Abstract
Flagellar propulsion of swimming Escherichia coli produces circling clockwise motions near planar solid surfaces. Counterclockwise motion was first reported near air-TN medium interfaces, showing that slip at the interface is a key parameter of bacterial swimming.
Most bacteria are endowed with several types of motility adapted to the variety of their habitats. Swimming, swarming, gliding, twitching, and floating are carried out by specific machineries (7). Swimming and swarming motilities both rely on flagellar propulsion produced by motors that rotate helical flagellin filaments (2). Swimming is the effective way to move and spread in bulk. Swarming, a cooperative multicellular swimming, is currently viewed as specific to moist surfaces (6). While swarming leads to bacterial dissemination, single-cell swimming motility is of uncertain advantage for bacterial dispersal on surfaces. Indeed, the random walk-like trajectory (1) of a swimming bacterium tends to become confined to a clockwise (CW) circular motion near solid surfaces (3, 4, 10, 13). Hydrodynamics accounts for this CW circular motion (8, 9, 11) by assuming it to be impossible for the fluid to slip along the surface. This “no-slip” boundary condition implies an increase in the drag that the confined fluid exerts at the bottom of a moving body. The flagellar bundle and the cell body counterrotate near a surface; therefore, they experience opposite sideways forces that bend the trajectory CW. Whether this framework is valid for all interfaces, particularly for slipping interfaces, is the issue addressed experimentally in this study. Since maximal slip occurs at a free surface, an experimental set-up was designed to (i) compare the trajectories of bacteria swimming near an air-culture medium interface with those near a solid-culture medium interface and (ii) check diffusivity in air-culture medium interfaces (shown to be nonslipping in swarming experiments [14]).
E. coli cells of the AW574 strain (wild type for motility [12]) were grown overnight in TN medium (4 g·liter−1 Bacto tryptone, 2.5 g·liter−1 NaCl, 0.4% [vol] glycerol) at 32°C under gyration at 300 rpm, then diluted to an optical density at 600 nm (OD600) of 0.05 and grown again at 32°C. Bacteria were sampled in the exponential phase and diluted 1:10 in TN at 32°C without or with 260-nm-radius polystyrene beads (2 × 10−4% solids). A silicon square frame fixed on a cleaned glass coverslip was filled with this solution (∼1.5 μl), locating the air-TN interface 200 μm above the glass surface. The Si frame was mounted in the 200-μl well of a WillCo dish glass bottom dish in which drops of water (15 μl) were deposited to reach the saturation vapor pressure once closed. This setup was fixed on the microscope stage to observe at room temperature the air-TN interface from either above or below. A volume of 15 μl of bacterial solution was deposited on various glass surfaces: untreated, siliconized (Hampton Research), and 1-octenyltrichlorosilane coated. Dark-field imaging was performed with a DMIRBE Leica microscope (40× air objective) and an electron-bombarded charge-coupled device (EB-CCD) Hamamatsu camera (30 frames per second). Tracks over 2.3 s long and those over 10 s long were determined by following the centroid of the cell body and the beads throughout a 15-s acquisition sequence using the particle tracking feature of SimplePCI. Circular motion of a swimming bacterium was characterized by the median value of the curvature along its trajectory. Raw instantaneous curvatures of a trajectory were treated twice with a moving 5-point-average filter. By convention, a positive sign was attributed to clockwise rotation when viewed from above and a negative sign was attributed to counterclockwise (CCW) rotation in an interface having the TN medium on top of the glass or of the air. Diffusion coefficients (D) were determined from the slope of the mean square distance according to the equation <(xi − xj)2> = 4Dt, where xi and xj are the displacements of two close beads trapped at the air-TN interfaces taken at 3 s. (see Fig. SI1 in the supplemental material).
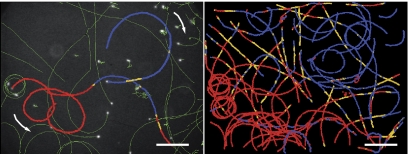
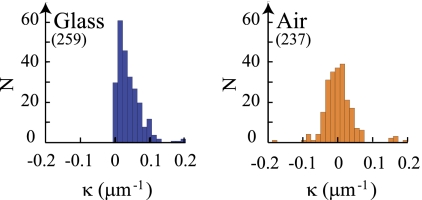
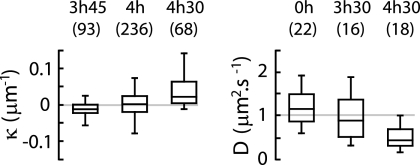
Bacteria swim CW on all glass surfaces (see Fig. SI2 in the supplemental material) but can swim CW or CCW or can alternate CW and CCW motions near the air-TN interface (Fig. 1, left) as demonstrated by the distribution of the median curvatures (Fig. 2). CW and CCW motions are observed at different spatial regions in the same air-TN interface (Fig. 1, right). The fraction of CW trajectories increases with growth time, while the average diffusion coefficient of the beads in the air-TN interface decreases (Fig. 3). Diffusion of the beads varies with their spatial location in the interface: beads having a low diffusion coefficient were observed associated with CW trajectories, and beads having a high diffusion coefficient were observed with CCW trajectories (see Fig. SI3 in the supplemental material).
FIG. 1.
(Left) Image of the trajectories (green) of E. coli (4-h growth time) swimming in the air-TN interfaces superimposed on the last frame of one acquisition (bacteria in white). Motion orientation of three highlighted trajectories is indicated by arrows, and values of instantaneous curvature (κi) are color coded. (Right) κi image of the trajectories of three consecutive acquisitions. CCW, red (κi <−0.007 μm−1); linear, yellow (−0.007 μm−1 < κi < 0.007 μm−1); CW, blue (0.007 μm−1 < κi). Bar, 35 μm.
FIG. 2.
Distribution of the median curvature (κ) of the trajectories of E. coli (4-h growth time) swimming in the glass-TN interface and air-TN interface. Bin size (0.013 μm−1) is twice the minimum detection limit. Numbers of trajectories are in parentheses.
FIG. 3.
Box plots showing κ of the trajectories of E. coli swimming (growth time specified) (left) and D of polystyrene beads in three air-TN interfaces (298 by 224 μm2) (right). Center lines show medians, and edges show the first and third quartiles. “Whiskers” extend to the largest and smallest data points within 1.5 interquartile ranges of the first and third quartiles. Numbers of trajectories are in parentheses.
CCW swimming of E. coli is first reported here at the air-TN interface, where the breaking of the no-slip boundary condition is evidenced by higher diffusion coefficients of beads trapped at the interface. The interfaces are heterogeneous and evolve in time, being progressively covered by areas of lower interfacial diffusivity and higher occurrence of CW motion. This progressive freezing occurs only in the presence of E. coli, pointing to the possibility that E. coli produces some compounds with surfactant properties.
The actuality of CCW motion opens new ways of controlling the motion of swimming bacteria in microfluidic devices (3, 5) by introducing adequate slip boundaries. The alternated CW and CCW motions that expand the surface displacement of a bacterium show that near some interfaces, in particular near the air-liquid interfaces common in the natural biotopes of prokaryotes, swimming contributes to bacterial dispersal and to strain dissemination.
Supplementary Material
Footnotes
Published ahead of print on 1 October 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Berg, H. C. 1993. Random walks in biology, p. 164. Princeton University Press, Princeton, NJ.
- 2.Darnton, N. C., L. Turner, S. Rojevsky, and H. C. Berg. 2007. On torque and tumbling in swimming Escherichia coli. J. Bacteriol. 189:1756-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiLuzio, W. R., L. Turner, M. Mayer, P. Garstecki, D. B. Weibel, H. C. Berg, and G. M. Whitesides. 2005. Escherichia coli swim on the right-hand side. Nature 435:1271-1274. [DOI] [PubMed] [Google Scholar]
- 4.Frymier, P. D., R. M. Ford, H. C. Berg, and P. T. Cummings. 1995. Three-dimensional tracking of motile bacteria near a solid planar surface. Proc. Natl. Acad. Sci. U. S. A. 92:6195-6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galajda, P., J. Keymer, P. Chaikin, and R. Austin. 2007. A wall of funnels concentrates swimming bacteria. J. Bacteriol. 189:8704-8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harshey, R. 2003. Bacterial motility on a surface: many ways to a common goal. Annu. Rev. Microbiol. 57:249-273. [DOI] [PubMed] [Google Scholar]
- 7.Jarrell, K. F., and M. J. McBride. 2008. The surprisingly diverse ways that prokaryotes move. Nat. Rev. Microbiol. 6:466-475. [DOI] [PubMed] [Google Scholar]
- 8.Lauga, E., W. R. DiLuzio, G. M. Whitesides, and H. A. Stone. 2006. Swimming in circles: motion of bacteria near solid boundaries. Biophys. J. 90:400-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li, G., L. K. Tam, and J. X. Tang. 2008. Amplified effect of Brownian motion in bacterial near surface swimming. Proc. Natl. Acad. Sci. U. S. A. 105:18355-18359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda, K., Y. Imae, J. I. Shioi, and F. Oosawa. 1976. Effect of temperature on motility and chemotaxis of Escherichia coli. J. Bacteriol. 127:1039-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramia, M., D. L. Tullock, and N. Phan-Thien. 1993. The role of hydrodynamic interaction in the locomotion of microorganisms. Biophys. J. 65:755-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tso, W.-W., and J. Adler. 1974. Negative chemotaxis in Escherichia coli. J. Bacteriol. 118:560-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vigeant, M. A., and R. M. Ford. 1997. Interactions between motile Escherichia coli and glass in media with various ionic strengths as observed with a three-dimensional-tracking microscope. Appl. Environ. Microbiol. 63:3474-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang, R., L. Turner, and H. C. Berg. 2010. The upper surface of an Escherichia coli swarm is stationary. Proc. Natl. Acad. Sci. U. S. A. 107:288-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.