Abstract
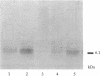
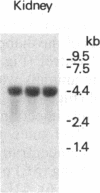
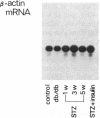
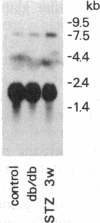
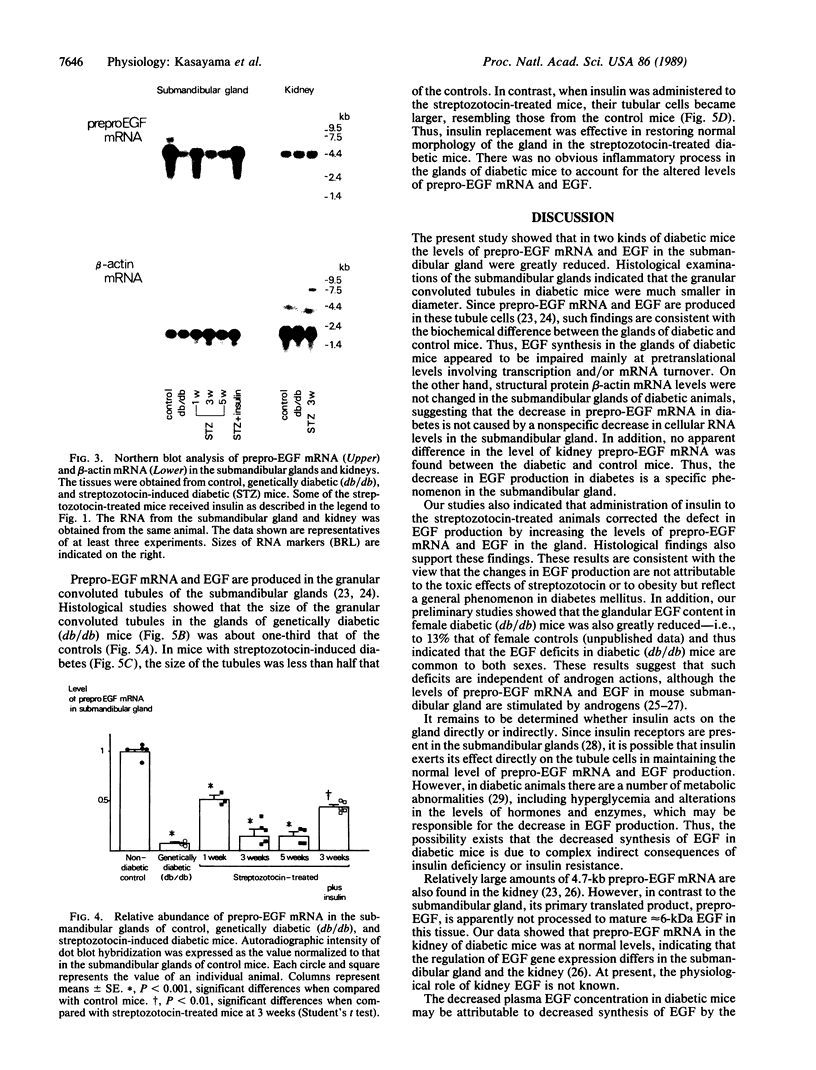
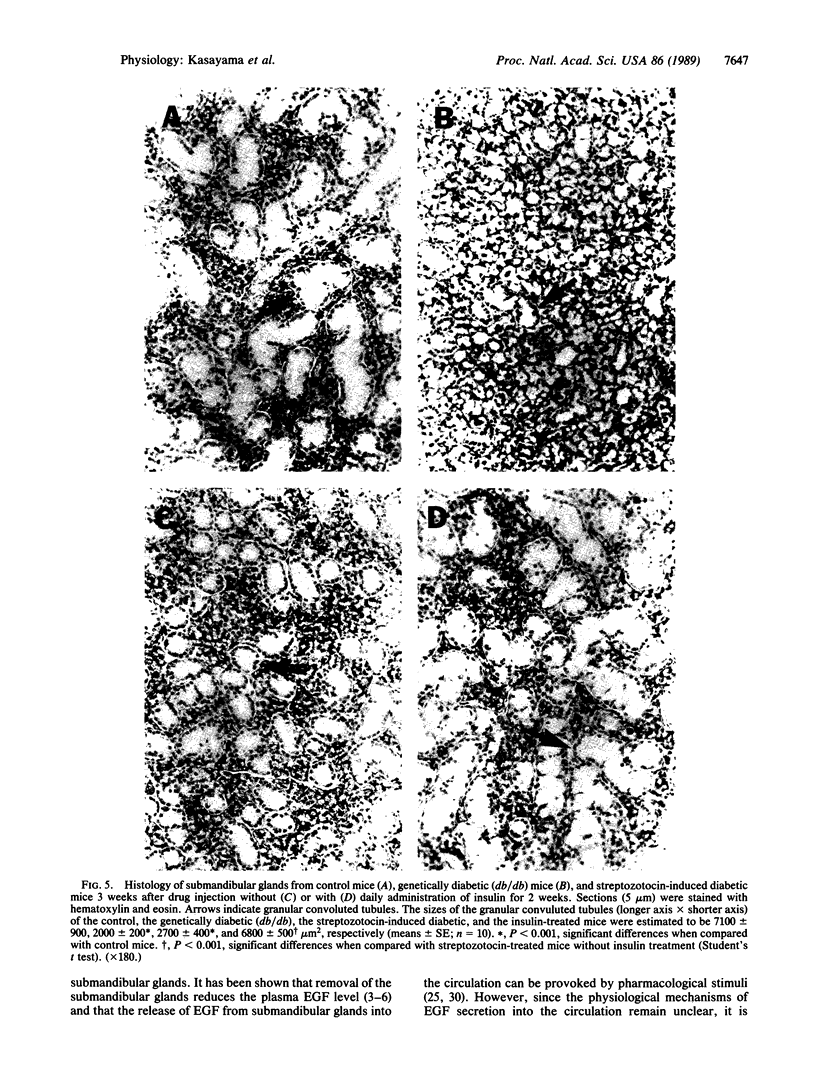
The production of epidermal growth factor (EGF) in the submandibular gland and its circulating level were studied in diabetic mice. In genetically diabetic (C57BL/KsJ db/db) mice, EGF concentrations in the submandibular gland and plasma were reduced to 13% and 30% of the control levels, respectively. In streptozotocin-treated diabetic mice, they were reduced to 18% and 20% of controls, respectively, 5 weeks after the drug injection. Furthermore, levels of submandibular prepro-EGF mRNA in these diabetic mice were decreased almost in parallel with the glandular EGF concentrations, while there was no change in the levels of submandibular beta-actin mRNA and kidney prepro-EGF mRNA. In addition, histological examination of the submandibular glands indicated that the size of the granular convoluted tubules, which produce EGF, was substantially reduced in the diabetic mice. Insulin administration to streptozotocin-treated mice almost completely reversed the decrease in EGF content in the submandibular gland, substantially elevated the level of the glandular prepro-EGF mRNA and plasma EGF concentration, and increased the size of the granular convoluted tubules in the gland. These results indicate that EGF deficiency occurs in diabetes mellitus and that insulin may be important in maintaining the normal level of EGF in the submandibular gland and plasma.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso S., Minty A., Bourlet Y., Buckingham M. Comparison of three actin-coding sequences in the mouse; evolutionary relationships between the actin genes of warm-blooded vertebrates. J Mol Evol. 1986;23(1):11–22. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]
- Barka T., Gresik E. W., vad der Noen H. Stimulation of secretion of epidermal growth factor and amylase of cyclocytidine. Cell Tissue Res. 1978 Jan 17;186(2):269–278. doi: 10.1007/BF00225536. [DOI] [PubMed] [Google Scholar]
- Byyny R. L., Orth D. N., Cohen S., Doyne E. S. Epidermal growth factor: effects of androgens and adrenergic agents. Endocrinology. 1974 Sep;95(3):776–782. doi: 10.1210/endo-95-3-776. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Danscher G., Nörgaard J. O. Light microscopic visualization of colloidal gold on resin-embedded tissue. J Histochem Cytochem. 1983 Dec;31(12):1394–1398. doi: 10.1177/31.12.6631001. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frati L., Daniele S., Delogu A., Covelli I. Selective binding of the epidermal growth factor and its specific effects on the epithelial cells of the cornea. Exp Eye Res. 1972 Sep;14(2):135–141. doi: 10.1016/0014-4835(72)90059-0. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D. Epidermal and nerve growth factors in mammalian development. Annu Rev Physiol. 1981;43:251–263. doi: 10.1146/annurev.ph.43.030181.001343. [DOI] [PubMed] [Google Scholar]
- Gray A., Dull T. J., Ullrich A. Nucleotide sequence of epidermal growth factor cDNA predicts a 128,000-molecular weight protein precursor. Nature. 1983 Jun 23;303(5919):722–725. doi: 10.1038/303722a0. [DOI] [PubMed] [Google Scholar]
- Gubits R. M., Shaw P. A., Gresik E. W., Onetti-Muda A., Barka T. Epidermal growth factor gene expression is regulated differently in mouse kidney and submandibular gland. Endocrinology. 1986 Sep;119(3):1382–1387. doi: 10.1210/endo-119-3-1382. [DOI] [PubMed] [Google Scholar]
- Hsu Y. H. Immunogold for detection of antigen on nitrocellulose paper. Anal Biochem. 1984 Oct;142(1):221–225. doi: 10.1016/0003-2697(84)90542-6. [DOI] [PubMed] [Google Scholar]
- Hummel K. P., Dickie M. M., Coleman D. L. Diabetes, a new mutation in the mouse. Science. 1966 Sep 2;153(3740):1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- Kasayama S., Yoshimura M., Oka T. Decreased expression of hepatic epidermal growth factor receptor gene in diabetic mice. J Mol Endocrinol. 1989 Jul;3(1):49–56. doi: 10.1677/jme.0.0030049. [DOI] [PubMed] [Google Scholar]
- Kasayama S., Yoshimura M., Oka T. The regulation by thyroid hormones and androgen of epidermal growth factor synthesis in the submandibular gland and its plasma concentrations in mice. J Endocrinol. 1989 May;121(2):269–275. doi: 10.1677/joe.0.1210269. [DOI] [PubMed] [Google Scholar]
- Kurachi H., Oka T. Changes in epidermal growth factor concentrations of submandibular gland, plasma and urine of normal and sialoadenectomized female mice during various reproductive stages. J Endocrinol. 1985 Aug;106(2):197–202. doi: 10.1677/joe.0.1060197. [DOI] [PubMed] [Google Scholar]
- Levine R. Insulin: the effects and mode of action of the hormone. Vitam Horm. 1982;39:145–173. doi: 10.1016/s0083-6729(08)61136-x. [DOI] [PubMed] [Google Scholar]
- Like A. A., Rossini A. A. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976 Jul 30;193(4251):415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- Niall M., Ryan G. B., O'Brien B. M. The effect of epidermal growth factor on wound healing in mice. J Surg Res. 1982 Aug;33(2):164–169. doi: 10.1016/0022-4804(82)90024-5. [DOI] [PubMed] [Google Scholar]
- Okamoto S., Oka T. Evidence for physiological function of epidermal growth factor: pregestational sialoadenectomy of mice decreases milk production and increases offspring mortality during lactation period. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6059–6063. doi: 10.1073/pnas.81.19.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall L. B., Scott J., Bell G. I., Crawford R. J., Penschow J. D., Niall H. D., Coghlan J. P. Mouse prepro-epidermal growth factor synthesis by the kidney and other tissues. Nature. 1985 Jan 17;313(5999):228–231. doi: 10.1038/313228a0. [DOI] [PubMed] [Google Scholar]
- Scott J., Urdea M., Quiroga M., Sanchez-Pescador R., Fong N., Selby M., Rutter W. J., Bell G. I. Structure of a mouse submaxillary messenger RNA encoding epidermal growth factor and seven related proteins. Science. 1983 Jul 15;221(4607):236–240. doi: 10.1126/science.6602382. [DOI] [PubMed] [Google Scholar]
- Sheffield L. G., Welsch C. W. Influence of submandibular salivary glands on hormone responsiveness of mouse mammary glands. Proc Soc Exp Biol Med. 1987 Dec;186(3):368–377. doi: 10.3181/00379727-186-42627. [DOI] [PubMed] [Google Scholar]
- Tsutsumi O., Kurachi H., Oka T. A physiological role of epidermal growth factor in male reproductive function. Science. 1986 Aug 29;233(4767):975–977. doi: 10.1126/science.3090686. [DOI] [PubMed] [Google Scholar]
- Tsutsumi O., Oka T. Epidermal growth factor deficiency during pregnancy causes abortion in mice. Am J Obstet Gynecol. 1987 Jan;156(1):241–244. doi: 10.1016/0002-9378(87)90245-6. [DOI] [PubMed] [Google Scholar]
- Tsutsumi O., Tsutsumi A., Oka T. Epidermal growth factor-like, corneal wound healing substance in mouse tears. J Clin Invest. 1988 Apr;81(4):1067–1071. doi: 10.1172/JCI113418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyn D., Scacchi G. E., Dellacha J. M. Unmasking of insulin receptors in rat submaxillary gland microsomes: effect of high ionic strength, phospholipase C and S-adenosyl-L-methionine. Biochim Biophys Acta. 1985 Jun 30;845(3):333–342. doi: 10.1016/0167-4889(85)90196-x. [DOI] [PubMed] [Google Scholar]
- Van Noorden S., Heitz P., Kasper M., Pearse A. G. Mouse epidermal growth factor: light and electron microscopical localisation by immunocytochemical staining. Histochemistry. 1977 Jun 24;52(4):329–340. doi: 10.1007/BF00508405. [DOI] [PubMed] [Google Scholar]