Abstract
The 8-aminoquinoline tafenoquine showed significant in vitro activity against Leishmania species, including L. donovani amastigotes in macrophages, with 50% inhibitory concentrations (IC50s) between 0.1 and 4.0 μM for both pentavalent antimony (SbV)-sensitive and SbV-resistant strains and by oral administration in BALB/c mice, with 50% effective dose (ED50) values of 1.2 to 3.5 mg/kg for 5 days. Tafenoquine was less active against intracellular Trypanosoma cruzi amastigotes, with an IC50 of 21.9 μM.
The neglected tropical diseases leishmaniasis, Chagas' disease, and human African trypanosomiasis (HAT), caused by trypanosomatid parasites, have a limited number of drugs for treatment and control, all with limitations of toxicity, variable efficacy, long dosing regimens, and/or parenteral administration. Recent reviews have outlined the advances made in the chemotherapy of these diseases over the past decade for visceral leishmaniasis (VL) (1), cutaneous leishmaniasis (CL) (18), Chagas' disease (22), and human African trypanosomiasis (2).
The search for new treatments for these diseases has adopted various strategies, including rational design of drugs (7, 15), screening libraries of synthetic and natural products (11), and therapeutic switching. The more rapid development of a new treatment by the latter approach has been recently demonstrated for Chagas' disease with ergosterol biosynthesis inhibitors (22) and for leishmaniasis with miltefosine and paromomycin (8, 20). The 8-aminoquinolines (Fig. 1) have a long history as antiprotozoal drugs, in particular as antimalarials. Since the 1950s, several have also been reported as being active against Leishmania and Trypanosoma parasites (13, 21). Interest in the activity of this class of compounds for these diseases has been kept in focus by the clinical trials of sitamaquine (WR6026) for VL (12, 23). Sitamaquine also has anti-Trypanosoma cruzi activity (6). Research on another 8-aminoquinoline, NPC1161, has identified an enantiomer with significant antileishmanial activity and a lower toxicity profile (17). Tafenoquine (TFQ) (WR238605), developed, like many agents of this class, by the Walter Reed Army Institute of Research (WRAIR), is now in clinical trials for the radical cure of Plasmodium vivax by GlaxoSmithKline (GSK) and the Medicines for Malaria Venture (MMV) (16). We present here the results of studies of the in vitro and in vivo activities of TFQ against Leishmania donovani and Trypanosoma cruzi. Studies on the mechanism of action of TFQ against Leishmania and activity against Trypanosoma brucei subsp. will be reported elsewhere.
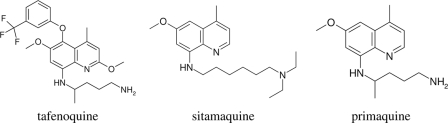
FIG. 1.
Structures of tafenoquine, sitamaquine, and primaquine.
Early tests of TFQ against the promastigotes of different Leishmania species demonstrated 50% inhibitory concentrations (IC50s) below 3 μM (data not shown). Of more clinical relevance, TFQ (GSK, United Kingdom) activity was evaluated, in vitro, against intracellular amastigotes of L. donovani MHOM/ET/67/HU3 (from East Africa), L. donovani MHOM/IN/82/DD8 (from India), and L. donovani BHU1 and BHU3 (antimony-resistant strains from India generously donated by Shyam Sundar). Infected murine peritoneal macrophages were exposed to the drug as previously described (24). The percent infection was calculated, and the IC50s were derived (Prism). Subsequently, TFQ was further evaluated in the BALB/c mouse-L. donovani model of infection (9). Eight-week-old, female mice (Charles River, United Kingdom) were infected with amastigotes harvested from a donor animal. After 7 days, the mice were treated with TFQ formulated in 10% Tween 80-ethanol (EtOH) 70:30 double-distilled water (ddH2O), at 5 mg/kg, by the oral route, for 5 consecutive days. On day 14, the mice were euthanized and liver impression smears were made at necropsy. The amastigote burden was calculated (Leishman-Donovan units [LDUs]) (4), the percent inhibition was derived, and 50% effective dose (ED50) values were calculated. TFQ hydrochloride (racemate batch R146390, positive enantiomer batch R206420, and negative enantiomer batch R206422) and sitamaquine tosylate (batch SLV3L004) were donated by GSK. Miltefosine was donated by Astra Zeneca, United Kingdom, and amphotericin B deoxycholate (Fungizone) was purchased from a commercial supplier. All in vivo experiments were carried out under license at the London School of Hygiene & Tropical Medicine (LSHTM) according to UK Home Office regulations.
The efficacy of TFQ against T. cruzi (Tulahuen-LacZ strain) (5) was tested against amastigotes in vitro. Peritoneal macrophages were infected with T. cruzi harvested from feeder cell layers and exposed to TFQ. β-Galactosidase activity was measured by the addition of Nonidet P-40 (detergent) and chlorophenol red β-d-thiogalactopyranoside (CPRG; developer). Ninety-six-well assay plates were read at 570 λ, and IC50s were calculated. Benznidazole (Roche, Switzerland) was used as a positive control.
Both the racemate and positive and negative enantiomers of TFQ were active against intracellular amastigotes of all of the L. donovani strains tested (see Table 1 for IC50s) and compared favorably with the standard drugs tested alongside. In the BALB/c mouse model, TFQ was equally active against both antimony-sensitive and antimony-resistant strains (BHU1 and BHU3), with no difference seen between the racemate and enantiomers. At 5 mg/kg, TFQ achieved 99% inhibition against all L. donovani species, with the enantiomers performing similarly. In a subsequent dose-response experiment, the ED50 values ranged from 1.01 to 3.5 mg/kg (Table 2) .
TABLE 1.
Activity of TFQ enantiomers against L. donovani SbVa-sensitive strains by the amastigote-peritoneal exudate macrophage model
| Drug (unit of activity) | IC50 (±95% confidence interval) for strainb: |
Dose achieving cytotoxicity in KB cells | |||
|---|---|---|---|---|---|
| HU3 | DD8 | BHU3 | BHU11 | ||
| TFQ (μM) | |||||
| Racemate | 1.75 ± 1.3 | 1.52 ± 1.02 | 2.26 ± 1.4 | 3.69 ± 0.9 | 6.6 |
| Positive enantiomer | 2.26 ± 1.8 | 3.28 ± 0.45 | 0.18 ± 3.3 | NDc | 7.4 |
| Negative enantiomer | 4.04 ± 2.2 | 2.56 ± 0.56 | 0.10 ± 0.85 | ND | 7.0 |
| Sitamaquine (μM) | 1.03 ± 1.02 | 1.86 ± 0.2 | 1.36 ± 0.56 | 2.35 ± 0.6 | 506 |
| SbV (μg/ml) | 4.49 ± 1.83 | 13.79 ± 10.17 | >50 | >50 | >300 |
| Miltefosine (μM) | 2.01 ± 0.84 | 4.85 ± 3.89 | 1.07 ± 0.2 | 1.77 ± 0.2 | 31 |
| Amphotericin B (μM) | 0.03 ± 0.01 | 0.04 ± 0.05 | 0.02 ± 0.01 | 0.07 ± 0.2 | 0.7 |
SbV, pentavalent antimony.
Results for HU3 represent an average of four tests, and those for DD8 represent an average of two tests.
ND, not done.
TABLE 2.
In vivo activities of TFQ, sitamaquine, and SbVa in L. donovani-BALB/c mouse models
| Drug (dose) | In vivo activity (% inhibition [±95% confidence interval]) for strain: |
ED50 (mg/kg) for strain DD8b | ||
|---|---|---|---|---|
| HU3 | BHU1 | BHU3 | ||
| TFQ (5 mg/kg)c | ||||
| Racemate | 99.32 ± 0.31 | 99.49 ± 0.66 | 100 | 1.47 ± 3.9 |
| Positive enantiomer | 99.12 ± 0.45 | NDd | 1.01 ± 9.7 | |
| Negative enantiomer | 99.03 ± 0.52 | ND | 3.5 ± 4.8 | |
| Sitamaquine (5 mg/kg) | 94.48 ± 0.29 | ND | 98.7 ± 0.6 | 2.2 ± 7.2 |
| SbV (15 mg/kg) | 70.93 ± 10.7 | Inactive at 100 mg/kg | Inactive at 100 mg/kg | 60% inhibition |
SbV, pentavalent antimony.
Note that amphotericin B (AmBisome) given intravenously at 1 mg/kg for 3 days gives 93% inhibition.
TFQ results represent oral administration for 5 days.
ND, not done.
We have shown that TFQ, an 8-aminoquinoline in development for the treatment of malaria (21) has, like other drugs of the same class, potential as an oral antileishmanial agent. In both in vitro and rodent models of Leishmania infection, TFQ had similar potency to sitamaquine, the drug currently in clinical development for VL, and NPC111B, which is in preclinical development (21). The limitation of this class has been toxicity, which is of special concern for glucose-6-phosphate dehydrogenase (G6PD)-deficient patients. The extensive antimalarial safety data for TFQ, along with clinical data on sitamaquine for VL, could support the design of appropriate treatment regimes for VL with TFQ. TFQ might also be an oral partner of interest in combination therapies for the treatment of VL (10, 19).
The activities of several series of 8-aminoquinolines against the causative pathogen of Chagas' disease have been published (13). Some have undergone preclinical development (23): for example, moxipraquine for Chagas' disease (3). Sitamaquine showed potential for prevention of T. cruzi transmission through blood transfusion, with activity against trypomastigotes at 4°C (6). We did not find TFQ to be as active in vitro against T. cruzi as other 8-aminoquinolines (Table 3), as others have previously reported (3, 13, 14).
TABLE 3.
In vitro activity of TFQ versus T. cruzi
| T. cruzi strain | IC50, μM (95% confidence interval) |
||||
|---|---|---|---|---|---|
| TFQ |
Sitamaquine | Benznidazole | |||
| Racemate | Positive enantiomer | Negative enantiomer | |||
| Tulahuen-LacZ | 21.9 (3.6-40.3) | 17.5 (5.5-29.5) | 15 (4.6-48.2) | 1.46 (0.93-2.31) | 6.6 (1.56-27.6) |
Acknowledgments
The work at LSHTM was generously supported by Diseases of the Developing World Group at GlaxoSmithKline, United Kingdom.
Footnotes
Published ahead of print on 13 September 2010.
REFERENCES
- 1.Alvar, J., S. Croft, and P. Olliaro. 2006. Chemotherapy in the treatment and control of leishmaniasis. Adv. Parasitol. 61:223-274. [DOI] [PubMed] [Google Scholar]
- 2.Barrett, M. P., D. W. Boykin, R. Brun, and R. R. Tidwell. 2007. Human African trypanosomiasis: pharmacological re-engagement with a neglected disease. Br. J. Pharmacol. 152:1155-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beveridge, E., I. C. Caldwell, V. S. Latter, R. A. Neal, V. Udall, and M. M. Waldron. 1980. The activity against Trypanosoma cruzi and cutaneous leishmaniasis, and toxicity, of moxipraquine (349C59). Trans. R. Soc. Trop. Med. Hyg. 74:43-51. [DOI] [PubMed] [Google Scholar]
- 4.Bradley, D. J., and J. Kirkley. 1977. Regulation of Leishmania populations within the host. I. the variable course of Leishmania donovani infections in mice. Clin. Exp. Immunol. 30:119-129. [PMC free article] [PubMed] [Google Scholar]
- 5.Buckner, F. S., C. L. Verlinde, A. C. La Flamme, and W. C. Van Voorhis. 1996. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob. Agents Chemother. 40:2592-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiari, E., A. B. Oliveira, M. A. Prado, R. J. Alves, L. M. Galvao, and F. G. Araujo. 1996. Potential use of WR6026 as prophylaxis against transfusion-transmitted American trypanosomiasis. Antimicrob. Agents Chemother. 40:613-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corrales, R. M., D. Sereno, and F. Mathieu-Daude. 2010. Deciphering the Leishmania exoproteome: what we know and what we can learn. FEMS Immunol. Med. Microbiol. 58:27-38. [DOI] [PubMed] [Google Scholar]
- 8.Croft, S. L., and J. Engel. 2006. Miltefosine—discovery of the antileishmanial activity of phospholipid derivatives. Trans. R. Soc. Trop. Med. Hyg. 100(Suppl. 1):S4-S8. [DOI] [PubMed] [Google Scholar]
- 9.Croft, S. L., and V. Yardley. 1999. Animal models of visceral leishmaniasis, p. 783-787. In O. Zak (ed.), Handbook of animal models of infection. Academic Press, New York, NY.
- 10.Guerin, P. J., P. Olliaro, S. Sundar, M. Boelaert, S. L. Croft, P. Desjeux, M. K. Wasunna, and A. D. Bryceson. 2002. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect. Dis. 2:494-501. [DOI] [PubMed] [Google Scholar]
- 11.Ioset, J.-R. 2008. Natural products for neglected diseases: a review. Curr. Org. Chem. 12:643-666. [Google Scholar]
- 12.Jha, T. K., S. Sundar, C. P. Thakur, J. M. Felton, A. J. Sabin, and J. Horton. 2005. A phase II dose-ranging study of sitamaquine for the treatment of visceral leishmaniasis in India. Am. J. Trop. Med. Hyg. 73:1005-1011. [PubMed] [Google Scholar]
- 13.Kinnamon, K. E., B. T. Poon, W. L. Hanson, and V. B. Waits. 1997. Evidence that certain 8-aminoquinolines are potentially effective drugs against Chagas disease. Ann. Trop. Med. Parasitol. 91:147-152. [DOI] [PubMed] [Google Scholar]
- 14.Kinnamon, K. E., E. A. Steck, P. S. Loizeaux, W. L. Hanson, W. L. Chapman, Jr., and V. B. Waits. 1978. The antileishmanial activity of lepidines. Am. J. Trop. Med. Hyg. 27:751-757. [DOI] [PubMed] [Google Scholar]
- 15.Mandal, S., M. Moudgil, and S. K. Mandal. 2009. Rational drug design. Eur. J. Pharmacol. 625:90-100. [DOI] [PubMed] [Google Scholar]
- 16.Medicines for Malaria Venture. 2009. Tafenoquine: phase 1. Medicines for Malaria Venture, Geneva, Switzerland.
- 17.Nanayakkara, N. P. D., A. L. Alger, Jr., M. S. Bartlett, V. Yardley, S. L. Croft, I. A. Khan, J. D. McChesney, and L. A. Walker. 2008. Antiparasitic activities and toxicities of individual entantiomers of the 8-aminoquinoline 8-[(4-amino-1-methylbuyl)amino]-6-methoxy-4-methyl-5-[3,4-dichorophenoxy]quinoline succinate. Antimicrob. Agents Chemother. 52:2130-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reithinger, R. 2008. Leishmaniases' burden of disease: ways forward for getting from speculation to reality. PLoS Negl. Trop. Dis. 2:e285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seifert, K., and S. L. Croft. 2006. In vitro and in vivo interactions between miltefosine and other antileishmanial drugs. Antimicrob. Agents Chemother. 50:73-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sundar, S., N. Agrawal, R. Arora, D. Agarwal, M. Rai, and J. Chakravarty. 2009. Short-course paromomycin treatment of visceral leishmaniasis in India: 14-day vs 21-day treatment. Clin. Infect. Dis. 49:914-918. [DOI] [PubMed] [Google Scholar]
- 21.Tekwani, B. L., and L. A. Walker. 2006. 8-Aminoquinolines: future role as antiprotozoal drugs. Curr. Opin. Infect. Dis. 19:623-631. [DOI] [PubMed] [Google Scholar]
- 22.Urbina, J. A. 2010. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop. 115:55-68. [DOI] [PubMed] [Google Scholar]
- 23.Wasunna, M. K., J. R. Rashid, J. Mbui, G. Kirigi, D. Kinoti, H. Lodenyo, J. M. Felton, A. J. Sabin, and J. Horton. 2005. A phase II dose-increasing study of sitamaquine for the treatment of visceral leishmaniasis in Kenya. Am. J. Trop. Med. Hyg. 73:871-876. [PubMed] [Google Scholar]
- 24.Yardley, V., and S. L. Croft. 2000. A comparison of the activities of three amphotericin B lipid formulations against experimental visceral and cutaneous leishmaniasis. Int. J. Antimicrob. Agents 13:243-248. [DOI] [PubMed] [Google Scholar]