Abstract
The recently described species Aspergillus lentulus exhibits differential and reduced susceptibilities to echinocandins and other antifungal drugs in vitro. A. lentulus isolates overall are less susceptible to caspofungin, although they maintain susceptibility to anidulafungin and micafungin. Mutations or polymorphisms in fks, the gene encoding the catalytic subunit of β-1,3-glucan synthase, are known to confer decreased susceptibility to echinocandins in Candida spp. and Aspergillus fumigatus. The analysis of the A. lentulus fks sequence did not reveal a polymorphism at any of the known hot-spot regions of the gene. Caspofungin and micafungin kinetic inhibition profiles of the A. lentulus glucan synthase were comparable to those from susceptible A. fumigatus enzymes. Although the basal cell wall chitin levels in A. lentulus averaged 60% of those in A. fumigatus, echinocandin treatment promoted the increase of cell wall chitin in both organisms, indicating that A. lentulus displays a compensatory chitin response similar to that of A. fumigatus. The data suggest that differential echinocandin susceptibilities in A. lentulus are independent of the echinocandin target, Fksp, and they emphasize the potential that the drugs' capacity to inhibit the target enzyme is unequal at the cellular level.
Invasive aspergillosis (IA) is an infection that continues to be associated with high mortality among numerous patient populations despite the availability of multiple antifungal drugs, which include newer-generation azoles and echinocandins. The most common causative agent of IA is Aspergillus fumigatus. Recently, with the use of molecular identification methods, “cryptic” species within this group have been reported as important causes of disease (1, 3); these findings emphasize a new understanding that morphotype largely distinguishes a group of organisms but does not define species.
We described a new species of Aspergillus, called A. lentulus (3, 5), that has morphological characteristics indistinguishable from those of A. fumigatus. This organism now has been identified within clinical isolate collections in centers worldwide (1, 43). A salient phenotype of A. lentulus is its reduced susceptibility to multiple antifungal drugs (1-4, 43). In general, A. lentulus isolates are less susceptible to amphotericin B (AMB), itraconazole (ITRA), voriconazole (VORI), and caspofungin (CAS) than A. fumigatus isolates in vitro. A curious aspect of this phenotype is the reduced susceptibility to CAS while maintaining relatively high susceptibility to the other echinocandins, micafungin (MICA) and anidulafungin (ANID).
Echinocandins are a new class of antifungals, with agents approved for the treatment of candidiasis and aspergillosis. The three available echinocandins are lipopeptides that contain a cyclic hexapeptide core linked to variably configured lipid side chains. These drugs inhibit glucan synthase (GS), an enzyme complex responsible for the synthesis of β-1,3-linked glucan, a major component of the cell wall. The GS complex typically is composed of two subunits, a plasma membrane-bound catalytic subunit (Fksp) and an activating subunit (Rho1p). Inhibition is thought to be noncompetitive, but the precise mechanism by which these drugs interact with the enzyme complex to inhibit the synthesis of glucan is not well defined. Results of new studies suggest a model in which the drugs bind into the cell wall, with intercalated lipid side chains having functional effect (David Perlin, personal communication). High-level cross-resistance with the echinocandin class can be found in organisms that have amino acid substitutions within the Fks protein (8, 30, 33), with intrinsic or acquired resistance to echinocandins in yeasts associated with amino acid changes at hot-spot conserved regions of the catalytic Fks protein.
β-1,3-Glucan is the main component of the fungal cell wall. However, other polymers, such as chitin, galactomannan, and β-1,4-glucan, are integral parts of the organization of the A. fumigatus cell wall (15, 25). Chitin, a linear polymer of β-1,4 N-acetylglucosamine, has been associated with echinocandin susceptibilities, because the level of the polymer increases in the presence of echinocandins in yeasts and in A. fumigatus (10, 17, 27, 38, 42). This compensatory response is thought to allow the fungi to escape from lethality when the β-1,3-glucan levels drop during treatment with echinocandins. The calcineurin signaling pathway is associated with this compensatory increase in chitin, and A. fumigatus mutants deleted for the calcineurin catalytic subunit cnaA or the downstream transcriptional factor crzA are more susceptible to echinocandins than the wild-type counterpart (17).
We investigated whether these mechanisms are associated with the unusual differential echinocandin susceptibility of A. lentulus. In these studies, we show that the differential echinocandin susceptibilities are not correlated with alterations of the drug target and are not directly associated with increased chitin levels after echinocandin treatment. Our results suggest that other cellular factors contribute to the A. lentulus echinocandin phenotype that allows for an adaptation or tolerance to CAS but not to ANID or MICA.
MATERIALS AND METHODS
Fungal strains and media.
The sequenced A. fumigatus strain Af293 (28) was used as the echinocandin-susceptible Aspergillus strain. Isolate FH5 (3) (ATCC MYA-3566) was used as the prototypical A. lentulus strain. Other A. lentulus isolates within our collection (4, 36) also were used in antifungal susceptibility testing and chitin assays. The A. fumigatus strain B-5233, obtained from June Kwon-Chung (National Institutes of Health, Bethesda, MD), also was used in chitin assays. All organisms were routinely maintained on potato dextrose agar (PDA; Difco, Becton Dickinson and Co.) at 37°C. Isolates' conidia were suspended in 8% skim milk and kept frozen at −80°C for long-term storage.
Antifungal susceptibility testing.
Broth microdilution susceptibility testing was performed according to the method described in document M38-A2 (9). Aspergillus spp. were grown on PDA for 3 to 4 days at 37°C prior to harvesting the conidia by suspension in phosphate-buffered saline (PBS; Mediatech, Inc.), 0.05% Tween 20 (Sigma), followed by passage through sterile gauze to trap hyphal elements. Inocula were prepared by adjusting conidial suspensions to a concentration of approximately 1 × 104 per ml by using a spectrophotometer or by counting and delivering 100 μl to wells containing 100 μl of antifungal drug dilutions in RPMI-1640, 165 mM morpholinepropanesulfonic acid (MOPS), pH 7.0, at twice their final concentration in 96-well plates. The antifungals used in this were caspofungin (CAS; Merck and Co.), anidulafungin (ANID; Pfizer), and micafungin (MICA; Astellas Pharma Inc.). The minimal effective concentration (MEC) was determined as the lowest concentration of echinocandin that led to the growth of small, rounded, compact hyphae relative to growth in the control growth well (9). The recent definition of MEC instituted by the Clinical and Laboratory Standards Institute (CLSI) in document M38-A2 is referred to here as the macro MEC. Prior to document M38-A2, the MEC was defined as the lowest concentration of lipopeptides (i.e., CAS) that induced the formation of aberrant hyphae, such as increased the branching and ballooning of hyphal tips (24). We refer to this echinocandin concentration as the micro MEC.
Assessment of cell morphology by microscopy.
The cellular effect of echinocandins was visualized by the phase-contrast microscopy of fungi grown in RPMI 1640 as described for microdilution susceptibility testing. Forty-eight-hour cultures were observed under 20× magnification with a Zeiss Axio observer inverted microscope equipped with a QImaging Micropublisher camera. Images were captured with QCapture 2.54 (Quantitative Image Corp.) and manipulated with Photoshop CS4 (Adobe Systems Inc.).
Sequencing of the A. lentulus fks gene.
Sequence data for the A. fumigatus Af293 (28) fks gene (gene locus AFUA_6G12400) was used to design oligonucleotides to amplify fks from FH5 genomic DNA (36) as follows: amplification reaction mixtures contained 0.2 μM each oligonucleotide 5.8F 5′-ATGTCGGGATATCAACAAGGGG-3′ and 5.8R 5′-GCACCGATGACGTTGCTCGATC-3′, 0.2 mM deoxynucleoside triphosphates (dNTPs), 2 mM MgSO4, 1× high-fidelity PCR buffer (Invitrogen), 50 ng of FH5 genomic DNA (36), and 1.0 U high-fidelity platinum Taq DNA polymerase (Invitrogen). The reaction mixtures were incubated in a thermal cycler (Techne TC-512) at 94°C for 2 min, followed by 35 cycles of 94°C for 30s, 59°C for 30s, and 68°C for 6 min. The 5.8-kb amplicon corresponding to the fks gene subsequently was cloned into pCR-Blunt-II according to the manufacturer's recommendations (Invitrogen). The A. lentulus fks gene was sequenced on both strands by primer walking. The sequence data were assembled, translated, and aligned to the A. fumigatus Fksp sequence (GenBank accession number XP_751118) using ClustalW algorithm within the software MacVector 8.0 (Accelrys). The FH5 fks sequence was deposited at GenBank (http://www.ncbi.nlm.nih.gov/GenBank/) and given the accession number GU815239.
Glucan synthase inhibition assays.
Yeast malt medium (YME; 0.4% yeast extract, 1.0% malt extract, 0.4% glucose) was inoculated with 1 × 106 to 5 × 106 FH5 conidia per ml, and the culture was grown for 24 h at 30°C with shaking. Mycelia were harvested, washed, and disrupted with a French press while suspended in extraction buffer (50 mM HEPES, pH 7.5, 1 mM EDTA, 1 mM dithiothreitol, 10% glycerol, 1 μg/ml aprotinin, 5 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 10 μM GTPγS). Crude membranes were prepared by centrifugation as described before (22). The glucan synthase was purified from the crude membrane by-product entrapment (22), and the sensitivity to CAS and MICA was measured in a polymerization assay utilizing [3H]UDP-glucose as the substrate (30). The incorporated counts per minute were analyzed with the Prism software (GraphPad Software, Inc.) using the sigmoidal dose-response (variable slope) curve-fitting algorithm.
Chitin assays.
The chitin content was determined by the method of Lehmann and White (26) as modified by Fortwendel et al. (17). Sabouraud's broth (SAB), Aspergillus minimal medium with glucose (MMG) (35), or RPMI 1640 was inoculated with freshly harvested conidia at 1 × 106 per ml and incubated at 37°C with shaking for 48 h. The mycelia were harvested between Miracloth sheets (Calbiochem) and washed once with 0.1N NaOH and twice with sterile water before being freeze-dried overnight. Two 5-mg samples were taken from each lyophilized mycelia and heated to 130°C for 1 h in 3 ml of saturated KOH. After the mixture was cooled to room temperature, 8 ml of ice-cold 75% ethanol was added and the mixture vortexed until the phases were miscible. The tubes were incubated in an ice-water bath for 15 min, followed by the addition of 0.3 ml of a freshly prepared 13.3% (wt/vol) Celite (Celite 545, Fisher) suspension in 75% ethanol and vortexing. The tubes were centrifuged (1,500 × g for 5 min, 2°C) and the pellets washed once with 10 ml ice-cold 40% ethanol and twice with ice-cold water. The pellets were brought up to 0.5 ml with water prior to adding 0.5 ml of 5% (wt/vol) NaNO2 and 0.5 ml (wt/vol) of KHSO4. Standard reaction mixtures were set up in parallel consisting of 0.2 ml of 10 μg/ml glucosamine or water plus 0.2 ml NaNO2 and 0.2 ml of KHSO4. All reactions were gently mixed three times during a 15-min period at room temperature before centrifuging the unknowns at 1,500 × g for 2 min at 2°C. A portion of each unknown (25 to 50 μl) was transferred to new glass tubes and brought to a volume of 0.6 ml with water. This was followed by the addition of 0.2 ml of 12.5% (wt/vol) ammonium sulfamate to all tubes and vortexing for a 5-min period. A volume of 0.2 ml of freshly prepared 5 mg/ml of 3-methyl-2-benzothiazolinone hydrazone hydrochloride hydrate (MBTH; Sigma) was added, and the tubes were incubated at 130°C for 3 min. After the mixture was cooled to room temperature, 0.2 ml of FeCl3 (0.49% [wt/vol]; 30.7 mM) was added and the tubes incubated at room temperature for 25 min. The absorbance of the neat colored solutions was measured at 650 nm, or dilutions were made in water to bring the reading within the spectrophotometer's range. The absorbance value of the water standard was subtracted from all sample values before calculating the chitin content by the following equation: (A650 unknown/A650 2 μg glucosamine) × correction for the fraction used in the assay × 2 μg × dilution of the colored solution (when necessary). The values are reported as glucosamine equivalents per 5 mg of mycelia. In cultures treated with echinocandins, 1 × 106 conidia per ml were incubated in SAB with echinocandins at 0.5 μg/ml for 48 h at 37°C with shaking. The mycelia were harvested and processed for chitin content determinations exactly as described above. The values shown are averages from at least two independent biological replicates sampled in duplicate. Statistical analyses were performed in Prism (GraphPad Software, Inc.) using the two-tailed Student's t test assuming unequal variance.
Calcofluor white staining and microscopy.
Chitin levels also were assessed by staining A. fumigatus and A. lentulus hyphal elements with calcofluor white (20). Conidia from Af293 and FH5 were plated at a concentration of 2 × 105 per ml onto coverslips submerged in SAB and allowed to germinate and grow at 37°C for 15 h. The coverslips with adherent mycelia were transferred to 3.7% formaldehyde in PBS and 0.2% Triton X-100 for fixation at room temperature for 45 min. The coverslips subsequently were rinsed with distilled water and placed in a solution of 5 μM calcofluor white (Molecular Probes, Invitrogen) for 10 min at room temperature. Finally, the coverslips were rinsed twice with distilled water and mounted onto clean slides in VECTASHIELD (Vector Labs) solution. The stained mycelia were observed at 20× magnification with a Zeiss Axio observer inverted microscope under UV wavelength equipped with a QImaging Micropublisher camera. Images were captured with DP2-BSW (Olympus) software and manipulated with ImageJ (version 1.41o; http://rsbweb.nih.gov/ij/).
RESULTS
Microscopic evaluation of A. lentulus morphology after treatment with echinocandins.
The current determination of echinocandin MEC relies on the macroscopic interpretation of slowed growth as apparent by the formation of small, compact hyphal forms (spider colonies) visible to the naked eye or with the aid of a mirror (9). Our initial A. lentulus MEC determinations (4, 5) were based on the criteria described by Kurtz et al. (24) that rely upon the microscopic evaluation of mycelia for the appearance of abnormal hyphal morphologies. Since our earlier reports of A. lentulus (3-5), the echinocandins anidulafungin (ANID) and micafungin (MICA) have become available in the United States (40). The reevaluation of our A. lentulus isolates for susceptibility to CAS, utilizing the recommended methods, resulted in disparate values for CAS (Table 1).
TABLE 1.
Echinocandin susceptibility testing
| Species | Strain | MEC (μg/ml) |
||
|---|---|---|---|---|
| ANID | CAS | MICA | ||
| A. lentulus | FH1 | 0.06c | 0.25a/16b | 0.12c |
| A. lentulus | FH4 | <0.03 | 0.25/32 | <0.03 |
| A. lentulus | FH5 | 0.06 | 0.25/32 | 0.06 |
| A. lentulus | FH6 | 0.12 | 0.25/4 | 0.25 |
| A. lentulus | FH7 | 0.06 | 0.25/16 | 0.06 |
| A. lentulus | FH84 | 0.12 | 0.5/2 | 0.12 |
| A. lentulus | FH85 | 0.25 | 0.12/16 | 0.25 |
| A. lentulus | FH86 | 0.06 | 0.25/16 | <0.03 |
| A. lentulus | FH219 | 0.25 | 0.25/16 | 0.25 |
| A. lentulus | FH220 | 0.12 | 0.25/16 | 0.25 |
| A. lentulus | FH231 | 0.12 | 0.25/2 | 0.12 |
| A. lentulus | FH238 | 0.06 | 0.25/2 | 0.06 |
| A. lentulus | FH239 | 0.12 | 0.12/2 | 0.12 |
| A. lentulus | FH265 | 0.06 | 0.12/2 | 0.06 |
| A. fumigatus | Af293 | 0.06 | 0.12 | 0.06 |
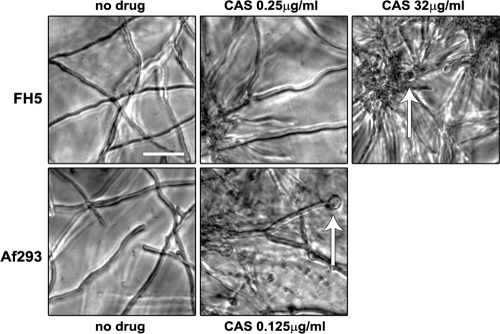
Upon the careful microscopic examination of A. lentulus FH5 and other A. lentulus isolates (data not shown), we observed that slowed growth and aberrant hyphal forms do not coincide at the same CAS concentration (Fig. 1). In contrast, the same CAS concentration elicited both slowed growth and the formation of aberrant hyphae in A. fumigatus Af293. Odabasi et al. also noted equivalent A. fumigatus echinocandin MEC readings between macroscopically and microscopically determined MEC endpoints (29). The separation of slowed growth and aberrant hyphal morphology was not apparent when either FH5 or Af293 was treated with the other two echinocandins (Table 1 and data not shown). These observations suggest that the inhibition of A. lentulus glucan synthesis by CAS follows a bimodal hyphal phenotype in which slowed growth is followed by the increased branching and ballooning of hyphal tips. This also suggests that the glucan synthase inhibition activity of CAS differs from that of the other two echinocandins at the cellular level.
FIG. 1.
Microscopic evaluation of A. fumigatus and A. lentulus grown with or without CAS. A. fumigatus (Af293) and A. lentulus (FH5) conidia were plated in RPMI 1640 with increasing concentrations of CAS per the microdilution susceptibility testing method (9). Hyphal morphologies at the micro MECs were captured under bright field at 20× magnification using an inverted microscope. Arrows point to ballooned hyphal tips, a criterion of the micro MEC. A. lentulus is shown at both the macro MEC (0.25 μg/ml) and at the micro MEC (32 μg/ml). Size bar, 50 μm.
Sequence analysis of A. lentulus fks.
Amino acid changes at three regions of the echinocandin drug target Fks1p in yeasts are known to confer resistance to all three drugs (8, 18, 19, 21, 30, 33). We investigated whether mutations within the homologous hot-spot regions of the A. lentulus Fksp could account for the differential echinocandin susceptibilities. The entire fks gene was amplified by PCR using A. fumigatus sequence data (Af293; gene locus AFUA_6G12400) and sequenced on both strands by primer walking. The translated sequence of A. lentulus fks (AlFksp) shows high homology to the Af293 Fksp amino acid sequence; AlFksp is 98% identical and 99% similar to AfFksp. Further, the amino acids that make up the Fksp hot-spot regions do not differ between the organisms (data not shown), indicating that the AlFksp hot-spot genotype is that of an echinocandin-sensitive Aspergillus spp.
The A. lentulus glucan synthase complex is sensitive to both CAS and MICA.
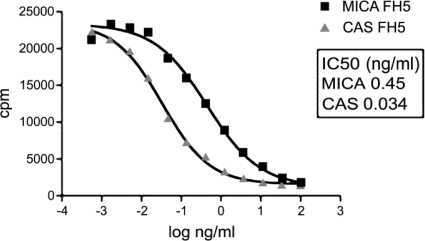
The translated amino acid sequence of A. lentulus did not reveal differences relative to the AfFksp sequence at known hot-spot regions. However, several nonconserved amino acids changes outside the hot spots were noted that could influence susceptibility to CAS. To determine whether other amino acid differences outside the hot-spot regions influenced the susceptibility of the enzyme complex, glucan synthesis inhibition assays were performed with GS isolated from FH5 (Fig. 2). The inhibition curves and 50% inhibitory concentrations (IC50s) for CAS and MICA were comparable to curves and values obtained for the GS inhibition of A. fumigatus Af293 (33), indicating that the A. lentulus GS was fully susceptible to both CAS and MICA. We conclude from these results that the few amino acid differences found in AlFksp do not contribute to the differential echinocandin susceptibility profile, and that AlFksp is sensitive to both CAS and MICA once isolated from its native cellular localization. These data also suggest that other factors outside the AlFksp influence the activity of CAS but do not appear to affect the activity of the other two echinocandins.
FIG. 2.
Kinetics of A. lentulus (FH5) GS inhibition by CAS and MICA. Echinocandin inhibition profiles of GS from FH5. Activity was assessed by the incorporation of [3H]glucose into the radiolabeled product. Concentrations yielding 50% activity (IC50s) were obtained from the inhibition curves and are expressed in ng/ml of echinocandin. Similar IC50s were obtained for the echinocandin-sensitive A. fumigatus (33).
Echinocandins increase the chitin content in A. lentulus.
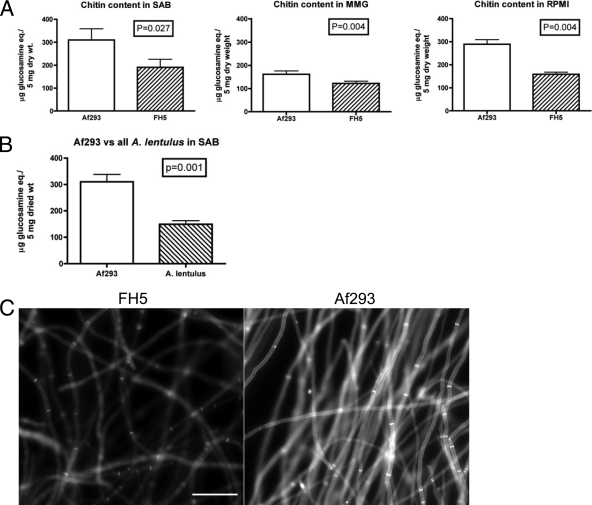
In previous studies with A. fumigatus, treatment with echinocandins results in an increase in chitin synthesis that is correlated with the paradoxical effect observed when the organism is grown in the presence of increasing concentrations of CAS (16, 17). Because chitin content has been associated with survival in the presence of CAS in A. fumigatus and in Candida spp. (38, 41), we investigated whether the chitin content of A. lentulus was affected by echinocandin treatment. Initially, we measured the chitin content of Af293 and FH5 in the absence of drugs to establish baseline values. When Af293 and FH5 were grown in three different media (SAB, RPMI 1640, and MMG), we consistently measured less chitin in FH5 mycelia than in those of Af293 samples, and these differences were statistically significant regardless of medium (Fig. 3A). The strain B-5233, an echinocandin-susceptible A. fumigatus strain, displayed chitin levels similar to those of Af293 (P = 0.74) (data not shown). To ensure that the measurements were not unique to a particular A. lentulus isolate, we determined the chitin content of four other A. lentulus isolates. We found similarly reduced chitin amounts in other A. lentulus isolates that have various micro CAS MEC values (FH1, FH6, FH86, and FH239; Table 1) when grown in SAB (Fig. 3B). This suggests that the reduced chitin content of A. lentulus is a phenotype of the organism and not a unique feature of FH5. It also suggests that the altered chitin content is not a singular explanation for variable CAS susceptibilities.
FIG. 3.
Decreased amounts of chitin in A. lentulus relative to A. fumigatus. (A) Comparison of the total chitin content in A. lentulus (FH5) and A. fumigatus (Af293) grown at 37°C for 48 h in three different media. The values are means of independent biological replicates sampled in duplicate (n = 4). (B) Comparison of five A. lentulus isolates (FH1, FH5, FH6, FH86, and FH239) to A. fumigatus (Af293) grown in SAB. The A. lentulus value is the mean from two independent samples of each isolate (n = 10). The A. fumigatus value is the mean from six independent samples (n = 6). Error bars represent ± standard deviations. Significance was determined by the two-tailed Student's t test assuming unequal variance. (C) Calcofluor white staining of A. fumigatus and A. lentulus hyphal elements to visualize relative chitin levels. Fifteen-hour SAB cultures of A. fumigatus (Af293) and A. lentulus (FH5) grown on coverslips were stained for calcofluor white and observed under UV light. The images were captured at a set exposure time (700 ms) to reflect the difference in calcofluor white staining between the organisms. Size bar, 50 μm.
The chitin levels in the cell wall of Af293 and FH5 also were assessed by calcofluor white staining, a fluorescent dye that predominantly binds to chitin in fungal cell walls (32). Because calcofluor white directly interacts with chitin (12), the degree (brightness) of fluorescence reflects the amount of chitin in the cell wall on a qualitative basis. The cell walls of FH5 stained with calcofluor white fluoresced at a reduced intensity relative to those of Af293, reflecting and supporting our biochemical findings (Fig. 3C). These results further indicate that the A. lentulus cell walls contain less chitin than those of A. fumigatus.
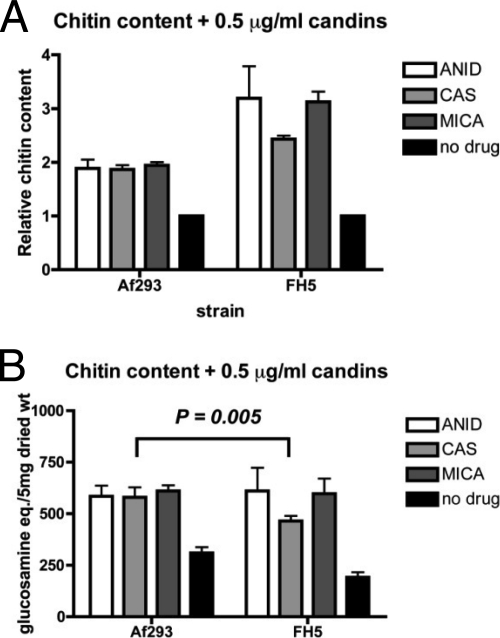
We next measured the effect of echinocandins on the chitin content of Af293 and FH5. Aspergillus spp. were grown in SAB for 48 h in the presence of 0.5 μg/ml of each echinocandin before harvesting the mycelia for chitin assays. In two independent experiments, we measured an increase in the chitin content in both organisms regardless of the echinocandin used. Af293 chitin levels increased to approximately 1.8× that of untreated cells (Fig. 4A), a change that recapitulated the results of previous studies (16). In FH5, the echinocandins induced larger increases in chitin content relative to that of untreated cultures (Fig. 4A). Interestingly, the relative fold increase was not uniform, in that CAS elicited less of an increase in A. lentulus chitin relative to the other two echinocandins. When we compared the measured amounts of chitin between the organisms, the values were not statistically different except for those of the CAS-grown cultures (Fig. 4B). Thus, A. lentulus responds to echinocandins in a manner similar to that of A. fumigatus and other fungi by increasing chitin levels in their cell walls. Ultimately the data do not directly link chitin measurements with differential susceptibilities to the echinocandins, as all three drugs elicited a similar response in both the CAS-susceptible A. fumigatus and in A. lentulus.
FIG. 4.
Increase in total chitin content in both A. fumigatus and A. lentulus upon echinocandin treatment. Equal numbers of A. fumigatus (Af293) and A. lentulus (FH5) conidia were inoculated into SAB with 0.5 μg/ml of each echinocandin (anidulafungin, ANID; caspofungin, CAS; and micafungin, MICA), and total chitin was measured at 48 h. The values are means of independent biological replicates sampled in duplicate (n = 4). (A) Fold change in chitin content in cultures incubated with echinocandins relative to no treatment (black bars). (B) Total chitin contents in cultures grown in the presence or absence of echinocandins (black bars). Significantly less chitin was measured in FH5 CAS samples relative to Af293 CAS. Error bars represent ± standard deviations. Significance was determined by the two-tailed Student's t test assuming unequal variance.
DISCUSSION
Results of these studies bring to light several novel findings. First, we observed a disconnect between susceptibilities interpreted by morphological and microscopic growth parameters, raising concern regarding the validity of the definitions for organisms other than A. fumigatus; importantly, the clinical significance of observed differential MECs have not been tested in vivo. The detailed testing of previously defined mechanisms of echinocandin resistance failed to demonstrate a likely mechanism of differential susceptibilities, in that high CAS MECs do not appear to be caused by mutations in the GS enzyme complex itself. Finally, and perhaps most importantly, the observation of differential echinocandin susceptibilities in A. lentulus emphasizes that these drugs may not access and inhibit the cellular GS enzyme complex in a similar fashion; potential explanations include differential drug uptake, GS complex exposure, the avidity of the drugs for the GS complex in the cell, and drug sequestration.
Echinocandins are cyclic lipopeptides with similar chemical structures derived from naturally occurring fungal metabolites. They have approximately the same molecular mass of 1,200 Da and mainly are distinguished by the composition of their N-linked acyl lipid side chains (6, 13). Early investigators determined that the calculated partition or distribution coefficient (measure of solubility) of the lipophilic side chain correlated with whole-cell antifungal activity (23). The chemistry of how echinocandins interact with the cellular GS complex is not completely understood (31). The results of recent studies suggest that the drugs function by an outside-in mechanism, with the cyclic peptide nucleus remaining in the cell wall and the side chain appearing to inhibit the GS enzyme complex by intercalation into the plasma membrane (David Perlin, personal communication). If the lipid side chains affect membrane solubility and subsequently the activity of the drug, the local environment (cell membrane/cell wall) is predicted to influence the antifungal potency of each echinocandin to different degrees depending upon the nature of the lipid side chain. Perhaps CAS activity is most susceptible to chemical properties imparted by the fungal cell membrane/cell wall, potentially explaining differential echinocandin susceptibility in A. lentulus and the paradoxical growth effect, which is predominantly observed with CAS (7, 14, 16, 37-39, 42). The A. lentulus GS complex isolated from the cell is sensitive to both CAS and MICA, supporting the notion that the environment of the cellular GS complex influences the effectiveness of CAS but not that of MICA.
The activity of echinocandins against filamentous fungi, including Aspergillus spp., can be difficult to quantify compared to that of yeasts, and recommended in vitro testing methods have changed recently. The echinocandins cause aberrant growth of hyphae at apical tips and the increased branching of hyphal elements, a phenotype that initially was used to establish the endpoint of the MEC (24). More recently, the CLSI has modified the definition of MEC to be the concentration that leads to the growth of small, rounded, compact hyphal forms upon visual inspection (9), an indication that growth has slowed relative to that of the no-drug control. One assumption of this definition is that the inhibition of the GS will affect hyphal growth equally across species of filamentous fungi and generate rounded, compact hyphal forms. A. lentulus displays a separation of slowed growth (macro MEC) and the induction of aberrant hyphal morphologies (micro MEC) that results in disparate CAS MECs, depending upon methodology. The micro/macro MEC data suggest that the activity of CAS at the cellular level is not identical to that of the other two echinocandins, and that slowed growth and aberrant hyphae formation are not directly related. It raises questions regarding whether the microscopic morphological changes and slowed growth necessarily reflect similar biologic pathways, and whether they accurately reflect susceptibility to echinocandin drugs in this Aspergillus species.
It is possible that in A. lentulus CAS does not induce a stress response(s) equivalent to that of the other echinocandins, and this appears phenotypically as the separation of MEC parameters. As noted above, the paradoxical growth of Aspergillus and Candida spp. in the presence of high levels of echinocandins is more commonly observed with CAS (7, 14, 16, 37-39, 42), also potentially implying that the precise mechanism by which these drugs inhibit the cellular GS complex is not the same. Consistently with our conclusions, Fortwendel et al. hypothesize that the paradoxical growth recovery of A. fumigatus reflects separate mechanisms that allow survival and permit radial growth in the presence of high CAS concentrations but not in ANID or MICA (16). Because the nature of the interaction between the echinocandins and the GS at the cell membrane is not fully understood (31), we cannot presently appreciate what specific factors impact the interaction between the echinocandins and the GS at the cellular level. However, we speculate that the cell wall architecture impacts echinocandin activity and likely influences the nature of the cellular response(s) to echinocandin-induced cell wall perturbations.
The fungal cell wall is a dynamic structure that responds to environmental stressors. Echinocandins induce a cellular stress response that is mediated by calcineurin signaling and by the protein kinase C cell wall integrity pathway (11, 31). Although most prior studies have been performed in yeasts, similar signaling and cell integrity pathways appear to exist in molds. In A. fumigatus, calcineurin signaling is implicated in the compensatory increase in cell wall chitin in response to all three echinocandins and in paradoxical growth in high CAS concentrations (16, 17). Similarly, CAS shows strong in vitro synergism with the calcineurin inhibitor tacrolimus (FK-506) against CAS-resistant Fusarium spp. (34), demonstrating an analogous need for calcineurin signaling in response to cell wall perturbation in these fungi. Since changes in chitin content are associated with the compensatory response to echinocandins, we wished to determine whether chitin could be associated with the differential echinocandin susceptibilities of A. lentulus. When we tested the baseline chitin content in A. lentulus, we found smaller amounts of the polymer that were medium and isolate independent compared to amounts measured in A. fumigatus. These findings suggest that lower chitin levels is a phenotype of A. lentulus and not unique to a particular isolate. The data also imply that the cell wall composition differs from that of A. fumigatus. We corroborated the biochemical findings by staining A. fumigatus and A. lentulus hyphae with calcoflour white, a fluorescent molecule that preferentially stains chitin in the cell wall of fungi (32).
All three echinocandins induced an increase in cell wall chitin in both A. fumigatus and in A. lentulus, showing that both organisms remodel their wall upon cell wall stress. Although the relative change in chitin content was largest in A. lentulus, the measured amounts were not statistically different between the organisms, except for the cultures grown in the presence of CAS. It is possible that the lower compensatory response of A. lentulus is due to decreased cell stress signaling, stemming from the reduced susceptibility of the organism to CAS. The differences in macro and micro MEC values of A. lentulus for CAS may phenotypically indicate a difference in cell signaling relative to the other echinocandins. Alternatively, cell wall architecture and/or composition influence either CAS binding or access to the GS directly. Although we measured differences in the chitin content between species, an association with differential echinocandin susceptibilities was not evident.
These results, specifically, the absence of amino acid changes and the full in vitro sensitivity of the A. lentulus Fkp to CAS (and MICA), suggest that other cellular factors impact CAS MEC phenotypes, and that our observations reflect a type of tolerance to CAS that is not achieved in the presence of the other echinocandins. Our data also highlight the difficulty in quantifying echinocandin susceptibilities, especially for CAS, because the results are methodology dependent. Because the mechanistic nature of the echinocandin inhibition of glucan synthesis is not understood (31), the cellular factors and variables that influence the activity of these drugs are unknown. A further understanding of the processes that generate the micro and macro MEC phenotypes will provide insights into the mechanistic nature of echinocandins and help define a more precise echinocandin MEC and understanding of Fksp-independent reduced susceptibility to, or tolerance of, these drugs.
Acknowledgments
We thank Guillermo Garcia-Effron (David Perlin laboratory) and Ming-Jo Hsu (Merck Laboratories) for technical assistance.
This work was supported by grants to K.A.M. from the NIAID (AI06791) and from Merck Research Laboratories.
Footnotes
Published ahead of print on 20 September 2010.
REFERENCES
- 1.Alcazar-Fuoli, L., E. Mellado, A. Alastruey-Izquierdo, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2008. Aspergillus section Fumigati: antifungal susceptibility patterns and sequence-based identification. Antimicrob. Agents Chemother. 52:1244-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alhambra, A., M. Catalan, M. D. Moragues, S. Brena, J. Ponton, J. C. Montejo, and A. del Palacio. 2008. Isolation of Aspergillus lentulus in Spain from a critically ill patient with chronic obstructive pulmonary disease. Rev. Iberoam. Micol. 25:246-249. [DOI] [PubMed] [Google Scholar]
- 3.Balajee, S. A., J. L. Gribskov, E. Hanley, D. Nickle, and K. A. Marr. 2005. Aspergillus lentulus sp. nov., a new sibling species of A. fumigatus. Eukaryot. Cell 4:625-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balajee, S. A., D. Nickle, J. Varga, and K. A. Marr. 2006. Molecular studies reveal frequent misidentification of Aspergillus fumigatus by morphotyping. Eukaryot. Cell 5:1705-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balajee, S. A., M. Weaver, A. Imhof, J. Gribskov, and K. A. Marr. 2004. Aspergillus fumigatus variant with decreased susceptibility to multiple antifungals. Antimicrob. Agents Chemother. 48:1197-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cappelletty, D., and K. Eiselstein-McKitrick. 2007. The echinocandins. Pharmacotherapy 27:369-388. [DOI] [PubMed] [Google Scholar]
- 7.Chamilos, G., R. E. Lewis, N. Albert, and D. P. Kontoyiannis. 2007. Paradoxical effect of echinocandins across Candida species in vitro: evidence for echinocandin-specific and candida species-related differences. Antimicrob. Agents Chemother. 51:2257-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleary, J. D., G. Garcia-Effron, S. W. Chapman, and D. S. Perlin. 2008. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrob. Agents Chemother. 52:2263-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 10.Cota, J. M., J. L. Grabinski, R. L. Talbert, D. S. Burgess, P. D. Rogers, T. D. Edlind, and N. P. Wiederhold. 2008. Increases in SLT2 expression and chitin content are associated with incomplete killing of Candida glabrata by caspofungin. Antimicrob. Agents Chemother. 52:1144-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowen, L. E., and W. J. Steinbach. 2008. Stress, drugs, and evolution: the role of cellular signaling in fungal drug resistance. Eukaryot. Cell 7:747-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elorza, M. V., H. Rico, and R. Sentandreu. 1983. Calcofluor white alters the assembly of chitin fibrils in Saccharomyces cerevisiae and Candida albicans cells. J. Gen. Microbiol. 129:1577-1582. [DOI] [PubMed] [Google Scholar]
- 13.Eschenauer, G., D. D. Depestel, and P. L. Carver. 2007. Comparison of echinocandin antifungals. Ther. Clin. Risk Manag. 3:71-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleischhacker, M., C. Radecke, B. Schulz, and M. Ruhnke. 2008. Paradoxical growth effects of the echinocandins caspofungin and micafungin, but not of anidulafungin, on clinical isolates of Candida albicans and C. dubliniensis. Eur. J. Clin. Microbiol. Infect. Dis. 27:127-131. [DOI] [PubMed] [Google Scholar]
- 15.Fontaine, T., C. Simenel, G. Dubreucq, O. Adam, M. Delepierre, J. Lemoine, C. E. Vorgias, M. Diaquin, and J. P. Latge. 2000. Molecular organization of the alkali-insoluble fraction of Aspergillus fumigatus cell wall. J. Biol. Chem. 275:27594-27607. [DOI] [PubMed] [Google Scholar]
- 16.Fortwendel, J. R., P. R. Juvvadi, B. Z. Perfect, L. E. Rogg, J. R. Perfect, and W. J. Steinbach. 2010. Transcriptional regulation of chitin synthases by calcineurin controls paradoxical growth of Aspergillus fumigatus in response to caspofungin. Antimicrob. Agents Chemother. 54:1555-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fortwendel, J. R., P. R. Juvvadi, N. Pinchai, B. Z. Perfect, J. A. Alspaugh, J. R. Perfect, and W. J. Steinbach. 2009. Differential effects of inhibiting chitin and 1,3-β-D-glucan synthesis in ras and calcineurin mutants of Aspergillus fumigatus. Antimicrob. Agents Chemother. 53:476-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Effron, G., S. K. Katiyar, S. Park, T. D. Edlind, and D. S. Perlin. 2008. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 52:2305-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Effron, G., S. Park, and D. S. Perlin. 2009. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob. Agents Chemother. 53:112-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris, S. D., J. L. Morrell, and J. E. Hamer. 1994. Identification and characterization of Aspergillus nidulans mutants defective in cytokinesis. Genetics 136:517-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, M., and T. D. Edlind. 2009. A new Fks hotspot for echinocandin resistance in yeast: genetic and topological analysis. Abstr. 109th Gen. Meet. Am. Soc. Microbiol., abstr. F-057/380.
- 22.Kelly, R., E. Register, M. J. Hsu, M. Kurtz, and J. Nielsen. 1996. Isolation of a gene involved in 1,3-beta-glucan synthesis in Aspergillus nidulans and purification of the corresponding protein. J. Bacteriol. 178:4381-4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein, L. L., and L. Li. 1999. Design and preparation of cyclopeptamine antifungal agents. Curr. Pharm. Des. 5:57-71. [PubMed] [Google Scholar]
- 24.Kurtz, M. B., I. B. Heath, J. Marrinan, S. Dreikorn, J. Onishi, and C. Douglas. 1994. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1, 3)-beta-D-glucan synthase. Antimicrob. Agents Chemother. 38:1480-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latgé, J. P. 2007. The cell wall: a carbohydrate armour for the fungal cell. Mol. Microbiol. 66:279-290. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann, P. F., and L. O. White. 1975. Chitin assay used to demonstrate renal localization and cortisone-enhanced growth of Aspergillus fumigatus mycelium in mice. Infect. Immun. 12:987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markovich, S., A. Yekutiel, I. Shalit, Y. Shadkchan, and N. Osherov. 2004. Genomic approach to identification of mutations affecting caspofungin susceptibility in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 48:3871-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nierman, W. C., A. Pain, M. J. Anderson, J. R. Wortman, H. S. Kim, J. Arroyo, M. Berriman, K. Abe, D. B. Archer, C. Bermejo, J. Bennett, P. Bowyer, D. Chen, M. Collins, R. Coulsen, R. Davies, P. S. Dyer, M. Farman, N. Fedorova, N. Fedorova, T. V. Feldblyum, R. Fischer, N. Fosker, A. Fraser, J. L. Garcia, M. J. Garcia, A. Goble, G. H. Goldman, K. Gomi, S. Griffith-Jones, R. Gwilliam, B. Haas, H. Haas, D. Harris, H. Horiuchi, J. Huang, S. Humphray, J. Jimenez, N. Keller, H. Khouri, K. Kitamoto, T. Kobayashi, S. Konzack, R. Kulkarni, T. Kumagai, A. Lafon, J. P. Latge, W. Li, A. Lord, C. Lu, W. H. Majoros, G. S. May, B. L. Miller, Y. Mohamoud, M. Molina, M. Monod, I. Mouyna, S. Mulligan, L. Murphy, S. O'Neil, I. Paulsen, M. A. Penalva, M. Pertea, C. Price, B. L. Pritchard, M. A. Quail, E. Rabbinowitsch, N. Rawlins, M. A. Rajandream, U. Reichard, H. Renauld, G. D. Robson, S. Rodriguez de Cordoba, J. M. Rodriguez-Pena, C. M. Ronning, S. Rutter, S. L. Salzberg, M. Sanchez, J. C. Sanchez-Ferrero, D. Saunders, K. Seeger, R. Squares, S. Squares, M. Takeuchi, F. Tekaia, G. Turner, C. R. Vazquez de Aldana, J. Weidman, O. White, J. Woodward, J. H. Yu, C. Fraser, J. E. Galagan, K. Asai, M. Machida, N. Hall, B. Barrell, and D. W. Denning. 2005. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438:1151-1156. [DOI] [PubMed] [Google Scholar]
- 29.Odabasi, Z., V. Paetznick, J. H. Rex, and L. Ostrosky-Zeichner. 2007. Effects of serum on in vitro susceptibility testing of echinocandins. Antimicrob. Agents Chemother. 51:4214-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park, S., R. Kelly, J. N. Kahn, J. Robles, M. J. Hsu, E. Register, W. Li, V. Vyas, H. Fan, G. Abruzzo, A. Flattery, C. Gill, G. Chrebet, S. A. Parent, M. Kurtz, H. Teppler, C. M. Douglas, and D. S. Perlin. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 49:3264-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perlin, D. S. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 10:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pringle, J. R. 1991. Staining of bud scars and other cell wall chitin with calcofluor. Methods Enzymol. 194:732-735. [DOI] [PubMed] [Google Scholar]
- 33.Rocha, E. M., G. Garcia-Effron, S. Park, and D. S. Perlin. 2007. A Ser678Pro substitution in Fks1p confers resistance to echinocandin drugs in Aspergillus fumigatus. Antimicrob. Agents Chemother. 51:4174-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shalit, I., Y. Shadkchan, G. Mircus, and N. Osherov. 2009. In vitro synergy of caspofungin with licensed and novel antifungal drugs against clinical isolates of Fusarium spp. Med. Mycol. 47:457-462. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu, K., and N. P. Keller. 2001. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics 157:591-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staab, J. F., S. A. Balajee, and K. A. Marr. 2009. Aspergillus section Fumigati typing by PCR-RFLP. J. Clin. Microbiol. 47:2079-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevens, D. A. 2009. Frequency of paradoxical effect with caspofungin in Candida albicans. Eur. J. Clin. Microbiol. Infect. Dis. 28:717. [DOI] [PubMed] [Google Scholar]
- 38.Stevens, D. A., M. Ichinomiya, Y. Koshi, and H. Horiuchi. 2006. Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for beta-1,6-glucan synthesis inhibition by caspofungin. Antimicrob. Agents Chemother. 50:3160-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens, D. A., T. C. White, D. S. Perlin, and C. P. Selitrennikoff. 2005. Studies of the paradoxical effect of caspofungin at high drug concentrations. Diagn. Microbiol. Infect. Dis. 51:173-178. [DOI] [PubMed] [Google Scholar]
- 40.Wagner, C., W. Graninger, E. Presterl, and C. Joukhadar. 2006. The echinocandins: comparison of their pharmacokinetics, pharmacodynamics and clinical applications. Pharmacology 78:161-177. [DOI] [PubMed] [Google Scholar]
- 41.Walker, L. A., C. A. Munro, I. de Bruijn, M. D. Lenardon, A. McKinnon, and N. A. Gow. 2008. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 4:e1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiederhold, N. P. 2007. Attenuation of echinocandin activity at elevated concentrations: a review of the paradoxical effect. Curr. Opin. Infect. Dis. 20:574-578. [DOI] [PubMed] [Google Scholar]
- 43.Yaguchi, T., Y. Horie, R. Tanaka, T. Matsuzawa, J. Ito, and K. Nishimura. 2007. Molecular phylogenetics of multiple genes on Aspergillus section Fumigati isolated from clinical specimens in Japan. Nippon Ishinkin Gakkai Zasshi 48:37-46. [DOI] [PubMed] [Google Scholar]