Abstract
MzrA was identified as a modulator of the EnvZ/OmpR two-component signal transduction system. Previous evidence indicated that MzrA interacts with EnvZ and modulates its enzymatic activities to influence OmpR phosphate (OmpR∼P) levels. Moreover, MzrA was shown to connect the bacterial envelope stress response systems CpxA/CpxR and σE to EnvZ/OmpR to widen the defensive response regulatory network. In this study, experiments were carried out to establish whether the membrane or periplasmic domain of MzrA is critical for MzrA-EnvZ interactions and to reveal MzrA residues that play an important role in these interactions. Data obtained from chimeric constructs, in which the transmembrane domain of MzrA was replaced with the unrelated transmembrane domain of NarX or signal sequence of PhoA, showed that the transmembrane domain residues of MzrA do not play a critical role in MzrA-EnvZ interactions. The importance of the periplasmic domain of MzrA in MzrA-EnvZ interactions was revealed by characterizing bifunctional, fully soluble, and periplasmically localized MalE::MzrA chimeras. This was further corroborated through the isolation of loss-of-function, single-amino-acid substitutions in the conserved periplasmic domain of MzrA that interfered with MzrA-EnvZ binding in a bacterial two-hybrid system. Together, the data suggest that the binding of MzrA to EnvZ influences the ability of EnvZ to receive and/or respond to environmental signals in the periplasm and modulate its biochemical output to OmpR.
Two-component signal transduction regulatory systems profoundly affect bacterial physiology (18) and pathogenesis (4). The archetypal two-component system involves an inner membrane-bound sensor kinase and a cytoplasmic response regulator, which is often a transcription factor. One of the prototypal two-component systems is comprised of EnvZ/OmpR, where EnvZ is a sensor kinase and OmpR is a response regulator. Together, they regulate the expression of a large number of genes (25), of which the regulation of the ompF and ompC porin genes has been extensively studied and is best understood (26). Growth medium osmolarity has opposite effects on the expression of these porin genes; ompF expression is elevated in low-osmolarity medium, while ompC is preferentially expressed in high-osmolarity medium (35).
Recently, a modulator of the EnvZ/OmpR system, dubbed MzrA, was identified (13). MzrA is an inner membrane protein that interacts with EnvZ (13). Enhanced MzrA-EnvZ interactions lead to high levels of OmpR phosphate (OmpR∼P) due to elevated EnvZ kinase activity, diminished EnvZ phosphatase activity, or both (13). mzrA was discovered among plasmid clones which overcame the conditional lethal phenotype of a bamB degP double deletion mutant (13). BamB is a component of the β-barrel outer membrane protein (OMP) assembly machine (Bam) (36, 38), while DegP provides the major protease activity in the periplasm (33). Defects in OMP assembly activate the CpxA/CpxR two-component system and the σE regulon (14). These two envelope stress response systems increase the transcription of genes whose products help restore envelope homeostasis by improving protein assembly, degrading misfolded OMPs (for a review, see reference 29), and inhibiting OMP synthesis (21). Transcription of mzrA is induced by the activated CpxA/CpxR and σE regulons (10, 14, 27, 39). Elevated MzrA levels increase MzrA-EnvZ interactions and modulate EnvZ's enzymatic activities to elevate OmpR∼P levels (13). This profoundly affects the transcription of a large number of genes, including ompF and lamB, which are repressed, and omrAB, which are activated. The omrAB genes encode small regulatory RNA molecules that inhibit translation of several OMPs, including CirA, FecA, FepA, and OmpT (15). Reduced OMP levels, as a result of a direct action of activated OmpR or an indirect consequence through omrAB overexpression, lead to reduced envelope stress by unburdening the defective Bam complex (9, 13, 14, 15).
The focus of this study is to investigate MzrA-EnvZ interactions. MzrA is predicted to have a single transmembrane (TM) domain, and the experimental data suggest that the N and C termini of the protein are exposed to the cytoplasm and periplasm, respectively (13). Given the short length of the N terminus (residues 1 to 10), it is predicted that MzrA interacts with EnvZ either through its TM domain (residues 11 to 31) or periplasmic domain (residues 32 to 127). Isolation and characterization of deletion and chimeric constructs revealed that the N terminus and the TM domain play no significant role in MzrA-EnvZ interactions. Through site-directed mutagenesis and a novel mutant isolation approach, two residues of the conserved periplasmic domain of MzrA that play an important role in MzrA-EnvZ interactions were identified. Together, the data indicated that MzrA interacts with EnvZ through its soluble periplasmic domain to influence the EnvZ/OmpR regulon.
MATERIALS AND METHODS
Bacterial strains and chemicals.
The Escherichia coli K-12 strains used here were derived primarily from MC4100 (7) or MC4100 Δara714 (37); for a list of these strains, see Table S1 in the supplemental material. The bacterial adenylate cyclase-based two-hybrid system strains and plasmids were purchased from Euromedex. SuperSignal West Pico chemiluminescent substrate was purchased from Thermo Scientific. Rabbit anti-FLAG polyclonal and goat anti-mouse IgG secondary antibodies and were purchased from Sigma-Aldrich. Anti-GroEL and PhoA polyclonal antibodies were purchased from Stressgen and 5 Prime-3 Prime, Inc., respectively. ONPG (2-ortho-nitrophenyl-β-d-galactopyranoside) and BCIP (5-bromo-4-chloro-3-indolylphosphate) were purchased from Acros and Sigma-Aldrich, respectively. All other chemicals were of analytical grade. The LB and LB agar media were prepared as described previously (30) and, when required, supplemented with ampicillin (50 μg/ml), kanamycin (25 μg/ml), or arabinose (0.2%).
DNA methods.
Chromosomal fragments containing either full-length mzrA or mzrA fragments were amplified using gene-specific cloning primers (see Table S2 in the supplemental material for all primer sequences). Restricted PCR-amplified fragments were cloned into appropriately digested pBAD24 (16), pKT25, pUT18, pUT18C (22) (Euromedex), or pMalp2x (New England BioLabs). The mzrA transmembrane domain, narX transmembrane domain, and phoA signal sequence were amplified with specific primers, digested with EcoRI and XbaI, and ligated into appropriately digested pBAD24 vectors to create pBAD24mzrATM, pBAD24narXTM, or pBAD24phoASS. The DNA corresponding to the OmpF signal sequence was amplified with specific primers, digested with BspHI and XbaI, and ligated into pBAD24 digested with NcoI and XbaI to give pBAD24ompFss. mzrA fragments were amplified with specific primers and digested with XbaI and HindIII and ligated into pBAD24, pBAD24mzrATM, pBAD24narXTM, or pBAD24phoASS to create the desired gene fusions.
Selected alterations in MzrA, including the D51A, K67A, D74A, I78F, Q85A, Δ13 to 28 (Δ13-28), and Δ8-31 in various plasmid clones, were generated using a QuikChange Lightning site-directed mutagenesis (SDM) kit from Stratagene following the manufacturer's protocol. Megaprimer mutagenesis of the DNA encoding amino acid residues 31 to 105 of MzrA was carried out using a GeneMorph II EZClone domain mutagenesis kit from Stratagene per the manu- facturer's instructions. Briefly, the mzrA gene was amplified from the pBAD24mzrA::phoA clone, with the mzrA fragment to be mutagenized calculated as 1/27 of the entire plasmid. One hundred nanograms of template (2.7 μg of plasmid) and 30 amplification cycles were used with the mzrA mutagenesis forward and reverse primers, to target a medium mutational frequency (4.5 to 9 mutations per kb of DNA). PCR products were purified with a QIAquick PCR purification kit (Qiagen) and used as the megaprimer for the EZClone reaction. Two hundred fifty nanograms of megaprimer and 50 ng of pBAD24mzrA::phoA were amplified in the EZClone reaction, followed by digestion of parent plasmids using DpnI enzyme. One-fifth (10 μl) of the EZClone reaction was transformed into competent RAM1800 cells per the standard laboratory protocol.
Protein methods.
For Western blot analysis, overnight cultures were diluted 100-fold into appropriately supplemented media and grown 3 h. Cell pellets were resuspended in sample buffer and heated at 95°C for 5 min and analyzed by SDS-PAGE. Urea (4 M) was added to the SDS-polyacrylamide running gel in order to better resolve OmpC and OmpF. Following electrophoresis, proteins were transferred onto Immobilin-P (Millipore) polyvinylidene difluoride (PVDF) membrane using a mini-transblot (Bio-Rad) and incubated in primary antibody for 1.5 h. The primary rabbit antibodies and dilutions used were: OmpF/OmpC/OmpA, 1:16,000; GroEL, 1:50,000; LamB, 1:10,000; MalE, 1:10,000; FLAG, 1:1,000; TolC, 1:5000; and PhoA, 1:10,000. Goat anti-rabbit horseradish peroxidase (HRP)-conjugated immunoglobulin G secondary antibodies were incubated for 1 h. Membrane blots were incubated with Immun-Star HRP substrate or SuperSignal West Pico chemiluminescent substrate for 5 min, and protein bands were visualized with a Molecular Imager ChemiDoc XRS system from Bio-Rad. Protein bands were quantified using Quantity One software from Bio-Rad.
Enzymatic assays.
β-Galactosidase activity from cultures grown to mid-log phase (optical density at 600 nm [OD600] of 0.7 to 0.9) was determined by a published method (23). Kinetic analysis of enzyme activity was carried out using a VersaMax (Molecular Dynamics) microtiter plate reader in quadruplicate, and activity was calculated as the rate of ONPG (β-galactosidase) hydrolysis.
Cell fractionation.
Overnight cultures were diluted 1:100 and grown 3 h with appropriate supplements. Equivalent amounts of cells, based on OD600, were pelleted and resuspended in a periplasmic extraction buffer (10 mM Tris-HCl [pH 7.5], 0.5 M sucrose, 10 mM EDTA, 0.2 mg/ml lysozyme), and periplasm was extracted by the gentle osmotic shock method (2). Following extraction of the periplasm, cells were pelleted and washed before resuspension in a lysis buffer (100 mM Tris [pH 7.5], 10 mM MgCl2, 0.25 mg/ml DNase I, and 2 mM phenylmethylsulfonyl fluoride [PMSF]) and lysed using a French press. Cell lysates were centrifuged for 1 h at 105,000 × g at 4°C to separate soluble (cytoplasm and residual periplasm) and insoluble (inner and outer membrane) fractions.
RESULTS
Deletion analysis to delineate the functionally important regions of MzrA.
MzrA is a 127-residue-long inner membrane protein that contains a single TM domain, extending from residues 11 to 31. In our previous analysis, we showed that the TM domain of MzrA is crucial for its membrane localization and that the C terminus of MzrA is exposed to the periplasm (13). Here, we carried out a systematic mutagenesis analysis to delineate the region of MzrA that is important for its activity. All constructs described below and shown in Fig. 1 were expressed from the arabinose-inducible PBAD promoter of pBAD24 and contained a FLAG tag at the C-terminal end for detection by Western blotting. Production of the wild-type FLAG-tagged MzrA protein from the plasmids results in decreased expression of OmpF and MalE. The steady-state levels of these proteins, as well the tagged MzrA constructs, were monitored from whole-cell extracts by Western blot analysis (Fig. 2).
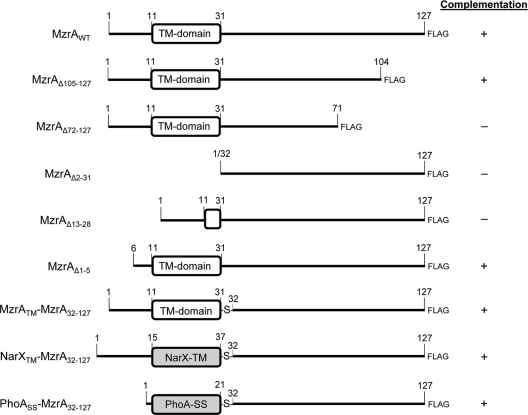
FIG. 1.
Various constructs of MzrA proteins and their abilities to complement a ΔmzrA mutation. The transmembrane (TM) or the signal sequence (SS) regions are shown as rounded rectangles. Shaded rectangles show the NarX TM domain and the PhoA signal sequence. The last three recombinant proteins contained an extra serine (S) residue that was inserted due to the creation of a restriction site in the corresponding DNA. Complementation was tested by examining the ability of the expressed proteins to inhibit (+) or not inhibit (−) OmpF and MalE synthesis, as shown in Fig. 2 and 3. WT, wild type.
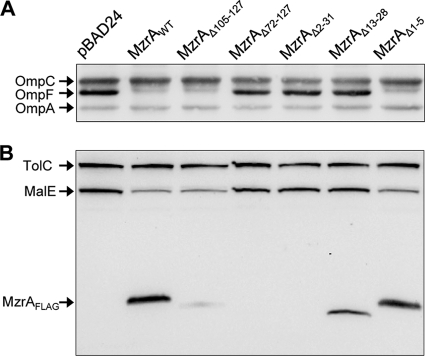
FIG. 2.
Effects of N- and C-terminal deletions of MzrA on OmpF, MalE, and MzrAFLAG. (A and B) Western blot analysis of whole-cell protein extracts isolated from freshly grown cultures containing plasmids, as shown in the figure. Expression of various recombinant proteins from pBAD24 was induced by the addition of 0.2% l-arabinose for 3 h. Membrane blots were probed with OmpF/OmpC/OmpA (A) or TolC/MalE and FLAG (B) antibodies.
The sequence alignment between MzrA and its orthologs from other members of Enterobacteriaceae showed little conservation at the extreme N- and C-terminal ends (13). Consequently, deletion of the last 23 residues from MzrA (Δ105 to 127) did not interfere with the mutant protein's ability to reduce OmpF and MalE levels; thus, it showed full activity (Fig. 2A and B). On the other hand, deletion of the last 55 residues of MzrA (Δ72 to 127 [MzrAΔ72-127]) completely abolished the protein's activity (Fig. 2A and B). Figure 2B shows that both of the C-terminal deletions significantly reduced mutant MzrA levels, with the effect of the larger deletion (Δ72 to 127) being greater than that of the smaller deletion (Δ105 to 127). Thus, the lost activity of MzrAΔ72-127 could stem from the highly unstable nature of the mutant protein.
Next, we probed the importance of the N-terminal region of MzrA. Three N-terminal deletions removed residues 1 to 5, 2 to 31, or 13 to 28 of MzrA (Fig. 1). The MzrAΔ1-5 mutant was constructed because we wanted to assess the relevance of two N-terminal methionine residues present at positions one and six (Fig. 3A). Deletion of the first five residues, including the first methionine, had no effect on the protein's activity (Fig. 2A and B), although a slight reduction in the MzrAΔ1-5 level was observed (Fig. 2B). On the other hand, a partial (Δ13 to 28) or complete (Δ2 to 31) deletion of the MzrA TM domain abolished the protein's activity (Fig. 2A and B), with the complete deletion severely reducing the protein's level (Fig. 2B).
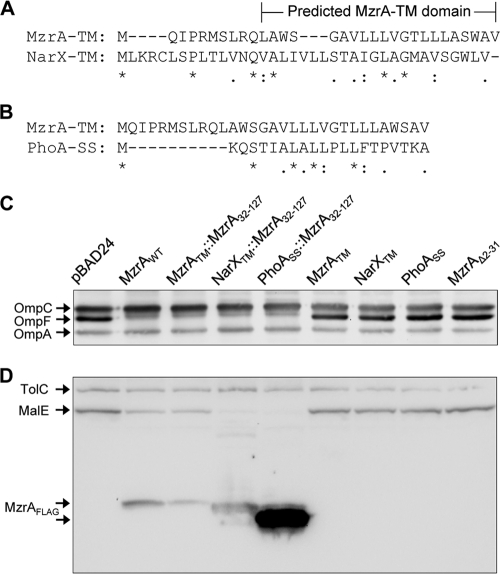
FIG. 3.
Importance of the transmembrane (TM) domain and the C-terminal region of MzrA in the protein's activity. (A and B) Amino acid sequence alignments between the first 31 amino acids of MzrA, which includes its TM domain, the putative TM domain of NarX (A), and the signal sequence (SS) of PhoA (B). Identical (*), highly similar (:), and weakly similar (.) amino acids are indicated. (C and D) Western blot analysis of cell lysates prepared from strains expressing no MzrA (pBAD24), wild-type MzrA, and other recombinant proteins as shown on top of the gel in panel C. Expression of recombinant proteins from pBAD24 was induced by the addition of 0.2% l-arabinose. Membrane blots were probed with OmpF/OmpC/OmpA antibodies (C) or TolC/MalE and FLAG antibodies (D).
Together, these deletion analyses narrowed the functionally important region to between the 6th and 104th residues of the MzrA protein. This region includes the N-terminal TM domain and a highly conserved segment of the MzrA protein, stretching from residues 46 to 95 (see Fig. S1 in the supplemental material).
Sequence of the putative TM domain of MzrA is not important for its function.
The above analysis reiterated the importance of the TM domain of MzrA, as previously suggested from an MzrA::PhoA chimeric construct in which the N terminus was composed of MzrA, including its TM domain (13). A deletion removing most of the MzrA TM domain of this chimera abolished both MzrA and PhoA activities, presumably due to mislocalization of the protein (13). It is not known whether MzrA interacts with EnvZ through its TM domain or the region that extends into the periplasm. To determine this, we constructed chimeras in which the TM domain of MzrA was replaced by that of NarX or the signal sequence from PhoA (Fig. 1). NarX is an inner membrane sensor kinase protein that phosphorylates NarL, the response regulator protein of the NarX-NarL two-component signal transduction system (32). PhoA is a periplasmic protein that expresses alkaline phosphatase. It contains an N-terminal signal sequence for translocation across the inner membrane (20). No data exist showing interactions between these two proteins and MzrA or EnvZ. Direct sequence alignments between the TM domain of MzrA and the TM domain of NarX or PhoA signal sequence showed no significant homology (Fig. 3A and B). Indeed, all three sequences contain a preponderance of hydrophobic residues which are presumably required for translocation across (PhoA) or localization into (MzrA and NarX) the inner membrane.
Expression of NarXTM::MzrA32-127 and PhoASS::MzrA32-127 chimeras drastically reduced OmpF and MalE levels (Fig. 3C and D); thus, they showed full activities. In fact, their effects on MalE levels appeared to be greater than that of the full-length, wild-type MzrA protein or the reconstructed MzrA that had an extra serine residue inserted during cloning (Fig. 3C and D). Expression of the MzrA and NarX TM domains or PhoA signal sequence alone had no effect on OmpF and MalE levels (Fig. 3C and D), showing that the MzrA sequence extending beyond the first 32 residues is required for the activity of the chimeras.
Examination of MzrA levels, through probing for the FLAG tag present at the C terminus of all these proteins, showed that whereas the reconstructed MzrATM::MzrA32-127 and NarXTM::MzrA32-127 protein levels were comparable to that of wild-type MzrA, the PhoASS::MzrA32-127 was present at significantly higher levels (Fig. 3D). Thus, the strongest activity of PhoASS::MzrA32-127 could in part be due to its extremely high levels. At present, it is not known why PhoASS::MzrA32-127 levels are significantly higher than those of MzrATM::MzrA32-127, but this may point to a possible regulatory role of MzrATM in translation efficiency or protein stability. Note that the levels of other secreted proteins, OmpA, OmpC, and TolC, were unaffected by the high expression levels of PhoASS::MzrA32-127, showing that the chimeras maintained functional specificity and that the inhibitory effects on OmpF and MalE were not due to a general shutdown of translocation across the inner membrane. These studies showed that MzrA activity is not dependent on its native TM domain sequence, thus suggesting that MzrA-EnvZ interactions likely occur via their periplasmic domains.
Membrane anchoring of MzrA is not required for its activity.
Given that MzrA's TM domain could be exchanged with unrelated sequences without the loss of MzrA activity (Fig. 3), we sought to establish whether the role of the TM domain is merely to localize the rest of the C-terminal segment into the periplasm. If so, this would indicate that the periplasmic domain of MzrA most likely interacts with EnvZ to influence gene expression.
In Fig. 3, we show a chimera in which residues 32 to 127 of MzrA were fused downstream of the PhoA signal sequence. Despite the presence of a cleavable signal sequence, however, localization studies showed that PhoASS::MzrA32-127 was present in both the periplasmic and the membrane fractions (data not shown). Therefore, this construct was not suitable for drawing a conclusion as to whether the soluble periplasmic form was active or the bulk of the activity came from membrane-bound species of PhoASS::MzrA32-127. A chimera resulting from fusion of the OmpF signal sequence to the MzrA32-127 segment met the same fate as PhoASS::MzrA32-127, meaning that the chimera was fully active but was present in both the periplasmic and envelope fractions.
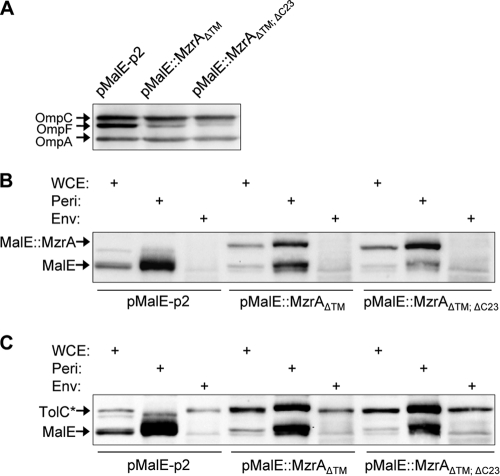
To avoid a possible inefficiency in chimera signal sequence processing, which could result in localization artifacts, we fused the MzrA32-127 (MzrAΔTM) or MzrA32-104 (ΔTM, ΔC23) segment of MzrA downstream of the MalE protein expressed from a pMalE-p2 plasmid. MalE, a periplasmic protein, from this plasmid is expressed from a Ptac promoter and is localized to the periplasm through its native signal sequence. The two resulting MalE::MzrA chimeras were active (Fig. 4A) and localized exclusively in the periplasm (Fig. 4B and C). The retention of normal activity by fully soluble, periplasmic forms of MzrA supported the notion that MzrA and EnvZ interact with each other by virtue of their periplasmic domains.
FIG. 4.
Activity and localization of MalE::MzrA fusion proteins. (A) Western blot analysis of cell lysates obtained from strains expressing either no MzrA (pMalE-p2) or MalE::MzrA fusion proteins. Membrane blots were probed with OmpF/OmpC/OmpA antibodies. (B and C) Localization of MalE and MalE::MzrA fusion proteins. Cells were fractioned into whole-cell lysate (WCE), periplasm (Peri), or envelope (Env), as described in Materials and Methods. Protein samples from various fractions were subjected to Western blot analysis, and membrane blots were probed with MalE antibodies (B) and MalE and TolC antibodies (C). TolC, an outer membrane protein, serves as a control for the envelope fraction; TolC* refers to close approximation of TolC and MalE::MzrA fusion bands. ΔTM and Δ23 refer to the absence of the TM domain and the last 23 C-terminal residues from MzrA, respectively. Due to the leaky nature of the Ptac promoter, the effect of the recombinant proteins on OmpF and their cellular localization were studied from uninduced (without IPTG) cells.
Isolation of point mutations that affect MzrA's activity.
Based on the deletion and chimeric constructs described above, we narrowed the functionally relevant part of MzrA to residues 32 to 104. To identify individual residues of functional relevance, we took two additional genetic approaches; in the first approach, we performed site-directed mutagenesis to replace four conserved polar residues of MzrA, D51, K67, D74, and Q85, with alanine (see Fig. S1 in the supplemental material). Of these four substitutions, only D51A severely abolished MzrA's activity (Fig. 5A) without significantly affecting its level (Fig. 5B). Proteins with the K67A and D74A alterations appeared to retain full activities (Fig. 5A) despite the fact that these alterations resulted in low MzrA levels (Fig. 5B).
FIG. 5.
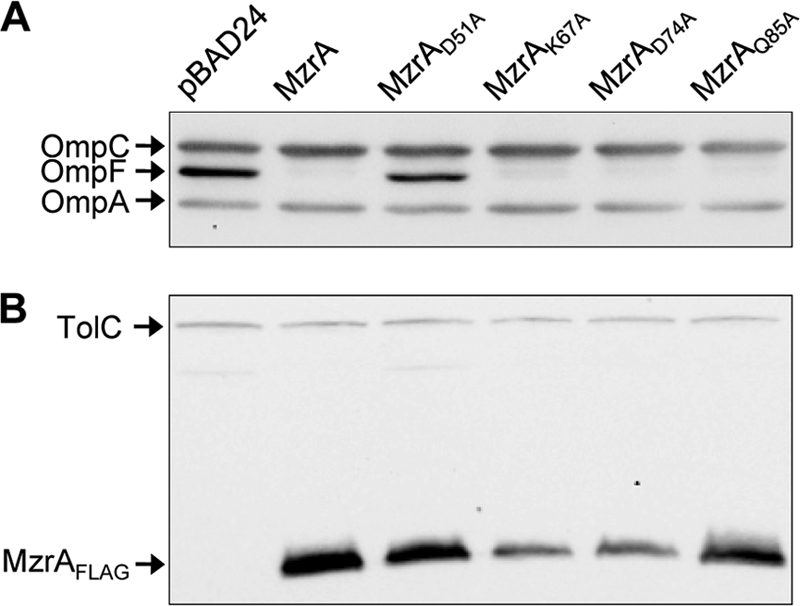
Effects of single-amino-acid substitutions on the activity and level of MzrA. Western blot analysis of cell lysates obtained from strains either not producing MzrA (pBAD24) or expressing the wild type or various mutant forms of MzrA from pBAD24. Membrane blots were probed with OmpF/OmpC/OmpA (A) or TolC and FLAG (B) antibodies. OmpA and TolC served as gel loading controls.
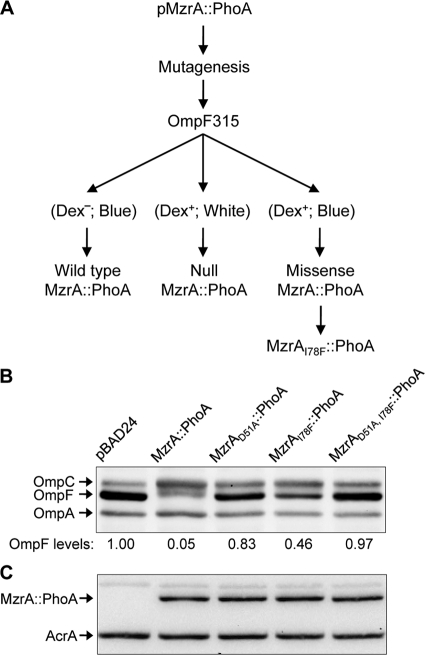
In our second approach, we devised a genetic strategy for isolating spontaneous MzrA mutants. This strategy, outlined in Fig. 6A, exploited a fully active and bifunctional MzrA::PhoA chimera in which the N terminus was derived from the entire MzrA protein, including its TM domain, while the C terminus was composed of the mature PhoA sequence (13). The expression of the MzrA portion of the chimera inhibits OmpF synthesis, while the PhoA region folds into a fully active alkaline phosphatase enzyme. The cleavage of 5-bromo-4-chloro-3-indolylphosphate (BCIP), present in the medium, by alkaline phosphatase gives bacterial colonies a blue color.
FIG. 6.
Isolation and characterization of a functionally inactive MzrA mutant. (A) Genetic strategy for isolating functionally null, nonpolar missense mutations in mzrA. The mzrA sequence, corresponding to amino acid residues 32 to 104 of the protein, from a plasmid expressing MzrA::PhoA fusion was subjected to random, megaprimer-based mutagenesis. The mutagenized plasmid DNA was transformed into a ΔlamB strain expressing OmpF315, a variant OmpF protein that confers a temperature-sensitive Dex+ phenotype (24). Transformed cells were spread on maltodextrin minimal medium containing BCIP to detect the alkaline phosphatase activity of PhoA (blue color), 0.2% l-arabinose for MzrA::PhoA expression, and ampicillin to select for plasmids. Expression of wild-type MzrA inhibits OmpF315 synthesis and causes a Dex− or down phenotype. The resulting small colonies appear blue due to normal PhoA synthesis from the plasmid. A null mutation in mzrA produces a polar effect on phoA, resulting in Dex+ PhoA− (white) colonies. The desired class of plasmid will carries a missense, nonpolar mutation in mzrA that interferes with the normal function of MzrA. These plasmids will result in Dex+ PhoA+ (blue) colonies. (B and C) Western blot analysis to determine OmpF and MzrA::PhoA levels. Proteins were analyzed from a strain expressing no MzrA (pBAD24), wild-type MzrA::PhoA, or its derivative containing single-amino-acid substitutions, as shown. OmpA (B) and AcrA (C) served as gel loading controls. In panel B, OmpF levels were quantified relative to those of OmpA, and the values were normalized to that determined from the pBAD24 vector control lane.
The wild-type OmpF protein does not confer a suitable positive selectable phenotype, so we used OmpF315, a variant of OmpF that confers a temperature-sensitive phenotype with the ability to grow on maltodextrins (Dex+) in the absence of LamB (24). Therefore, expression of MzrA::PhoA in an OmpF315 ΔlamB background confers a Dex− or down phenotype due to the inhibition of OmpF315 synthesis. A mutation in the mzrA region of the chimera may eliminate the inhibitory effect on OmpF315 synthesis, allowing for a Dex+ phenotype. The presence of BCIP helps differentiate Dex+ colonies which are formed due to either a null mutation in mzrA that also prevents phoA expression (white colonies) or a missense mutation in mzrA that allows normal phoA expression (blue colonies).
Since we have already established that residues 32 to 104 of MzrA are important for its activity, only the corresponding region of DNA in the pmzrA::phoA clone was subjected to random, megaprimer-based mutagenesis. Mutagenized plasmids were transformed into a strain expressing OmpF315 and cells were spread on maltodextrin minimal medium supplemented with BCIP, arabinose, and ampicillin. Out of several dozen Dex+ PhoA+ (blue) colonies, in only one case were we able to restore the desired phenotype upon reintroducing the extracted plasmid into a fresh OmpF315 background. DNA sequencing of the isolated plasmid from this colony revealed the presence of a single point mutation in mzrA, resulting in an I78 to F substitution.
The OmpF inhibitory activity of MzrAI78F::PhoA was examined by introducing this plasmid into a wild-type OmpF background. Two additional variants were constructed by site-directed mutagenesis, yielding MzrAD51A::PhoA and MzrAD51A, I78F::PhoA proteins. Figure 6B shows that MzrAI78F::PhoA exerted a somewhat weaker effect than MzrAD51A::PhoA on OmpF; however, the double mutant behaved just like a null mutant. Examination of MzrA::PhoA constructs by Western blotting using PhoA antibodies showed that the presence of missense mutations in mzrA had no effect on the chimera levels (Fig. 6C). Therefore, D51A and I78F, when present in the MzrA::PhoA backbone, affected principally MzrA activity.
Effects of D51A and I78F on MzrA-EnvZ interactions.
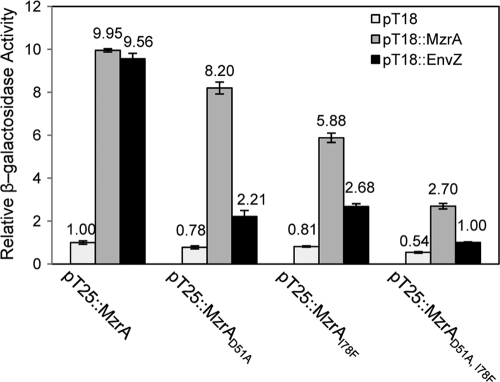
We considered the possibility that D51A and I78F substitutions in MzrA interfere with MzrA's ability to properly interact with EnvZ, thus not activating a regulatory pathway that inhibits OmpF and MalE synthesis (13). To examine MzrA-EnvZ interactions in vivo, we resorted to a bacterial two-hybrid system (Fig. 7) (13). Here, the synthesis of hybrids between T18 and T25 fragments of adenylate cyclase (Cya) from Bordetella pertussis and two interacting proteins of interest lead to fully active Cya (22). The activity of Cya is indirectly measured by assaying for β-galactosidase, whose synthesis is dependent on Cya. The N terminus of fusion constructs was composed of the T18 or T25 fragment of Cya, while the C terminus was provided from MzrA or EnvZ. Interactions between MzrA and EnvZ in the periplasm will bring the T18 and T25 fragments in close proximity in the cytoplasm, resulting in an active adenylate cyclase enzyme.
FIG. 7.
In vivo examination of MzrA-MzrA and MzrA-EnvZ interactions. Protein-protein interactions were monitored using a bacterial two-hybrid system involving transcomplementation of T18 and T25 adenylate cyclase fragments from Bordetella pertussis. The two adenylate cyclase fragments, expressed separately from two compatible plasmids, were produced as fusion proteins containing MzrA or EnvZ (13). Interactions between fusion proteins restored the adenylate cyclase activity, allowing the production of chromosomally expressed LacZ in a Δcya background. Thus, measurement of the β-galactosidase activity reflects the extent of MzrA-MzrA and MzrA-EnvZ interactions. Maximum β-galactosidase activity occurs in the presence of T18::MzrA-T25::MzrA (MzrA dimer formation) or T18::EnvZ-T25::MzrA (MzrA-EnvZ heteromer formation) proteins. Single-amino-acid substitutions in the MzrA periplasmic domain interfere with either the MzrA-EnvZ (D51A) interaction or both MzrA-MzrA and MzrA-EnvZ (I78F) interactions.
The results in Fig. 7 showed strong evidence for MzrA-MzrA dimer and MzrA-EnvZ heteromer formation when the MzrA sequence was the wild type. The presence of D51A in MzrA reduced the ability of MzrA to form dimers by about 20%, but MzrA-EnvZ interactions were reduced by almost 80% (Fig. 7), indicating that the MzrA-EnvZ interface is likely affected by D51A. I78F reduced MzrA dimer and MzrA-EnvZ heteromer formation by almost 40% and 70%, respectively. Thus, reduced MzrA-EnvZ interactions in the MzrAI78F mutant could be due primarily to reduced MzrA dimerization, which is possibly required for efficient MzrA-EnvZ interactions. The presence of both alterations in MzrA abolished MzrA-EnvZ interactions and drastically inhibited MzrA dimer formation (Fig. 7). These results are in good agreement with the protein data shown in Fig. 6 and demonstrate that D51A and I78F substitutions in MzrA interfere with MzrA-EnvZ interactions. In part, this defect appears to stem from the mutant MzrA protein's inability to form homodimers.
DISCUSSION
We have shown here that the N-terminal TM domain of MzrA is not required for its function; the MzrA TM domain was not only replaceable with an unrelated TM domain or a cleavable signal sequence but was also found to be completely dispensable for MzrA activity as long as the MzrAΔTM protein could be targeted to the periplasm via a cleavable signal sequence of an unrelated protein. The retention of MzrA activity by a fully soluble, periplasmic MalE-MzrAΔTM chimera indicated that the periplasm-exposed region of MzrA interacts with EnvZ. Consistent with this notion, two single-amino-acid substitutions, D51A and I78F, located in the conserved region of the periplasmic domain of MzrA (see Fig. S1 in the supplemental material) reduced MzrA's ability to interact with EnvZ; the D51A substitution inhibited principally the interaction with EnvZ, while I78F appeared to also interfere with MzrA dimerization. Together, these data indicated that MzrA-EnvZ interactions take place in the soluble periplasmic compartment of the bacterial envelope.
Although the precise biochemical nature of the environmental signals to which EnvZ responds is not known, growth medium osmolarity-dependent fluctuation in OmpF and OmpC porin synthesis is commonly used to assess the in vivo status of the EnvZ/OmpR system (26). In the absence of MzrA or its overexpression, the medium osmolarity-dependent fluctuation in porin synthesis still occurs but not to the same extent as in the wild type, since the ratios of OmpF/OmpC are significantly altered in favor of OmpF (without MzrA) or OmpC (with MzrA overexpression) (13). Therefore, while MzrA may not function as a primary receptor for medium osmolarity and other environmental signals, its interactions with EnvZ appear to influence EnvZ's ability to receive or respond to signals in the periplasm and modulate biochemical output to OmpR. By fine-tuning the activities of EnvZ, MzrA helps optimize EnvZ/OmpR-mediated cellular response to various environmental cues.
Based on the results obtained in this study and those reported previously (13), we propose a model which envisages that under normal physiological growth conditions, MzrA and EnvZ maintain a steady-state interaction through their periplasmic domains. These interactions increase or decrease depending on the MzrA level which, in part, fluctuates in response to the status of envelope stress and perhaps other environmental cues. Conditions that activate the Cpx and σE regulons will increase MzrA expression, resulting in elevated MzrA-EnvZ interactions and thus a strongly activated EnvZ/OmpR system (i.e., high OmpR∼P levels). Such an OmpR∼P status resembles that which exists in the presence of EnvZ mutants with a diminished phosphatase activity (1, 13, 19, 34) or when MzrA is overexpressed (13). On the other hand, reduced envelope stress or other environmental conditions that lower Cpx/σE activities will also lower MzrA expression, resulting in decreased MzrA-EnvZ interactions and a less active EnvZ/OmpR system (i.e., low OmpR∼P levels). We have reported previously that certain alterations in EnvZ, which lead to constitutively elevated OmpR∼P levels, lower mzrA expression (13). This suggests the existence of a feedback regulatory loop that allows for the resumption of the steady-state MzrA-EnvZ interaction.
While data presented here strongly suggest a direct MzrA-EnvZ interaction, they do not reveal how such interactions modulate various enzymatic activities of EnvZ. In fact, this is generally true for most modulators. In E. coli, at least six envelope-localized modulators, NlpE, RcsF, CpxP, TorT, B1500, and MzrA, which are thought to influence the activities of their inner membrane-bound sensor kinases have been identified (a description of currently known modulators can be found in reference 6). Of these, only CpxP is known to downregulate its cognate regulon of CpxA/CpxR (5) by inhibiting the autokinase activity of CpxA (12). The remaining five modulators influence the activity of their cognate sensor kinases to ultimately increase the phosphorylated states of the respective response regulators. In the cases of CpxP, TorT, B1500, and MzrA, the data exist for interactions between them and the cognate sensor kinases (3, 11, 12, 13; this work). Of the six modulators listed above, RcsF and NlpE are localized in the outer membrane, thus making it even more intriguing as to how they act on their cognate sensor kinases. RcsF, a modulator of the RcsC/RcsD/RcsB system, is an outer membrane lipoprotein (8). Because of its small size of 134 residues, it is difficult to envision a direct interaction between RcsF and the inner membrane-bound sensor kinase RcsC. Overproduction of an outer membrane lipoprotein, NlpE, activates the Cpx system (31). It was hypothesized that in its extended conformation, the 216-residue-long mature NlpE protein can interact directly with the CpxA sensor kinase (17). The same authors also offered a possibility of CpxA activation through an indirect mechanism involving unfolded NlpE-mediated sequestration of CpxP from CpxA (17).
Regardless of whether the envelope modulators interact with the sensor kinase directly or indirectly, the biochemical and molecular basis by which such interactions influence enzymatic activities of the sensor kinase is poorly understood. It is perhaps safe to assume that modulator-sensor kinase interactions lead to conformational changes in the TM and/or periplasmic domains of the sensor kinase, which would influence the various enzymatic activities of its cytoplasmic domain (autophosphorylation, phosphotransfer, or phosphatase). Consistent with this notion, mutational alterations in the TM or periplasmic domain of sensor kinases often constitutively lock their biochemical activities (13, 19, 28, 34). It is generally believed that most sensor kinases form homodimers, although little is known about the dynamics and stability of these homodimers. Therefore, a possibility exists that some envelope modulators affect sensor kinase activities by influencing their dimerization.
B1500 and MzrA represent a unique group of membrane-bound modulators that not only fine-tune the activities of their cognate sensor kinases but also connect two different sensor kinase/response regulator pairs. Synthesis of B1500 is positively controlled by the activated EvgS/EvgA system; B1500, in turn, activates the PhoQ/PhoP system by binding to the PhoQ sensor kinase and stimulating its activity (11). Similarly, MzrA synthesis is under the positive control of the CpxA/CpxR system; MzrA binds to EnvZ to activate the EnvZ/OmpR system (13). Thus, connecting the two independent two-component signal transduction systems by modulators expands the cellular response to a stimulus that may be specific to one of the systems.
A deeper understanding of the molecular mechanism by which MzrA influences EnvZ activities will be important toward understanding the critical roles the EnvZ/OmpR system play in combating cellular stress. Data obtained in this work raise a possibility that MzrA synthesis in addition to transcriptional control may be subject to posttranscriptional control. Therefore, our future efforts will also be directed at gaining a better understanding of MzrA biogenesis.
Supplementary Material
Acknowledgments
We thank Drew Bennion and Leanne Misra for reading the manuscript.
This work was supported by grant GM048167 from the National Institutes of Health.
Footnotes
Published ahead of print on 1 October 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Aiba, H., F. Nakasai, S. Mizushima, and T. Mizuno. 1989. Evidence for the physiological importance of the phosphotransfer between the two regulatory components, EnvZ and OmpR, in osmoregulation in Escherichia coli. J. Biol. Chem. 264:14090-14094. [PubMed] [Google Scholar]
- 2.Arié, J.-P., N. Sassoon, and J.-M. Betton. 2001. Chaperone function of FkpA, a heat shock prolyl isomerase, in the periplasm of Escherichia coli. Mol. Microbiol. 39:199-210. [DOI] [PubMed] [Google Scholar]
- 3.Baraquet, C., L. Théraulaz, M. Guiral, D. Lafitte, V. Méjean, and C. Jourlin-Castelli. 2006. TorT, a member of a new periplasmic binding protein family, triggers induction of the Tor respiratory system upon trimethylamine N-oxide electron-acceptor binding in Escherichia coli. J. Biol. Chem. 281:38189-38199. [DOI] [PubMed] [Google Scholar]
- 4.Beier, D., and R. Gross. 2006. Regulation of bacterial virulence by two-component systems. Curr. Opin. Microbiol. 9:143-152. [DOI] [PubMed] [Google Scholar]
- 5.Buelow, D. R., and T. L. Raivio. 2005. Cpx signal transduction is influenced by a conserved N-terminal domain in the novel inhibitor CpxP and the periplasmic protease DegP. J. Bacteriol. 187:6622-6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buelow, D. R., and T. L. Raivio. 2010. Three (and more) component regulatory systems—auxiliary regulators of bacterial histidine kinases. Mol. Microbiol. 75:547-566. [DOI] [PubMed] [Google Scholar]
- 7.Casadaban, M. J. 1976. Transposition and fusion of the lac genes to select promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 104:541-555. [DOI] [PubMed] [Google Scholar]
- 8.Castanié-Cornet, M.-P., K. Cam, and A. Jacq. 2006. RcsF is an outer membrane lipoprotein involved in the RcsCDB phosphorelay signaling pathway in Escherichia coli. J. Bacteriol. 188:4264-4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlson, E. S., J. N. Werner, and R. Misra. 2006. Differential effects of yfgL mutation on the biogenesis of Escherichia coli outer membrane proteins and lipopolysaccharide. J. Bacteriol. 188:7186-7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dartigalongue, C., D. Missiakas, and S. Raina. 2001. Characterization of the Escherichia coli σE regulon. J. Biol. Chem. 276:20866-20875. [DOI] [PubMed] [Google Scholar]
- 11.Eguchi, Y., J. Itou, M. Yamane, R. Demizu, F. Yamato, A. Okada, H. Mori, A. Kato, and R. Utsumi. 2007. B1500, a small membrane protein, connects the two-component systems EvgS/EvgA and PhoQ/PhoP in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 104:18712-18717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleischer, R., R. Heermann, K. Jung, and S. Hunke. 2007. Purification, reconstitution, and characterization of the CpxRAP envelope stress system of Escherichia coli. J. Biol. Chem. 282:8583-8593. [DOI] [PubMed] [Google Scholar]
- 13.Gerken, H., E. S. Charlson, E. M. Cicirelli, L. J. Kenney, and R. Misra. 2009. MzrA, a novel modulator of the EnvZ/OmpR two-component regulon. Mol. Microbiol. 72:1408-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerken, H., O. P. Leiser, D. Bennion, and R. Misra. 2010. Involvement and necessity of the Cpx regulon in the event of aberrant β-barrel outer membrane protein assembly. Mol. Microbiol. 75:1033-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guillier, M., and S. Gottesman. 2006. Remodeling of the Escherichia coli outer membrane by two small regulatory RNAs. Mol. Microbiol. 59:231-247. [DOI] [PubMed] [Google Scholar]
- 16.Guzman, L.-M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirano, Y., M. M. Hossain, K. Takeda, T. Tokuda, and K. Miki. 2007. Structural studies of the Cpx pathway activator NlpE on the outer membrane of Escherichia coli. Structure 15:963-976. [DOI] [PubMed] [Google Scholar]
- 18.Hoch, J. A., and T. J. Silhavy (ed). 1995. Two-component signal transduction. ASM Press, Washington, DC.
- 19.Hsing, W., F. D. Russo, K. K. Bernd, and T. J. Silhavy. 1998. Mutations that alter the kinase and phosphatase activities of the two-component sensor EnvZ. J. Bacteriol. 180:4538-4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inouye, H., W. Barnes, and J. Beckwith. 1982. Signal sequence of alkaline phosphatase of Escherichia coli. J. Bacteriol. 149:434-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansen, J., A. A. Rasmussen, M. Overgaard, and P. Valentin-Hansen. 2006. Conserved small non-coding RNAs that belong to the σE regulon: role in down-regulation of outer membrane proteins. J. Mol. Biol. 364:1-8. [DOI] [PubMed] [Google Scholar]
- 22.Karimova, G., J. Pidoux, A. Ullmann, and D. Ladant. 1998. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. U. S. A. 95:5752-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria, p 71-74. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 24.Misra, R. 1993. OmpF assembly mutants of Escherichia coli K-12: isolation, characterization and suppressor analysis. J. Bacteriol. 175:5049-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oshima, T., H. Aiba, Y. Masuda, S. Kanaya, M. Sugiura, B. L. Wanner, H. Mori, and T. Mizuno. 2002. Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol. Microbiol. 46:281-291. [DOI] [PubMed] [Google Scholar]
- 26.Pratt, L. A., and T. J. Silhavy. 1995. Porin regulation of Escherichia coli, p. 105-127. In J. A. Hoch and T. J. Silhavy (ed.), Two-component signal transduction. ASM Press, Washington, DC.
- 27.Price, N. L., and T. L. Raivio. 2009. Characterization of the Cpx regulon in Escherichia coli strain MC4100. J. Bacteriol. 191:1798-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raivio, T. L., and T. J. Silhavy. 1997. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J. Bacteriol. 179:7724-7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz, N., and T. J. Silhavy. 2005. Sensing external stress: watchdogs of the Escherichia coli cell envelope. Curr. Opin. Microbiol. 8:122-126. [DOI] [PubMed] [Google Scholar]
- 30.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 31.Snyder, W. B., L. J. B. Davis, P. N. Danese, C. L. Cosma, and T. J. Silhavy. 1995. Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J. Bacteriol. 177:4216-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart, V., and R. S. Rabin. 1995. Dual sensors and dual response regulators interact to control nitrate- and nitrite-responsive gene expression in Escherichia coli, p. 233-252. In J. A. Hoch and T. J. Silhavy (ed.), Two-component signal transduction. ASM Press, Washington, DC.
- 33.Strauch, K. L., K. Johnson, and J. Beckwith. 1989. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J. Bacteriol. 171:2689-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tokishita, S. I., A. Kojima, H. Aiba, and T. Mizuno. 1991. Transmembrane signal transduction and osmoregulation in Escherichia coli: functional importance of the periplasmic domain of the membrane-located protein kinase EnvZ. J. Biol. Chem. 266:6780-6785. [PubMed] [Google Scholar]
- 35.van Alphen, W., and B. Lugtenberg. 1977. Influence of osmolarity of the growth medium on the outer membrane protein pattern of Escherichia coli. J. Bacteriol. 131:623-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vuong, P., D. Bennion, J. Mantei, D. Frost, and R. Misra. 2008. Analysis of YfgL and YaeT interactions through bioinformatics, mutagenesis, and biochemistry. J. Bacteriol. 190:1507-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Werner, J., and R. Misra. 2005. YaeT (Omp85) affects the assembly of lipid-dependent and lipid-independent outer membrane proteins of Escherichia coli. Mol. Microbiol. 57:1450-1459. [DOI] [PubMed] [Google Scholar]
- 38.Wu, T., J. Malinverni, N. Ruiz, S. Kim, T. J. Silhavy, and D. Kahne. 2005. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell 121:235-245. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto, K., and A. Ishihama. 2006. Characterization of copper-inducible promoters regulated by CpxA/CpxR in Escherichia coli. Biosci. Biotechnol. Biochem. 70:1688-1695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.