Abstract
Tomopenem (formerly CS-023) is a novel carbapenem with broad-spectrum activities against diverse hospital pathogens, including Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus (MRSA). We examined the in vivo pharmacodynamic characteristics of tomopenem against P. aeruginosa and MRSA by using a neutropenic murine thigh infection model with P. aeruginosa 12467 (MIC, 1 μg/ml) and MRSA 12372 (MIC, 2 μg/ml). The mice had 106 to 107 CFU/thigh of each strain 2 h after inoculation and were treated for 24 h with a fractionated administration of tomopenem given at intervals of 3, 6, 12, and 24 h. The serum protein binding of tomopenem was 17.4%. The efficacy of tomopenem in both infection models was enhanced by frequent dosing, which indicates that the efficacy is driven by the time above MIC (TMIC). In a sigmoid model, the cumulative percentages of the 24-h period that the concentrations of free, unbound fractions of the drug exceeded the MIC under steady-state pharmacokinetic conditions (f%TMICs) were best correlated with efficacy when R2 was 0.79 and 0.86 against P. aeruginosa and MRSA, respectively. Other pharmacokinetic and pharmacodynamic (PK-PD) indexes for the free, unbound fractions, the area under the concentration-time curve over 24 h in the steady state divided by the MIC (AUC/MIC) and the maximum concentration of the drug in serum divided by the MIC (Cmax/MIC), showed poor correlation with efficacy when R2 was ≤0.42. The f%TMIC values required for a static effect, 1-log kill, and 2-log kill against P. aeruginosa were 29, 39, and 51, respectively, which were similar to those for meropenem, for which the values were 24, 33, and 45, respectively. Against MRSA, the values for tomopenem were 27, 35, and 47. In conclusion, the pharmacodynamic characteristics of tomopenem were similar to those of meropenem against P. aeruginosa, and there was no difference between the target values for P. aeruginosa and MRSA required for efficacy in this study.
Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus (MRSA) are well known as difficult-to-treat pathogens in nosocomial infections (5, 9, 19). Carbapenems are often used for empirical treatments, including those for P. aeruginosa infections. However, the activities of commercially available carbapenems against MRSA are not strong enough. Tomopenem (formerly CS-023) is a novel parenteral carbapenem with broad-spectrum activities against diverse hospital pathogens, including P. aeruginosa and MRSA. Tomopenem has been reported to show potent activities against clinical isolates of P. aeruginosa and MRSA due to its strong activities against the various mutants of P. aeruginosa, such as OprD-deficient P. aeruginosa, its high level of expression of efflux pumps, and its high level of affinity for MRSA PBP2a (14).
Pharmacokinetic and pharmacodynamic (PK-PD) analysis of antimicrobial agents is a useful method for predicting clinical efficacy. There are three PK-PD indexes, the time above MIC (TMIC), the maximum concentration of the drug in serum divided by the MIC (Cmax/MIC), and the area under the concentration-time curve over 24 h in the steady state divided by the MIC (AUC/MIC). The correlations between the PK-PD indexes and the efficacies of commercially available classes of antimicrobial agents have been well analyzed (2, 4, 7, 8). As the efficacies of beta-lactams are known to be correlated in terms of TMIC, the half-life is an important factor for this class (2, 7, 8). In addition to its strong broad-spectrum activity, tomopenem has a long half-life, which is almost twice as long as those of other carbapenems, such as imipenem, meropenem (MEM), and doripenem (18, 21). In this study, to provide information for its clinical efficacy, we evaluated the in vivo pharmacodynamic activities of tomopenem against P. aeruginosa and MRSA. For the purpose of comparison, we also evaluated the activity of MEM against P. aeruginosa.
(This work was presented at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 25 to 28 October 2008 [23].)
MATERIALS AND METHODS
Bacteria, media, and compounds.
Clinical isolates of P. aeruginosa 12467 and MRSA 12372 were used for this study. These strains were isolated from the sputum and blood, respectively, of patients in Japan in 1999. The MICs of tomopenem and MEM were tested by the broth microdilution method (6) and were 1 and 2 μg/ml, respectively, against P. aeruginosa 12467. The MIC of tomopenem against MRSA 12372 was 2 μg/ml. Mueller-Hinton agar (MHA) and Mueller-Hinton broth (MHB) were obtained commercially and used to culture the bacterial strains. Tomopenem was synthesized at Daiichi Sankyo Research Laboratories, Tokyo, Japan, and MEM was obtained from the National Institute of Infectious Diseases, Tokyo, Japan. Cilastatin sodium (Wako Pure Chemical Industries, Ltd., Tokyo, Japan) was obtained commercially.
Thigh infection model.
Specific-pathogen-free 5-week-old male ICR mice (Japan SLC, Inc., Shizuoka, Japan) were used throughout the experiments (n = 4). Neutropenia was induced by 150 and 100 mg/kg of cyclophosphamide (Shionogi & Co., Ltd., Osaka, Japan) injected intraperitoneally at 4 days and 1 day, respectively, before inoculation. Each bacterial colony of P. aeruginosa and MRSA grown on MHA plates was suspended in cation-adjusted MHB and incubated at 35°C for 2.5 h with shaking. Then both bacterial cultures were diluted with cation-adjusted MHB to obtain inoculum suspensions ranging from 106 to 107 CFU/ml. The neutropenic mice were inoculated intramuscularly with 0.1 ml of the bacterial suspension 2 h prior to the initiation of antimicrobial therapy. All experimental procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Daiichi Sankyo Co., Ltd.
Pharmacokinetic studies.
Tomopenem and MEM were dissolved in saline with a fixed dose of 40 mg/kg of cilastatin to obtain a longer half-life (20). Single subcutaneous doses of 50, 100, 200, 400, and 800 mg/kg of compounds of both drugs were administered at 0.004, 0.004, 0.008, 0.016, 0.032 ml/g body weight, respectively, in the same mice infected with P. aeruginosa. The mice (n = 4) were exsanguinated, and blood samples were collected by cardiopuncture with a heparinized syringe at 0.08, 0.25, 0.5, 1, 1.5, and 2 h after administration. After centrifugation of the heparinized blood samples, the plasma was immediately separated and diluted with an equal volume of 500 mM 3-morpholinopropanesulfonic acid buffer (pH 6.0). The plasma concentrations were determined by using high-performance liquid chromatography with UV detection as reported previously (17). The range of concentrations in plasma used for quantification of both compounds was from 0.5 μg/ml up to 1,000 μg/ml. The reproducibility and accuracy of this method were evaluated by using quality control samples, which ensured the accuracy of batch analysis, spiked at concentrations of 1.5, 150, and 750 μg/ml. The reproducibilities of the interassays for tomopenem and MEM did not exceed 6.9% and 2.9%, respectively. The accuracies of the interassays ranged from 95.6% to 111% for tomopenem and from 98.5% to 114% for MEM. The intra-assay reproducibilities ranged from 95.6% to 100% for tomopenem and 99.9% to 108% for MEM. The intra-assay precisions for tomopenem and MEM did not exceed 2.2% and 0.2%, respectively. Regarding the specificity of the method, the linearity of the standard curve and the stability of both compounds in plasma from preparation to the end of the analysis were confirmed.
Pharmacodynamic studies.
The mice were treated for 24 h with a fractionated administration of tomopenem and MEM given at intervals of 3, 6, 12, and 24 h from 2 h after inoculation. Viable cell counts in the thighs were determined following a 24-h treatment period. Untreated control mice received sterile saline and were sacrificed at the initiation of therapy and after 24 h of saline treatment.
Data analysis.
The concentrations of the free, unbound fractions of the drugs in the mouse plasma were calculated by using protein binding ratios of 17.4% and 33.8% for tomopenem and MEM, respectively (22, 24). The mean value of four plasma concentrations at each time point was used for calculation of the PK parameters. The free-drug concentration-time profiles were analyzed using WinNonlin professional software (version 4.0.1; Pharsight Corp.). A one-compartment model with the various dosages was fit to the observations. The best-fit model was determined by Akaike's information criteria. The cumulative percentage of the 24-h period that the drug concentration exceeded the MIC under steady-state pharmacokinetic conditions (%TMIC) was calculated using SAS System release 8.2 software (SAS Institute, Inc.), and the %TMIC for the free, unbound drug (f%TMIC) was also calculated with the ratios for protein binding in mouse sera described above. The values for half-life (t1/2), Cmax, and AUC for 0 h to infinity (AUC0-inf) at various dosages were determined using a noncompartmental model with extravascular input in WinNonlin professional software. A sigmoid maximum effect (Emax) model was used to examine the relationship between each PK-PD index, f%TMIC, fCmax/MIC, and fAUC/MIC, and the efficacy of the drug. For each PK-PD index, nonlinear regression analysis using the following sigmoid Emax model was applied: viable cell count in the thighs (log10 CFU/thigh) = E0 − (Emax × Dn)/[(ED50)n + Dn], where n is the sigmoidicity factor, E0 is the minimum effect (mean of the viable cell counts in the thighs of the saline group), Emax is the maximum effect, D is the value of the PK-PD parameter, and ED50 is the PK-PD index value required to achieve the 50% effective dose, (E0 − Emax)/2. From the sigmoid Emax model, the coefficient of the determinant (R2) and the magnitudes of the PK-PD index required for a static effect, 1-log kill, and 2-log kill related to efficacy were calculated. The analysis was performed using SAS System release 8.2 software.
RESULTS
Pharmacokinetics.
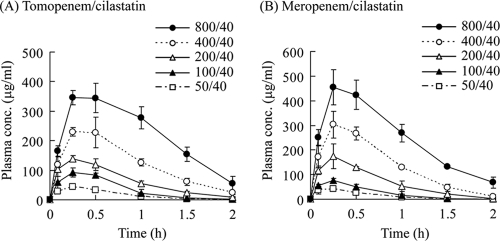
The concentration-time profiles of the free fractions of tomopenem and MEM in plasma administered subcutaneously at 50, 100, 200, 400, and 800 mg/kg in a murine thigh infection model by P. aeruginosa 12467 are shown in Fig. 1. The pharmacokinetic parameters of both compounds are shown in Table 1. Over the range of the single doses tested, the AUC0-inf of the free fraction of tomopenem increased proportionally with the dose, but the Cmax of the free drug did not and the t1/2 was not constant, suggesting that the pharmacokinetics of tomopenem were not linear after subcutaneous administration in the mice. The same characteristics were observed for MEM. The differences in the administration volume was thought to be one reason for these nonlinear pharmacokinetics; therefore, we used these 5 doses for multiple administrations of both compounds in the following studies. In order to investigate how to calculate the free fraction of the AUC0-inf after multiple administrations, we calculated how much greater the AUC0-inf for the free drug was after 8 administrations at 200 mg/kg than it was after a single administration of tomopenem or MEM at the same dose. As the increasing ratio was below 1%, the AUC0-inf of the free drug after multiple administrations was calculated by multiplying the AUC0-inf of the free drug for a single administration by the dose number. The Cmax of the free drug was not changed by multiple administrations (data not shown). Extrapolated ratios of the AUC0-inf at 800 mg/kg of tomopenem and MEM were 6.9% and 8.8%, respectively, compared to the AUC0-2 h, for which the ratios were calculated by WinNonlin software using a noncompartment model. Since the t1/2 at a higher dose had a tendency to be longer, as shown in Table 1, the extrapolation ratio should be higher at a higher dose. However, the extrapolation at the highest dose was below 8.8%, indicating that the calculated AUC0-inf was not overextrapolated and was suitable for the determination and comparison of PK-PD parameters of both compounds.
FIG. 1.
Concentration-time profiles of the free fractions of tomopenem (A) and meropenem (B) in plasma after single administrations at doses of 50, 100, 200, 400, and 800 mg/kg with a fixed dose of cilastatin at 40 mg/kg in a mouse infection model. Each symbol represents the mean ± the standard deviation (n = 4).
TABLE 1.
Pharmacokinetic parameters for tomopenem and meropenem after single administrations at different doses and a fixed dose of cilastatin in a mouse infection modela
| Drug and dose (mg/kg) | t1/2 (h) | fCmax (μg/ml) | AUC0-inf (μg·h/ml) |
|---|---|---|---|
| Tomopenem/cilastatin | |||
| 50/40 | 0.24 | 44.1 | 32.4 |
| 100/40 | 0.27 | 92.5 | 73.7 |
| 200/40 | 0.39 | 140 | 134 |
| 400/40 | 0.41 | 230 | 261 |
| 800/40 | 0.43 | 346 | 484 |
| Meropenem/cilastatin | |||
| 50/40 | 0.24 | 42.8 | 27.6 |
| 100/40 | 0.18 | 76.8 | 51.1 |
| 200/40 | 0.30 | 175 | 138 |
| 400/40 | 0.28 | 303 | 280 |
| 800/40 | 0.50 | 456 | 551 |
Cilastatin was given at a dosage of 40 mg/kg. All parameters are for free, unbound fractions of the drugs.
PK-PD index determination.
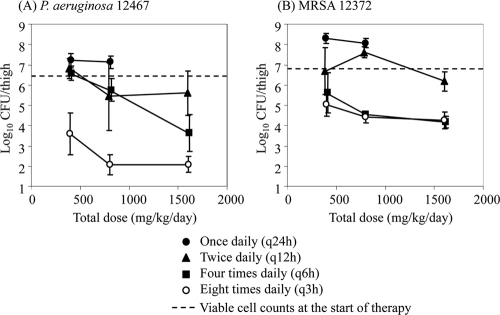
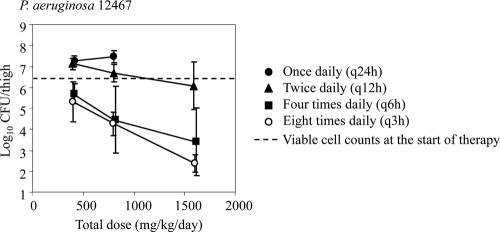
The relationships between the tomopenem dose level and the in vivo efficacy in a murine thigh infection model with P. aeruginosa 12467 and MRSA 12372 are shown in Fig. 2, and those between the MEM dose level and in vivo efficacy are shown in Fig. 3. At the start of therapy, the mice had 6.32 and 6.85 log10 CFU of P. aeruginosa 12467 and MRSA 12372, respectively. The two strains grew to 8.74 (P. aeruginosa 12467) and 8.24 log10 (MRSA 12372) CFU after 24 h of treatment in the untreated control mice. Even though a single dose of 800 mg/kg tomopenem did not reduce the viable cell counts, every-3-h doses of 100 mg/kg (a total of 800 mg/kg) reduced them by more than 4 and 2 log10 CFU against P. aeruginosa 12467 and MRSA 12372, respectively. Overall, as in the case of MEM against P. aeruginosa 12467, the efficacy of tomopenem against both strains was enhanced by frequent dosing, which indicates that the efficacy is driven by the TMIC.
FIG. 2.
Relationship between the tomopenem dose level and its in vivo efficacy in a murine thigh infection model with P. aeruginosa (A) and MRSA (B). Each symbol represents the mean ± the standard deviation (n = 4).
FIG. 3.
Relationship between the meropenem dose level and its in vivo efficacy in a murine thigh infection model with P. aeruginosa. Each symbol represents the mean ± the standard deviation (n = 4).
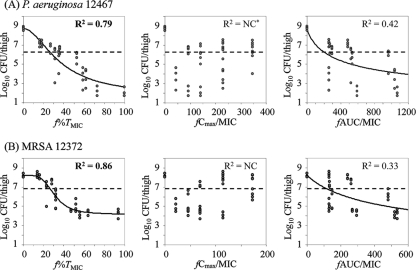
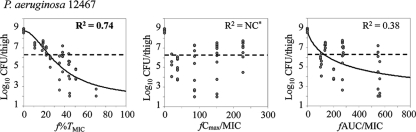
The relationships between each free-drug PK-PD index, f%TMIC, fCmax/MIC, and fAUC/MIC, and the efficacies of tomopenem against both strains are shown in Fig. 4 and those for MEM against P. aeruginosa 12467 are shown in Fig. 5. For tomopenem, the best relationships were observed when the results were correlated with f%TMIC with an R2 of 0.79 (for fCmax/MIC, R2 is not calculable; for fAUC/MIC, R2 = 0.42) in the P. aeruginosa infection model and with an R2 of 0.86 (for fCmax/MIC, R2 is not calculable; for fAUC/MIC, R2 = 0.33) in the MRSA infection model. The results for MEM also showed the best correlation with f%TMIC, with an R2 of 0.74 in the P. aeruginosa infection model.
FIG. 4.
Relationship between the PK-PD index and the in vivo efficacy of tomopenem against P. aeruginosa (A) and MRSA (B). The dotted lines indicate the viable cell counts at the start of therapy. NC*, not calculable (nonlinear regression using the sigmoid Emax model failed to converge).
FIG. 5.
Relationship between the PK-PD index and the in vivo efficacy of meropenem against P. aeruginosa. The dotted lines indicate the viable cell counts at the start of therapy. NC*, not calculable (nonlinear regression using the sigmoid Emax model failed to converge).
Magnitude of PK-PD index required for efficacy.
The magnitudes of f%TMIC required for the in vivo efficacies of tomopenem and MEM are shown in Table 2. The f%TMIC values of tomopenem required for a static effect, 1-log kill, and 2-log kill against P. aeruginosa 12467 were 29, 39, and 51, respectively, which were similar to those for MEM, which were 24, 33, and 45, respectively. Against MRSA 12372, the values for tomopenem were 27, 35, and 47, respectively. The values necessary to achieve a static effect, 1-log kill, or 2-log kill against P. aeruginosa 12467 and MRSA 12372 were almost equal, which indicates that there is no difference between the doses required for efficacy against P. aeruginosa and MRSA.
TABLE 2.
Percentages of time above the MICs of the free, unbound fractions of tomopenem and meropenem required for their in vivo efficacies
| Strain |
f%TMIC of indicated drug for effects indicateda |
|||||
|---|---|---|---|---|---|---|
| Tomopenem |
Meropenem |
|||||
| Static (95% CI) | 1-log kill (95% CI) | 2-log kill (95% CI) | Static (95% CI) | 1-log kill (95% CI) | 2-log kill (95% CI) | |
| P. aeruginosa 12467 | 29 (25-33) | 39 (35-43) | 51 (45-58) | 24 (21-28) | 33 (30-37) | 45 (39-52) |
| MRSA 12372 | 27 (24-29) | 35 (31-37) | 47 (39-52) | ND | ND | ND |
ND, not determined.
DISCUSSION
For antimicrobial agents, PK-PD analysis using a murine infection model has become a standard method to predict clinical efficacy and is often used for the determination of optimal doses for clinical trials. This method was established by Craig et al. (2, 4, 8). However, it remains difficult to evaluate in vivo efficacy for humans in a murine model because plasma exposure in humans may be higher than that in mice. Craig and Andes and Nicolau et al. used uranyl nitrate to induce renal impairment and obtain a long half-life for beta-lactams in mice (1, 13). As the half-lives of tomopenem and MEM were also as short in mice as those reported for other carbapenems (15, 26), we used cilastatin to obtain longer half-lives by inhibiting murine dehydropeptidase I. Moreover, we used doses equal to or higher than 50 mg/kg to obtain an adequate amount of TMIC at each administration. To evaluate the PK-PD index required for efficacy and its target value properly, these approaches are necessary in case the pharmacokinetics in mice is much shorter than in humans.
The PK-PD indexes of beta-lactams required for efficacy are known to be the TMIC. In this study, we demonstrated that the efficacy of tomopenem is driven by the TMIC and that the magnitude necessary for a static effect against P. aeruginosa is similar to that of MEM. In the in vitro study, the bactericidal activity and postantibiotic effect (PAE) of tomopenem against P. aeruginosa were shown to be as strong as those of MEM (16, 25). The values of magnitude required for efficacy against both P. aeruginosa and MRSA were almost the same in this study. In the in vitro study, tomopenem also showed strong bactericidal activity and PAE against MRSA, similar to that against P. aeruginosa. These in vitro activities may support the results of our study.
The exposure to tomopenem required for a static effect is similar to those for commercially available carbapenem and doripenem against P. aeruginosa and MRSA (10, 13). On the other hand, current anti-MRSA beta-lactams seem to require less exposure for a static effect against MRSA than tomopenem. The anti-MRSA cephalosporins, RWJ-54428, ceftobiprole, and ceftaroline (PPI-0903), as well as carbapenem SMP-601, were shown to achieve static effects with fTMICs of 14 to 20%, 23 to 25%, 15 and 21%, and 23%, respectively, whereas tomopenem required a fTMIC of 27% (2, 8, 11, 12). Although it is not clear whether this difference is significant or not, the different strains used in these experiments might be one of the reasons for the difference. It is important to directly compare these compounds and to evaluate the efficacies such as killing ratios and PAEs in depth if there are any differences.
To improve the efficacies of compounds related to the TMIC, it is clear that the length of the half-life and a low MIC are important factors. Considering the longer elimination half-life of tomopenem and its potent activity, it is expected to be more effective than other carbapenems against diverse hospital pathogens, including P. aeruginosa and MRSA. Although further study is needed to provide more detailed information regarding the clinical efficacy of tomopenem against various strains, it is thought to be a promising compound for nosocomial infections.
Footnotes
Published ahead of print on 4 October 2010.
REFERENCES
- 1.Andes, D., and W. A. Craig. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andes, D., and W. A. Craig. 2006. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob. Agents Chemother. 50:1376-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andes, D., S. Kiem, and W. A. Craig. 2003. In vivo pharmacodynamic activity of a new carbapenem, doripenem (DOR), against multiple bacteria in a murine thigh infection model. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-308.
- 4.Andes, D., M. L. van Ogtrop, J. Peng, and W. A. Craig. 2002. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob. Agents Chemother. 46:3484-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodmann, K. F. 2005. Current guidelines for the treatment of severe pneumonia and sepsis. Chemotherapy 51:227-233. [DOI] [PubMed] [Google Scholar]
- 6.Clinical and Laboratory Standards Institute. 2006. M7-A7. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 7th ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 7.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 8.Craig, W. A., and D. R. Andes. 2008. In vivo pharmacodynamics of ceftobiprole against multiple bacterial pathogens in murine thigh and lung infection models. Antimicrob. Agents Chemother. 52:3492-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeRyke, C. A., D. Maglio, and D. P. Nicolau. 2005. Defining the need for new antimicrobials: clinical and economic implications of resistance in the hospitalized patient. Expert Opin. Pharmacother. 6:873-889. [DOI] [PubMed] [Google Scholar]
- 10.DeRyke, C. A., M. A. Banevicius, H. W. Fan, and D. P. Nicolau. 2007. Bactericidal activities of meropenem and ertapenem against extended-spectrum-β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a neutropenic mouse thigh model. Antimicrob. Agents Chemother. 51:1481-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eguchi, K., K. Kanazawa, Y. Eriguchi, and Y. Ueda. 2009. Pharmacodynamics of SMP-601 (PTZ601) against vancomycin-resistant Enterococcus faecium and methicillin-resistant Staphylococcus aureus in neutropenic murine thigh infection models. Antimicrob. Agents Chemother. 53:3391-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffith, D. C., D. Rodriguez, E. Corcoran, and M. N. Dudley. 2008. Pharmacodynamics of RWJ-54428 against Staphylococcus aureus, Streptococcus pneumoniae, and Enterococcus faecalis in a neutropenic mouse thigh infection model. Antimicrob. Agents Chemother. 52:244-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim, A., M. A. Banevicius, and D. P. Nicolau. 2008. In vivo pharmacodynamic profiling of doripenem against Pseudomonas aeruginosa by simulating human exposures. Antimicrob. Agents Chemother. 52:2497-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koga, T., N. Masuda, M. Kakuta, E. Namba, C. Sugihara, and T. Fukuoka. 2008. Potent in vitro activity of tomopenem (CS-023) against methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 52:2849-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koga, T., T. Abe, H. Inoue, T. Takenouchi, A. Kitayama, T. Yoshida, N. Masuda, C. Sugihara, M. Kakuta, M. Nakagawa, T. Shibayama, Y. Matsushita, T. Hirota, S. Ohya, Y. Utsui, T. Fukuoka, and S. Kuwahara. 2005. In vitro and in vivo antibacterial activities of CS-023 (RO4908463), a novel parenteral carbapenem. Antimicrob. Agents Chemother. 49:3239-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masuda, N., T. Takenouchi, M. Kakuta, C. Ishii, E. Sakagawa, T. Abe, Y. Utsui, S. Ohya, and S. Kuwahara. 2000. R-115685, a novel parenteral carbapenem: mode of action and resistance mechanisms. Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1231.
- 17.Morinaga, Y., K. Yanagihara, S. Nakamura, K. Yamamoto, K. Izumikawa, M. Seki, H. Kakeya, Y. Yamamoto, Y. Yamada, S. Kohno, and S. Kamihira. 2008. In vivo efficacy and pharmacokinetics of tomopenem (CS-023), a novel carbapenem, against Pseudomonas aeruginosa in a murine chronic respiratory tract infection model. J. Antimicrob. Chemother. 62:1326-1331. [DOI] [PubMed] [Google Scholar]
- 18.Nicolau, D. P. 2008. Carbapenems: a potent class of antibiotics. Expert Opin. Pharmacother. 9:23-37. [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal, V. D., D. G. Maki, A. Mehta, C. Alvarez-Moreno, H. Leblebicioglu, F. Higuera, L. E. Cuellar, N. Madani, Z. Mitrev, L. Duenas, J. A. Navoa-Ng, H. G. Garcell, L. Raka, R. F. Hidalgo, E. A. Medeiros, S. S. Kanj, S. Abubakar, P. Nercelles, and R. D. Pratesi of the International Nosocomial Infection Control Consortium. 2008. International Nosocomial Infection Control Consortium report, data summary for 2002-2007, issued January 2008. Am. J. Infect. Control 36:627-637. [DOI] [PubMed] [Google Scholar]
- 20.Shibata, K., R. Nagano, T. Hashizume, and H. Morishima. 2000. Therapeutic efficacy of J-111,225, a novel trans-3,5-disubstituted pyrrolidinylthio-1β-methylcarbapenem, against experimental murine systemic infections. J. Antimicrob. Chemother. 45:379-382. [DOI] [PubMed] [Google Scholar]
- 21.Shibayama, T., Y. Matsushita, T. Hirota, T. Ikeda, and S. Kuwahara. 2006. Pharmacokinetics of CS-023 (RO4908463), a novel parenteral carbapenem, in healthy male Caucasian volunteers. Antimicrob. Agents Chemother. 50:4186-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibayama, T., Y. Matsushita, A. Kurihara, T. Hirota, and T. Ikeda. 2007. Prediction of pharmacokinetics of CS-023 (RO4908463), a novel parenteral carbapenem antibiotic, in humans using animal data. Xenobiotica 37:91-102. [DOI] [PubMed] [Google Scholar]
- 23.Sugihara, K., C. Sugihara, Y. Matsushita, N. Yamamura, M. Uemori, A. Tokumitsu, H. Inoue, M. Kakuta, E. Namba, H. Nasu, and T. Koga. 2008. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-027. [DOI] [PMC free article] [PubMed]
- 24.Sumita, Y., H. Nouda, E. Tada, T. Kohzuki, M. Kato, T. Okuda, and M. Fukasawa. 1992. Pharmacokinetics of meropenem, a new carbapenem antibiotic, parenterally administered to laboratory animals. Chemotherapy 40(Suppl. 1):123-131. [Google Scholar]
- 25.Tomozawa, T., C. Sugihara, M. Kakuta, K. Sugihara, and T. Koga. 2010. In vitro postantibiotic effects of tomopenem (CS-023) against Staphylococcus aureus and Pseudomonas aeruginosa. J. Med. Microbiol. 59:438-441. [DOI] [PubMed] [Google Scholar]
- 26.Tsuji, M., Y. Ishii, A. Ohno, S. Miyazaki, and K. Yamaguchi. 1998. In vitro and in vivo antibacterial activities of S-4661, a new carbapenem. Antimicrob. Agents Chemother. 42:94-99. [DOI] [PMC free article] [PubMed] [Google Scholar]