Abstract
Oritavancin is an investigational lipoglycopeptide in clinical development for the treatment of acute bacterial skin and skin structure infections. In this study, we demonstrate that oritavancin causes bacterial membrane depolarization and permeabilization leading to cell death of Gram-positive pathogens and that these effects are attributable to the 4′-chlorobiphenylmethyl group of the molecule.
Vancomycin-resistant Staphylococcus aureus (VRSA) and enterococci (VRE) exhibit high-level resistance to vancomycin that is caused by alteration of the bacterial cell wall target C-terminal acyl-d-alanyl-d-alanine dipeptide to the depsipeptide d-alanyl-d-lactate (6). Intermediate-level resistance to vancomycin in S. aureus, referred to as vancomycin-intermediate S. aureus (VISA) and heterogeneous VISA (hVISA), has also been described and poses a clinical challenge (1, 17). The investigational lipoglycopeptide oritavancin demonstrates in vitro activity against vancomycin-nonsusceptible isolates (hVISA, VISA, VRSA, and VRE), exhibiting MIC90 values between 0.25 μg/ml and 2 μg/ml for the various phenotypes in recent studies (2, 3). Its activity is unaffected by methicillin resistance in staphylococci (2). Oritavancin is derived from the addition of a 4′-chlorobiphenylmethyl side chain to the natural product chloroeremomycin (16). This functional group is believed to permit binding to a secondary site in peptidoglycan, the pentaglycyl bridge, and to account in part for its activity against VRSA and VRE isolates (15). Oritavancin typically exerts rapid and concentration-dependent killing against Gram-positive organisms (13), signifying a mechanism of action that is distinct from that of vancomycin. Recent studies showed that oritavancin interacts with isolated membrane protoplasts and increases the permeability of artificial liposomes composed of bacterial membrane phospholipids (7, 10, 15). Our earlier studies demonstrated that oritavancin probably kills S. aureus via perturbation of membrane integrity (4, 14). In this study, we characterize the effect of oritavancin on bacterial membrane potential (depolarization) and permeability by using intact cells of Gram-positive pathogens and reveal a correlation between membrane depolarization and cell death. Importantly, we demonstrate for the first time that these effects are attributable to the 4′-chlorobiphenylmethyl group of the molecule.
The bacterial isolates used in this study were hVISA ATCC 700698, VISA ATCC 700699, VRSA VRS5 (Network on Antimicrobial Resistance in Staphylococcus aureus), and the VRE Enterococcus faecalis (VREF) ATCC 51299 (VanB). The effects of oritavancin (The Medicines Company, Parsippany, NJ), chloroeremomycin (The Medicines Company), and vancomycin (Sigma-Aldrich, St. Louis, MO) on bacterial membrane depolarization and permeability were determined fluorometrically as described previously (4). Although the inclusion of 0.002% polysorbate-80 is recommended in susceptibility testing to prevent loss of oritavancin from solution due to binding to plastic (5), it was omitted because it interferes with fluorescence quantification (4). In the absence of polysorbate-80, the concentrations of antimicrobial agents tested (Fig. 1) represent the equivalent fold-above-MIC for the hVISA isolate (MIC of 1 μg/ml for each drug). The methodology for short-duration time-kill studies has also been described (4). The results, presented as percent depolarization or percent change in fluorescence, are relative to the maximal effect observed with oritavancin. The experimental results shown in Fig. 1A to C are presented as the means ± standard deviations of the results from three independent experiments. The correlation coefficients (r) between membrane depolarization (corrected for background and converted to percent relative to maximal effect observed with oritavancin) and cell killing (change in log CFU relative to time zero) caused by oritavancin were determined with Prism 5.02 software (Graphpad Software, Inc., La Jolla, CA), using the mean results from three (Fig. 1D) and two (Fig. 2) independent experiments.
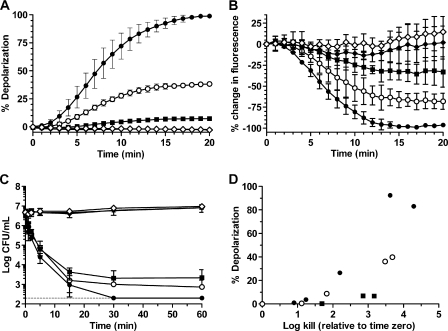
FIG. 1.
Oritavancin but not its precursor chloroeremomycin or vancomycin disrupts bacterial membrane integrity, leading to concomitant killing of hVISA ATCC 700698. (A to D) Effects of oritavancin and comparator agents on membrane depolarization (A), membrane permeability (B), cell viability (C), and the correlation between membrane depolarization and cell killing (D). Drug concentrations: control (✳); oritavancin at 4 μg/ml (▪), 8 μg/ml (○) and 16 μg/ml (•); 16 μg/ml chloroeremomycin (⋄); 16 μg/ml vancomycin (⧫). Note that the chloroeremomycin and vancomycin curves overlap in panel A and with the control curve in panel C. The limit of detection (200 CFU) is indicated as a dashed line in panel C.
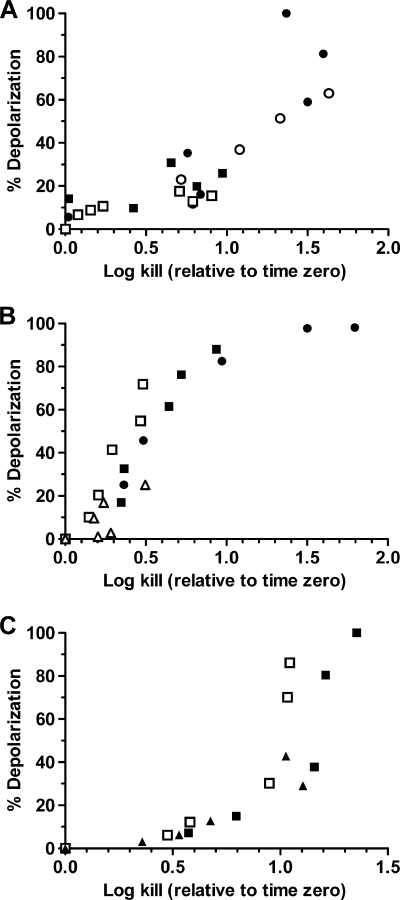
FIG. 2.
Oritavancin-induced membrane depolarization and cell killing of VISA, VRSA, and VREF isolates are tightly correlated. (A to C) Correlations were determined for VISA ATCC 700699 (A), VRSA VRS5 (B), and VanB VREF ATCC 51299 (C). Oritavancin was used at 0.5 μg/ml (▵), 1 μg/ml (▴), 2 μg/ml (□), 4 μg/ml (▪), 8 μg/ml (○), and 16 μg/ml (•).
The rapid bactericidal pharmacodynamics of oritavancin against S. aureus and enterococci in vitro differ from those of vancomycin and teicoplanin (13). To understand these differences, we assessed the effect of oritavancin challenge on membrane depolarization. The addition of oritavancin to exponential-phase hVISA caused an immediate and concentration-dependent increase in the fluorescence of DiSC3(5) (3,3′-dipropylthiadicarbocyanine iodide) that was preloaded into cell membranes (Fig. 1A), an indication of membrane depolarization. Depolarization has also been described with the lipoglycopeptide telavancin (8, 11). In contrast, fluorescent signals from cells exposed to 16 μg/ml chloroeremomycin or vancomycin remained constant over the exposure period, indicating that membrane potential was unaffected. These results demonstrate that the 4′-chlorobiphenylmethyl group of oritavancin is responsible for membrane depolarization in S. aureus. Further study of lipoglycopeptide side chains (16) could elucidate the structure-activity relationship required for depolarization.
Exposure of hVISA to oritavancin also caused immediate and concentration-dependent increases in membrane permeability, as measured by decreased SYTO 9 fluorescence (Fig. 1B). In contrast, these fluorescent signals were unchanged over the time course of the assay following the addition of 16 μg/ml chloroeremomycin or vancomycin. These results demonstrate that the 4′-chlorobiphenylmethyl group of oritavancin is also responsible for causing increased membrane permeability.
The effects of oritavancin on cell viability were monitored under conditions similar to those used in the membrane integrity assays. Oritavancin at 4, 8, and 16 μg/ml caused immediate killing of hVISA and was bactericidal (≥3 log CFU decrease) within 30 min (Fig. 1C). In contrast, the addition of 16 μg/ml of either chloroeremomycin or vancomycin had no effect on cell viability within the time frame of the assay. These results imply that loss of membrane potential and increased permeability, attributed to the 4′-chlorobiphenylmethyl group of oritavancin, are responsible for its rapid bactericidal activity. The correlation between membrane depolarization (timing and extent) and cell viability of hVISA yielded r = 0.78 (Fig. 1D). The correlations were also strong for the VISA (r = 0.85), VRSA (r = 0.90), and VRE (r = 0.82) isolates (Fig. 2). Unlike prior studies with telavancin (8) and the lipopeptide daptomycin (18), this is the first study to demonstrate and quantify a correlation between disruption of membrane potential and bacterial killing for both staphylococci and enterococci.
Although we cannot exclude the possibility that oritavancin inhibition of peptidoglycan cross-linking via binding of the pentaglycyl bridge (9) causes the rapid bactericidal effect, it seems unlikely in that oritavancin retains bactericidal activity against stationary-phase S. aureus inoculated into nutrient-depleted medium, conditions under which cell wall synthesis is expected to be minimal (4). This idea is further supported by the results of a study demonstrating negligible killing of stationary-phase S. aureus by vancomycin and the β-lactam nafcillin (12).
We conclude that the addition of the 4′-chlorobiphenylmethyl group to the natural product chloroeremomycin provides for profoundly increased antibacterial activity of the resultant molecule, oritavancin. Most notably, oritavancin effects rapid and concentration-dependent bactericidal killing against Gram-positive pathogens, including vancomycin-nonsusceptible phenotypes, via perturbation of membrane integrity. Although it remains to be determined whether the multiple mechanisms of action of oritavancin act simultaneously to effect cell death, it is possible that they reduce the propensity to select for resistance.
Acknowledgments
This work was initiated while the authors were employees of Targanta Therapeutics. Targanta Therapeutics is now a wholly owned subsidiary of The Medicines Company.
Footnotes
Published ahead of print on 27 September 2010.
REFERENCES
- 1.Appelbaum, P. C. 2007. Reduced glycopeptide susceptibility in methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Antimicrob. Agents 30:398-408. [DOI] [PubMed] [Google Scholar]
- 2.Arhin, F. F., D. C. Draghi, C. M. Pillar, T. R. Parr, Jr., G. Moeck, and D. F. Sahm. 2009. Comparative in vitro activity profile of oritavancin against recent gram-positive clinical isolates. Antimicrob. Agents Chemother. 53:4762-4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arhin, F. F., I. Sarmiento, T. R. Parr, Jr., and G. Moeck. 2009. Comparative in vitro activity of oritavancin against Staphylococcus aureus strains that are resistant, intermediate or heteroresistant to vancomycin. J. Antimicrob. Chemother. 64:868-870. [DOI] [PubMed] [Google Scholar]
- 4.Belley, A., E. Neesham-Grenon, G. McKay, F. F. Arhin, R. Harris, T. Beveridge, T. R. Parr, Jr., and G. Moeck. 2009. Oritavancin kills stationary-phase and biofilm Staphylococcus aureus cells in vitro. Antimicrob. Agents Chemother. 53:918-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CLSI. 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, CLSI document M7-A8, 8th ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Courvalin, P. 2006. Vancomycin resistance in gram-positive cocci. Clin. Infect. Dis. 42(Suppl. 1):S25-S34. [DOI] [PubMed] [Google Scholar]
- 7.Domenech, O., G. Francius, P. M. Tulkens, F. Van Bambeke, Y. Dufrene, and M. P. Mingeot-Leclercq. 2009. Interactions of oritavancin, a new lipoglycopeptide derived from vancomycin, with phospholipid bilayers: effect on membrane permeability and nanoscale lipid membrane organization. Biochim. Biophys. Acta 1788:1832-1840. [DOI] [PubMed] [Google Scholar]
- 8.Higgins, D. L., R. Chang, D. V. Debabov, J. Leung, T. Wu, K. M. Krause, E. Sandvik, J. M. Hubbard, K. Kaniga, D. E. Schmidt, Jr., Q. Gao, R. T. Cass, D. E. Karr, B. M. Benton, and P. P. Humphrey. 2005. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim, S. J., L. Cegelski, D. Stueber, M. Singh, E. Dietrich, K. S. Tanaka, T. R. Parr, Jr., A. R. Far, and J. Schaefer. 2008. Oritavancin exhibits dual mode of action to inhibit cell-wall biosynthesis in Staphylococcus aureus. J. Mol. Biol. 377:281-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim, S. J., M. Singh, and J. Schaefer. 2009. Oritavancin binds to isolated protoplast membranes but not intact protoplasts of Staphylococcus aureus. J. Mol. Biol. 391:414-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lunde, C. S., S. R. Hartouni, J. W. Janc, M. Mammen, P. P. Humphrey, and B. M. Benton. 2009. Telavancin disrupts the functional integrity of the bacterial membrane through targeted interaction with the cell wall precursor lipid II. Antimicrob. Agents Chemother. 53:3375-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mascio, C. T., J. D. Alder, and J. A. Silverman. 2007. Bactericidal action of daptomycin against stationary-phase and nondividing Staphylococcus aureus cells. Antimicrob. Agents Chemother. 51:4255-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKay, G. A., S. Beaulieu, F. F. Arhin, A. Belley, I. Sarmiento, T. Parr, Jr., and G. Moeck. 2009. Time-kill kinetics of oritavancin and comparator agents against Staphylococcus aureus, Enterococcus faecalis and Enterococcus faecium. J. Antimicrob. Chemother. 63:1191-1199. [DOI] [PubMed] [Google Scholar]
- 14.McKay, G. A., I. Fadhil, S. Beaulieu, S. Ciblat, A. R. Far, G. Moeck, and T. R. Parr, Jr. 2006. Oritavancin disrupts transmembrane potential and membrane integrity concomitantly with cell killing in Staphylococcus aureus and vancomycin-resistant enterococci, abstr. C1-682, p. 76. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 15.Patti, G. J., S. J. Kim, T. Y. Yu, E. Dietrich, K. S. Tanaka, T. R. Parr, Jr., A. R. Far, and J. Schaefer. 2009. Vancomycin and oritavancin have different modes of action in Enterococcus faecium. J. Mol. Biol. 392:1178-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez, M. J., N. J. Snyder, M. J. Zweifel, S. C. Wilkie, D. R. Stack, R. D. Cooper, T. I. Nicas, D. L. Mullen, T. F. Butler, and R. C. Thompson. 1998. Novel glycopeptide antibiotics: N-alkylated derivatives active against vancomycin-resistant enterococci. J. Antibiot. (Tokyo) 51:560-569. [DOI] [PubMed] [Google Scholar]
- 17.Rybak, M., B. Lomaestro, J. C. Rotschafer, R. Moellering, Jr., W. Craig, M. Billeter, J. R. Dalovisio, and D. P. Levine. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 66:82-98. [DOI] [PubMed] [Google Scholar]
- 18.Silverman, J. A., N. G. Perlmutter, and H. M. Shapiro. 2003. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 47:2538-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]