3, 20). Here, we utilize heterologous expression in Saccharomyces cerevisiae to demonstrate that CBU1206 is an active Δ24 sterol reductase.
FIG. 1.
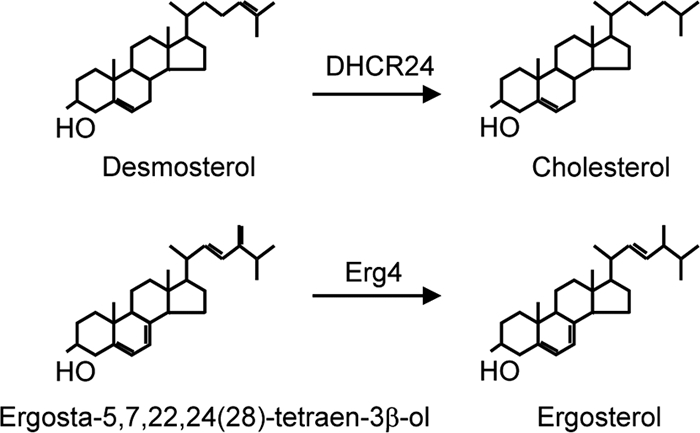
Schematic showing reduction of carbon-24 double bonds by Δ24 sterol reductases. In mammalian cells, the final step in cholesterol synthesis is reduction of the C24 bond in desmosterol by DHCR24, a Δ24 sterol reductase. Ergosterol is the final sterol in yeast, with the Erg4 Δ24 sterol reductase catalyzing the reduction of ergosta-5,7,22,24(28)-tetraen-3β-ol.
MATERIALS AND METHODS
Reagents.
Brefeldin A, cycloheximide, ergosterol, and high-performance liquid chromatography (HPLC)-grade methanol, acetonitrile, and hexane were from Sigma-Aldrich (St. Louis, MO). Yeast growth medium was obtained from Clontech (Mountain View, CA).
Construction of yeast expression plasmids.
All PCRs were conducted using Accuprime Pfx DNA Polymerase (Invitrogen, Carlsbad, CA) and gene-specific primers (Integrated DNA Technologies, Coralville, IA) (Table 1).
TABLE 1.
Primers used in this study
| Primer | Sequence (5′-3′) |
|---|---|
| YES-DEST52recFforGFP | TGAGTTTAAACCCGCTGATCCTAGAGGGC |
| YES-DEST52recRforGFP | ACCTTCGAAGGGCCCTCTAGATCGAACC |
| YES-GFP-recF | GGGCCCTTCGAAGGTATGGCTAGCAAAGGAGAAGAAC |
| YES-GFP-recR | GCGGGTTTAAACTCATTATTTGTAGAGCTCATCCATGC |
| yeastpENTR1206for | CACCAATAATGTCTCGCGTTACCCCACTTGATCAAAAATT |
| CBU1206rev | AAAAATAAACGGAATAAATAACCAAGG |
| yeastpENTRerg4for | CACCAATAATGGCAAAGGATAATAGTGAGAAGCTG |
| Erg4rev | GAAAACATAAGGAATAAAGACGTAAGGG |
| yeastpENTRDHCR24for | CACCAATAATGTCTGAGCCCGCCGTGTCGCTGGCC |
| DHCR24rev | GTGCCTGGCGGCCTTGCAGATC |
The pYES-DEST52 C-terminal V5/6×His tags (Invitrogen) were replaced with green fluorescent protein (GFP) to generate pYES-DEST-GFP. Briefly, the pYES-DEST52 backbone was amplified by inverse PCR using the primers YES-DEST52recFforGFP and YES-DEST52recRforGFP. GFP was amplified from pcDNA-DEST47 (Invitrogen) using the primers YES-GFP-recF and YES-GFP-recR. The resulting PCR products were gel purified by using a QiaQuick gel extraction kit (Qiagen, Valencia, CA). Equal amounts (150 ng) of each PCR product were ligated by using an In-Fusion reaction (Clontech) according to the manufacturer's protocol. Two microliters of the In-Fusion reaction was used to transform Escherichia coli One Shot ccdB Survival 2 T1R cells (Invitrogen). Transformants were selected on ampicillin, and individual clones were isolated and sequenced.
For generation of CBU1206 fusion constructs, the complete open reading frame of CBU1206 (accession AAO90715.2) was amplified from genomic DNA of the C. burnetii Nine Mile (RSA439) strain using the primers yeastpENTR1206for and CBU1206rev. Wild-type ERG4 was amplified from yeast wild-type genomic DNA (a generous gift of Gabriel Shaaf, Universität Tübingen, Tübingen, Germany) using the primers yeastpENTRerg4for and Erg4rev. The human Δ24 sterol reductase gene DHCR24 was amplified from the cDNA plasmid pCMV6-AC-DHCR24 (OriGene, Rockville, MD) using the primers yeastpENTRDHCR24for and DHCR24rev. All forward primers contained the sequence CACC for directional cloning into pENTR-D/TOPO (Invitrogen), and the consensus start sequence for CBU1206 and DHCR24 was changed to AATAATGTCT for optimal expression in yeast. PCR products were gel purified and cloned into pENTR-D/TOPO. Homologous recombination subcloning using LR Clonase II (Invitrogen) was utilized to move the PCR inserts into the galactose-inducible yeast plasmids pYES-DEST52 and pYES-DEST52-GFP. All plasmid constructs were confirmed by DNA sequencing.
Yeast strains and culture conditions.
The wild-type yeast parental strain BY4741 (MATa his3Δ1 leu2Δ met15Δ ura3Δ) and erg4 deletion strain YGL012W BY4741 (MATa his3Δ1 leu2Δ met15Δ ura3Δ erg4Δ) were obtained from Invitrogen. Yeast expressing a Sec13-red fluorescent protein (RFP) fusion protein was a generous gift of Peter Arvidson and Erin O'Shea, Harvard University (15). Yeast strains were grown on rich yeast-peptone-dextrose (YPD) agar at 30°C. Genetic transformation of yeast was conducted by using the lithium acetate/polyethylene glycol procedure (11). Transformants were selected on uracil-free minimal medium containing 2% glucose (−Ura/Glu). For galactose-induced gene expression, overnight cultures (30°C, 220 rpm) in −Ura/Glu broth were centrifuged at 6,000 × g for 5 min, and then the pellet resuspended to 0.4 optical density (OD) units/ml in uracil-free minimal medium containing 2% galactose (−Ura/Gal). For growth curves, cultures were induced for the entire time course. For immunoblotting, drug sensitivity testing, and sterol analysis, cultures were induced for 6 h prior to the assay.
Immunoblotting.
Broth cultures of uninduced and galactose-induced yeast transformed with pYES-DEST52 constructs were centrifuged at 6,000 × g for 5 min, and then the yeast pellet was suspended in water. An equal volume of fresh 0.2 M NaOH was added, and the samples were incubated for 5 min at room temperature. Treated yeast were then centrifuged at 15,000 × g for 15 min, and the pellet was suspended in 1× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Samples were heated at 99°C for 3 min, and the equivalent of 1 OD unit was separated by SDS-10% PAGE. The proteins were transferred to nitrocellulose, and the membrane was blocked for 1 h in Tris-buffered saline (150 mM NaCl, 100 mM Tris-HCl [pH 7.6]) containing 0.1% Tween 20 and 5% milk. The membrane was probed with mouse anti-V5 (Invitrogen) and anti-mouse immunoglobulin G secondary antibody conjugated to horseradish peroxidase (HRP) (Thermo Scientific, Rockford, IL). After development via chemiluminescence (Thermo Scientific), the membrane was treated for 1 h with 0.1% azide in phosphate-buffered saline (PBS; 1 mM KH2PO4, 155 mM NaCl, 3 mM Na2HPO4 [pH 7.2]) to kill the chemiluminescent signal and then reprobed with rat anti-alpha tubulin (Abcam, Cambridge, MA) and anti-rat-HRP (Thermo Scientific) to visualize alpha-tubulin as a loading control.
Fluorescence microscopy.
Yeast transformed with pYES-DEST52-GFP constructs were induced as described above in uracil minimal medium and then pelleted. Pelleted cells were suspended in 4% paraformaldehyde-PBS and incubated for 1 h at room temperature. Fixed cells were then washed three times with PBS, spotted onto glass coverslips, and visualized by using a modified Perkin-Elmer UltraView spinning disk confocal system connected to a Nikon Eclipse TE2000-S microscope, with a ×60 objective. Images were acquired by using Metamorph software (Molecular Devices, Downingtown, PA).
Drug sensitivity testing.
Transformed yeast were induced with galactose, diluted to 0.1 OD unit/ml, and then diluted further by two serial 10-fold dilutions. A portion (5 μl) of each dilution was then spotted onto YPD plates (wild-type and erg4 strains) or uracil-free galactose minimal medium plates (complemented erg4 strains) containing either ethanol, 100 μg of brefeldin A/ml (50 mg/ml stock in ethanol), or 0.05 μg of cycloheximide/ml (1 mg/ml stock in water). Plates were incubated for 2 days at 30°C.
Sterol analysis.
Transformed yeast were induced with galactose, and then the cells were pelleted by centrifugation. Sterols were extracted and saponified for 2 h at 85°C in a solution comprised of 2.5 ml of 0.2% pyrogallol (freshly made in methanol) and 1 ml of 60% potassium hydroxide. Sterols were extracted three times with 2.5 ml of hexane and dried under a nitrogen stream. Sterols were then resuspended in a small amount of methanol-acetonitrile (50:50) and analyzed by HPLC using a C18 reversed-phase column (Zorbax Eclipse XDB-C18, 4.6 by 150 mm; Agilent, Santa Clara, CA) thermostated at 51°C, with a mobile phase of methanol-acetonitrile (50:50) at 1 ml/min. Sterol fractions were detected with an evaporative light scattering detector set at 60°C. The retention time of ergosterol was determined by using a commercially available standard (Sigma).
RESULTS AND DISCUSSION
CBU1206 is closely related to an amoebal sterol reductase.
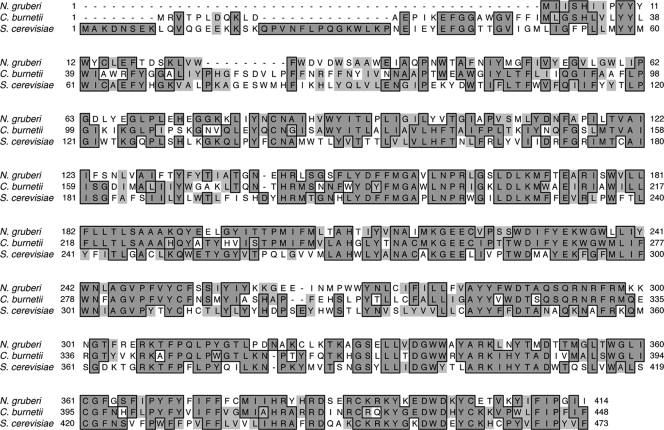
Analysis of the C. burnetii genome revealed two genes encoding putative sterol reductases: CBU1158 and CBU1206 (3, 20). CBU1206 displayed 46.2% amino acid identity with the Saccharomyces cerevisiae Δ24 sterol reductase, Erg4 (Fig. 2). The closest homolog of CBU1206 was from the free-living soil amoeba Naegleria gruberi (51.7% amino acid identity). This suggests that C. burnetii obtained CBU1206 through horizontal gene transfer from amoeba, an event that has also been proposed for C. burnetii's putative Δ7 sterol reductase, CBU1158 (18). The predicted CBU1206 protein is 52.5 kDa and contains nine potential transmembrane domains, and the encoding gene was transcribed during host cell infection (data not shown). The multiple membrane-spanning domains of CBU1206 and the observation that the protein was not secreted by C. burnetii's T4SS (data not shown) (22) suggest that CBU1206 is associated with the bacterial cell envelope. Thus far, we have not identified a Δ24 sterol reductase in other bacterial genomes, while two amoeba-associated bacteria, Legionella drancortii and Protochlamydia amoebophila, have putative Δ7 sterol reductases (18).
FIG. 2.
Protein sequence alignment of Δ24 sterol reductases. Saccharomyces cerevisiae Erg4, C. burnetii CBU1206, and amoebal Naegleria gruberii Δ24 sterol reductase proteins are highly homologous. Identical residues are outlined, and similar residues are shaded light gray.
CBU1206 rescues the drug sensitivity of a S. cerevisiae erg4 mutant.
We took advantage of a S. cerevisiae Δ24 sterol reductase mutant (erg4) to test the functionality of CBU1206. The yeast sterol synthesis pathway is similar to mammalian cells with two important exceptions: (i) yeasts do not have a Δ7 sterol reductase, and (ii) the final product is ergosterol, which is structurally and functionally similar to cholesterol (Fig. 1). The erg4 knockout accumulates ergosta-5,7,22,24(28)-tetraen-3β-ol in its cell wall in the place of ergosterol, making the cell hypersensitive to divalent cations and drugs such as brefeldin A, cycloheximide, and fluconazole (27).
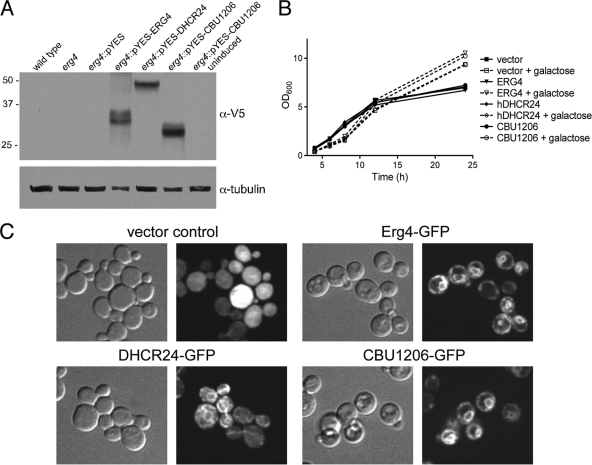
CBU1206, the human Δ24 sterol reductase DHCR24, and S. cerevisiae Erg4 were expressed in the erg4 yeast mutant under the control of a galactose-inducible promoter, with either C-terminal V5/6×His or GFP tags. Immunoblotting showed high expression after 6 h, with no expression in uninduced samples (Fig. 3A). Induced expression of fusion proteins had no effect on yeast growth over a 24-h period compared to the vector-only control (Fig. 3B). Like Erg4-GFP, both CBU1206-GFP and DHCR24-GFP localized to a structure resembling the ER, the location of yeast ergosterol synthesis (Fig. 3C) (27). Colocalization with the yeast ER marker Sec13 fused to red fluorescent protein (RFP) confirmed the ER localization of Erg4, DHCR24, and CBU1206 (data not shown) (15).
FIG. 3.
Heterologous expression in S. cerevisiae erg4. The yeast erg4 mutant was transformed with V5/6×His-tagged or GFP-tagged Erg4-, DHCR24-, or CBU1206-encoding constructs. Fusion protein expression was induced as described in Materials and Methods. (A) Immunoblotting with antibody directed against the V5 tag showed induced expression of Erg4, DHCR24, and CBU1206. Alpha-tubulin was probed as a loading control. (B) Heterologous expression of CBU1206 and the human Δ24 sterol reductase DHCR24 did not affect growth of a yeast erg4 mutant. The OD600 of cultures was measured at the indicated time points following galactose induction. (C) CBU1206-GFP localizes to the yeast ER. The yeast erg4 mutant expressing GFP fusion proteins was fixed and visualized by fluorescence microscopy. As previously described for Erg4 (27), DHCR24 and CBU1206 localized to the ER. GFP (vector control) was cytoplasmic.
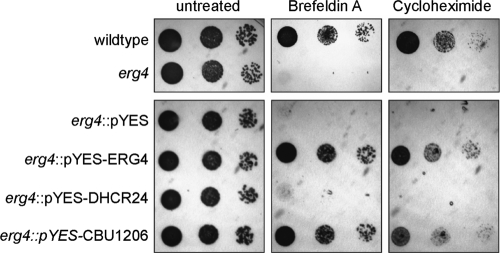
To test whether CBU1206 could rescue the erg4 mutant's sensitivity to brefeldin A and cycloheximide, complemented yeast cells under inducing conditions were spotted onto plates containing these compounds (Fig. 4). Expression of DHCR24 did not rescue erg4 growth, indicating that it does not recognize ergosterol intermediates as a substrate. In contrast, erg4 expressing CBU1206 grew in the presence of both brefeldin A and cycloheximide, demonstrating functional complementation of the yeast Δ24 reductase mutant.
FIG. 4.
CBU1206 functionally complements a yeast erg4 mutant. Cultures induced for 6 h with galactose were spotted in 10-fold dilutions onto untreated plates or plates containing 100 μg of brefeldin A/ml or 0.05 μg of cycloheximide/ml. S. cerevisiae erg4 cells with or without the empty vector pYES-DEST52 were sensitive to both brefeldin A and cycloheximide. In contrast, ERG4 and CBU1206, but not DHCR24, rescued growth.
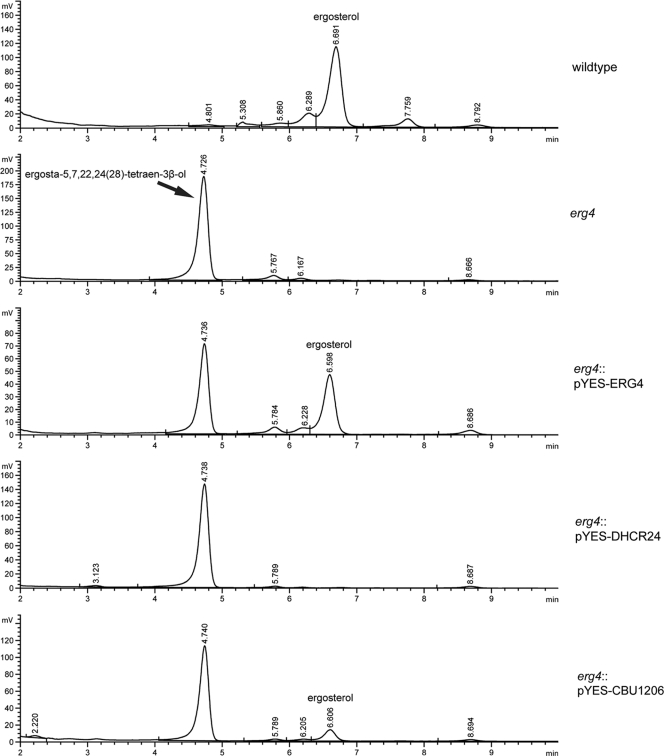
The yeast erg4 mutant complemented with CBU1206 generates ergosterol.
To investigate whether phenotypic rescue of S. cerevisiae erg4 by CBU1206 correlated with biosynthesis of ergosterol, we analyzed the sterol content of complemented yeast by reversed-phase HPLC. As expected, the major sterol in wild-type yeast and the erg4 mutant complemented with Erg4 was ergosterol (Fig. 5). Both uncomplemented erg4 and the erg4 mutant expressing DHCR24 accumulated the ergosterol precursor ergosta-5,7,22,24(28)-tetraen-3β-ol. The lack of ergosterol production in DHCR24-expressing yeast is consistent with the enzyme's inability to phenotypically rescue cells from drug sensitivity. In contrast, CBU1206 expression resulted in generation of ergosterol in the erg4 mutant, further demonstrating that CBU1206 is an active Δ24 sterol reductase.
FIG. 5.
CBU1206 generates ergosterol in yeast. HPLC trace of total sterols extracted from complemented yeast. The dominant sterol in wild-type yeast was ergosterol, while the erg4 mutant accumulated ergosta-5,7,22,24(28)-tetraen-3β-ol. Expression of ERG4 and CBU1206, but not DHCR24, in erg4 yeast resulted in the generation of ergosterol. A commercially available standard was used to determine the retention time of ergosterol.
In summary, our data demonstrate that the intracellular bacterial pathogen C. burnetii expresses a sterol reductase capable of reducing the C24 double bond of ergosterol precursors. Both C. burnetii sterol reductases are considered “orphaned enzymes” because the bacterium lacks the remaining terminal enzymes in the cholesterol biosynthetic pathway (9). However, the fact that C. burnetii has retained sterol reductase genes suggests they serve a critical function. It is unlikely that these proteins are needed to generate more cholesterol since the host cell has an ample supply of sterols from both biosynthetic and exogenous sources. The ability of CBU1206 to reduce a yeast sterol, which it would not encounter inside of a mammalian cell, suggests that CBU1206 has broad substrate specificity. One hypothesis is that the C. burnetii sterol reductases convert cholesterol precursors to novel sterols that serve structural or signaling roles. Another possibility is that these enzymes play a role during infection of a nonmammalian hosts, such as ticks (1).
Although our data demonstrate that CBU1206 has enzymatic activity, we cannot rule out an additional nonenzymatic function. The mammalian DHCR24 both reduces sterol intermediates and has a nonenzymatic role in conferring resistance to oxidative stress and apoptosis through interactions with the tumor suppressor p53 and the p53 negative regulator Mdm2 (16, 17, 26). C. burnetii inhibits host cell apoptosis (23) and also encounters reactive oxygen species in the phagolysosome-like PV (5). New advances in C. burnetii genetic systems (2) may soon allow generation of a CBU1206 knockout that will help define the enzyme's biological role.
Acknowledgments
We thank Dale Howe, Shelly Robertson, and Seth Winfree for critical reading of the manuscript and Anita Mora for graphic support.
This research was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 24 September 2010.
REFERENCES
- 1.Baca, O. G., and D. Paretsky. 1983. Q fever and Coxiella burnetii: a model for host-parasite interactions. Microbiol. Rev. 47:127-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beare, P. A., D. Howe, D. C. Cockrell, A. Omsland, B. Hansen, and R. A. Heinzen. 2009. Characterization of a Coxiella burnetii ftsZ mutant generated by Himar1 transposon mutagenesis. J. Bacteriol. 191:1369-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beare, P. A., N. Unsworth, M. Andoh, D. E. Voth, A. Omsland, S. D. Gilk, K. P. Williams, B. W. Sobral, J. J. Kupko III, S. F. Porcella, J. E. Samuel, and R. A. Heinzen. 2009. Comparative genomics reveal extensive transposon-mediated genomic plasticity and diversity among potential effector proteins within the genus Coxiella. Infect. Immun. 77:642-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beron, W., M. G. Gutierrez, M. Rabinovitch, and M. I. Colombo. 2002. Coxiella burnetii localizes in a Rab7-labeled compartment with autophagic characteristics. Infect. Immun. 70:5816-5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan, R. E., K. Russell, G. Zhang, and J. E. Samuel. 2004. Both inducible nitric oxide synthase and NADPH oxidase contribute to the control of virulent phase I Coxiella burnetii infections. Infect. Immun. 72:6666-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brzostek, A., B. Dziadek, A. Rumijowska-Galewicz, J. Pawelczyk, and J. Dziadek. 2007. Cholesterol oxidase is required for virulence of Mycobacterium tuberculosis. FEMS Microbiol. Lett. 275:106-112. [DOI] [PubMed] [Google Scholar]
- 7.Coppens, I., A. P. Sinai, and K. A. Joiner. 2000. Toxoplasma gondii exploits host low-density lipoprotein receptor-mediated endocytosis for cholesterol acquisition. J. Cell Biol. 149:167-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards, P. A., and J. Ericsson. 1999. Sterols and isoprenoids: signaling molecules derived from the cholesterol biosynthetic pathway. Annu. Rev. Biochem. 68:157-185. [DOI] [PubMed] [Google Scholar]
- 9.Frohlich, K. M., R. A. Roberts, N. A. Housley, and J. P. Audia. 2010. Rickettsia prowazekii uses an sn-glycerol-3-phosphate dehydrogenase and a novel dihydroxyacetone phosphate transport system to supply triose phosphate for phospholipid biosynthesis. J. Bacteriol. 192:4281-4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gatfield, J., and J. Pieters. 2000. Essential role for cholesterol in entry of mycobacteria into macrophages. Science 288:1647-1650. [DOI] [PubMed] [Google Scholar]
- 11.Gietz, R. D., R. H. Schiestl, A. R. Willems, and R. A. Woods. 1995. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11:355-360. [DOI] [PubMed] [Google Scholar]
- 12.Heinzen, R. A., M. A. Scidmore, D. D. Rockey, and T. Hackstadt. 1996. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect. Immun. 64:796-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howe, D., and R. A. Heinzen. 2006. Coxiella burnetii inhabits a cholesterol-rich vacuole and influences cellular cholesterol metabolism. Cell. Microbiol. 8:496-507. [DOI] [PubMed] [Google Scholar]
- 14.Howe, D., J. G. Shannon, S. Winfree, D. W. Dorward, and R. A. Heinzen. 2010. Coxiella burnetii phase I and II variants replicate with similar kinetics in degradative phagolysosome-like compartments of human macrophages. Infect. Immun. 78:3465-3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huh, W. K., J. V. Falvo, L. C. Gerke, A. S. Carroll, R. W. Howson, J. S. Weissman, and E. K. O'Shea. 2003. Global analysis of protein localization in budding yeast. Nature 425:686-691. [DOI] [PubMed] [Google Scholar]
- 16.Lu, X., F. Kambe, X. Cao, Y. Kozaki, T. Kaji, T. Ishii, and H. Seo. 2008. 3β-Hydroxysteroid-Δ24 reductase is a hydrogen peroxide scavenger, protecting cells from oxidative stress-induced apoptosis. Endocrinology 149:3267-3273. [DOI] [PubMed] [Google Scholar]
- 17.Lu, X., F. Kambe, X. Cao, T. Yoshida, S. Ohmori, K. Murakami, T. Kaji, T. Ishii, D. Zadworny, and H. Seo. 2006. DHCR24-knockout embryonic fibroblasts are susceptible to serum withdrawal-induced apoptosis because of dysfunction of caveolae and insulin-Akt-Bad signaling. Endocrinology 147:3123-3132. [DOI] [PubMed] [Google Scholar]
- 18.Moliner, C., D. Raoult, and P. E. Fournier. 2009. Evidence that the intra-amoebal Legionella drancourtii acquired a sterol reductase gene from eukaryotes. BMC Res. Notes 2:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandey, A. K., and C. M. Sassetti. 2008. Mycobacterial persistence requires the utilization of host cholesterol. Proc. Natl. Acad. Sci. U. S. A. 105:4376-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seshadri, R., I. T. Paulsen, J. A. Eisen, T. D. Read, K. E. Nelson, W. C. Nelson, N. L. Ward, H. Tettelin, T. M. Davidsen, M. J. Beanan, R. T. Deboy, S. C. Daugherty, L. M. Brinkac, R. Madupu, R. J. Dodson, H. M. Khouri, K. H. Lee, H. A. Carty, D. Scanlan, R. A. Heinzen, H. A. Thompson, J. E. Samuel, C. M. Fraser, and J. F. Heidelberg. 2003. Complete genome sequence of the Q.-fever pathogen Coxiella burnetii. Proc. Natl. Acad. Sci. U. S. A. 100:5455-5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voth, D. E., and R. A. Heinzen. 2009. Coxiella type IV secretion and cellular microbiology. Curr. Opin. Microbiol. 12:74-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voth, D. E., D. Howe, P. A. Beare, J. P. Vogel, N. Unsworth, J. E. Samuel, and R. A. Heinzen. 2009. The Coxiella burnetii ankyrin repeat domain-containing protein family is heterogeneous, with C-terminal truncations that influence Dot/Icm-mediated secretion. J. Bacteriol. 191:4232-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voth, D. E., D. Howe, and R. A. Heinzen. 2007. Coxiella burnetii inhibits apoptosis in human THP-1 cells and monkey primary alveolar macrophages. Infect. Immun. 75:4263-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waterham, H. R., J. Koster, G. J. Romeijn, R. C. Hennekam, P. Vreken, H. C. Andersson, D. R. FitzPatrick, R. I. Kelley, and R. J. Wanders. 2001. Mutations in the 3β-hydroxysterol Δ24-reductase gene cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis. Am. J. Hum. Genet. 69:685-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winberg, M. E., A. Holm, E. Sarndahl, A. F. Vinet, A. Descoteaux, K. E. Magnusson, B. Rasmusson, and M. Lerm. 2009. Leishmania donovani lipophosphoglycan inhibits phagosomal maturation via action on membrane rafts. Microbes Infect. 11:215-222. [DOI] [PubMed] [Google Scholar]
- 26.Wu, C., I. Miloslavskaya, S. Demontis, R. Maestro, and K. Galaktionov. 2004. Regulation of cellular response to oncogenic and oxidative stress by Seladin-1. Nature 432:640-645. [DOI] [PubMed] [Google Scholar]
- 27.Zweytick, D., C. Hrastnik, S. D. Kohlwein, and G. Daum. 2000. Biochemical characterization and subcellular localization of the sterol C-24(28) reductase, erg4p, from the yeast Saccharomyces cerevisiae. FEBS Lett. 470:83-87. [DOI] [PubMed] [Google Scholar]