Abstract
Although mitochondrial electron transport is a validated target of the antimalarial drug atovaquone, the molecular details underlying parasite demise are unclear. We have shown that a critical function of mitochondrial electron transport in blood-stage Plasmodium falciparum is to support pyrimidine biosynthesis. Here, we explore the effects of atovaquone, alone and in combination with proguanil, on P. falciparum viability. Our results suggest that the effects of inhibition depend upon the erythrocytic stage of the parasites and the duration of exposure. Ring- and schizont-stage parasites are most resilient to drug treatment and can survive for 48 h, with a fraction remaining viable even after 96 h. Survival of parasites does not appear to require nutrient uptake. Thus, intraerythrocytic parasites with inhibited mitochondrial electron transport and collapsed mitochondrial membrane potential do not undergo apoptosis but enter an apparent static state. These results have significant implications for desirable properties of antimalarials under development that target mitochondrial functions.
Malaria remains a major threat to human health and welfare in the world today (11, 21). Although the available antimalarial drugs inhibit parasite growth and development in vitro, the underlying molecular mechanisms of parasite demise are not always well understood. Since the goal of antimalarial treatment is parasite death, a deeper knowledge of the essential metabolic processes targeted by antimalarial drugs is of importance. In this regard, we are interested in studying the mitochondrial electron transport chain (mtETC), which is a validated target of antimalarial compounds. The critical functions of the mtETC appear to be (i) to serve as an electron disposal system for dihydroorotate dehydrogenase (DHOD), an essential mitochondrial enzyme in pyrimidine biosynthesis (18), and (ii) to generate the electro potential across the inner mitochondrial membrane (Δψm) that is necessary for transport. A number of metabolic pathways within mitochondria require maintenance of Δψm to assist the transport of molecules critical for protein synthesis, iron-sulfur cluster and heme biogenesis, and the biosynthesis of ubiquinone (reviewed in reference 30). In addition, the unusual branched tricarboxylic acid metabolism described recently (17) cannot function without several charge-dependent transporters. The antiparasitic drug atovaquone disrupts the mtETC at the cytochrome bc1 complex, thereby preventing both the regeneration of ubiquinone needed for the DHOD reaction and the generation of Δψm by the coupled transmembrane translocation of protons (8, 24). However, our previous experiments with transgenic Plasmodium falciparum expressing a yeast DHOD that does not require ubiquinone revealed an underlying mode of Δψm generation independent of the mtETC that was essential for long-term survival of the parasites in the presence of atovaquone or other electron transport inhibitors (18). We also provided evidence to suggest that this alternative mode of Δψm generation is the likely target of proguanil (18), a synergistic drug that is combined with atovaquone in the antimalarial drug Malarone (4, 25). Thus, atovaquone resistance in the transgenic parasites was completely reversed by the inclusion of proguanil.
Mitochondria are central to the life and death decisions of cells, especially in multicellular organisms. Inhibition of the mtETC and/or collapse of Δψm triggers a cascade of irreversible events that result in programmed cell death in many organisms. Such events occur quite rapidly and involve molecular entities that are conserved across a large evolutionary distance. However, in malaria parasites, the mitochondrial genome and functions are greatly reduced and divergent compared to their counterparts in more familiar organisms (30). A preliminary study has suggested that an irreversible programmed cell death cascade similar to that in metazoa does not ensue in malaria parasites consequent to mitochondrial inhibition (16). In addition, a number of studies have begun to address the stage specificity or static effects of a number of antimalarials (23, 27, 28, 33). In light of these studies, it is important to address the toxicity of various antimalarials and to define them in terms of how they affect parasite growth and development. Indeed, the most effective antimalarials are cytotoxic, inducing parasite death. However, cytostatic drugs have been quite successful for malaria treatment. One concern with cytostatic drugs is the development of resistance or recrudescence of the infectious agent once the concentration has decreased below effective levels.
Here we have examined the viability of the human malaria parasite Plasmodium falciparum when exposed to mitochondrial inhibitors. Our results indicate the absence of a programmed cell death pathway triggered by mitochondrial inhibition in these parasites. Instead, the parasites remain viable for a significant length of time with an inhibited mtETC and eventual collapse of their Δψm, resulting in our classifying atovaquone as a cytostatic inhibitor of P. falciparum growth and development in vitro. Several new chemical classes of antimalarial compounds that target the mtETC are under development (reviewed in reference 20). Our results suggest that for effective treatment of malaria, the pharmacokinetics of these compounds need to be such that inhibitory concentrations are maintained for durations longer than the parasite is able to remain viable.
MATERIALS AND METHODS
Parasite clones and culture conditions.
P. falciparum strain Dd2 was propagated in human erythrocytes by a modification of the method described previously (29). Parasite cultures were maintained at a hematocrit of human erythrocytes of 5% in RPMI 1640 supplemented with hypoxanthine and 0.5% Albumax II (Invitrogen).
Drug treatment and assessment of parasite viability.
To assess effects of atovaquone and an atovaquone-proguanil combination on different intraerythrocytic stages of P. falciparum, the parasitized erythrocytes (pRBC) were synchronized using a method described previously (12). Briefly, the parasite culture was centrifuged at low speed for 5 min, and the supernatant was removed. The cell pellet was then resuspended in an equal volume of synchronization solution (10 mM HEPES [pH 7.5], 0.3 M l-alanine) and incubated for 10 min at 37°C. This process results in osmotic lysis of the trophozoite- and schizont-stage pRBC, resulting in selection of the ring stage. This synchronization process was carried out at least two times during successive 48-h growth cycles. All synchronized cultures were allowed to complete a full 48-h cycle prior to drug treatment to ensure that the results were not affected by the synchronization procedure.
All drug treatments were carried out at 10× the 50% inhibitory concentration (IC50) of atovaquone or atovaquone and proguanil. This concentration is about the IC99 of these two compounds in a standard 48-h [3H]hypoxanthine incorporation assay for growth inhibition.
To assess the stage-specific effects of antimalarial drugs, synchronized parasites were cultured in medium containing either no drug or 10 nM atovaquone (a gift from Glaxo Wellcome, Research Triangle Park, NC) with or without 1 μM proguanil (a gift from Jacobus Pharmaceutical Company, Princeton, NJ) at about 2% parasitemia and a 5% hematocrit, beginning at various stages of development during the 48-h growth cycle. Ring-stage treatment began at 10 to 12 h postinvasion (hpi), early-trophozoite-stage treatment at 20 to 22 hpi, mid-trophozoite-stage treatment at 26 to 28 hpi, late-trophozoite-stage treatment at 32 to 34 hpi, and schizont-stage treatment at 40 to 44 hpi. The treatment continued for either 24 or 48 h, with the medium containing drug being changed every 24 h. Following the specified lengths of time, the medium was removed from treated and untreated cultures by centrifugation, followed by resuspension in a 10× volume of complete medium two times to remove the drugs. The infected cells were diluted to 1,000 parasite-infected cells per well in a 96-well plate, followed by serial dilution to about 16 parasite-infected cells per well in complete medium containing uninfected red cells at a 5% hematocrit. The medium of the recovering parasites was changed every 24 h until growth was observed. Growth was assessed by observing the concomitant color change in the medium, which contains phenol red, due to the acidification resulting from the secretion of lactic acid by the growing parasites. This observation was then verified by Giemsa-stained thin blood films. The number of days to observable growth was plotted versus the dilution of parasites for each stage.
The short- and long-term drug treatments utilized the same culturing techniques as described above. The concentrations and combination of drugs remained the same. Cultures were synchronized, and drug treatment began at 10 to 12 hpi to investigate ring stages or at 40 to 44 hpi to investigate schizont stages and was continued for 24, 48, 72, or 96 h. Following treatment, cultures were thoroughly washed to remove the drug(s) and incubated in complete medium that was refreshed every 24 h. Giemsa-stained thin blood films were used to determine parasitemia every 24 h following drug treatment, and percent parasitemia was plotted against the number of days posttreatment to indicate the emergence of surviving parasites following various lengths of drug treatment.
Growth inhibition assays.
All parasite growth inhibition assays were performed in triplicate in 96-well plates as described by Desjardins et al. (6). P. falciparum-infected erythrocytes at a 1.0% initial parasitemia and a 4% hematocrit were exposed to various concentrations of atovaquone (0.001 to 300 nM) in the presence or absence of proguanil (1 μM) for 24 h, followed by incubation with 0.5 μCi of [3H]hypoxanthine for an additional 24 h. Untreated parasitized and nonparasitized red blood cells were incubated similarly to serve as positive and negative controls. Incorporation of [3H]hypoxanthine served as a measure of parasite growth.
Parasite morphology.
The morphology of treated parasites was compared to that of untreated controls every 24 h during and following drug treatment by examining Giemsa-stained thin blood smears. Following staining, images were captured by an Olympus BX60 microscope using a 100× oil immersion lens.
Assessing the presence of new permeability pathways by osmotic hemolysis.
Utilizing a method adapted from Ginsburg et al. (10), the status of the new permeability pathways (NPP) displayed by the parasites under drug treatment was examined by determining the kinetics of osmotic hemolysis of pRBC incubated in an isosmotic l-alanine solution. At 6-h intervals during treatment, 1.5 × 104 pRBC were suspended in 10 mM HEPES and 0.3 M l-alanine. The parasites were incubated for 0, 10, 20, 30, 40, 50, or 60 min at 37°C and centrifuged at 10,000 × g for 30 s. The amount of hemoglobin in the supernatant was measured at 415 nm. This analysis was carried out at 0, 6, 12, 18, 24, 30, 36, 42, and 48 h throughout the length of the 10 nM atovaquone treatment, beginning with either ring- or schizont-stage P. falciparum, and results were compared to those for untreated parasitized erythrocytes to control for changes in hemoglobin content. Any nonisosmotic lysis was accounted for by subtracting the absorbance measurements of the wells with lysis buffer alone from the measurements for uninfected erythrocytes exposed to the lysis buffer at each time point. The kinetics of lysis was plotted using GraphPad software, and the time to 50% lysis was calculated by a nonlinear sigmoidal response curve. This value approximately represents the relative proportions of NPP channels displayed by the parasites at different stages of their intraerythrocytic development cycle.
RESULTS
Stage-specific response of P. falciparum to drug treatment.
To understand the parasite's response to antimitochondrial treatment and the possibility of resistance generation, Plasmodium falciparum strain Dd2, which has been demonstrated to rapidly develop resistance to multiple antimalarials (19), was chosen for this study. Highly synchronized cultures were treated with atovaquone alone or in combination with proguanil starting at the ring, early-trophozoite, mid-trophozoite, late-trophozoite, and schizont stages for 24 or 48 h. Viability of parasites following the drug treatments was assessed by serial dilution of the culture in drug-free medium and determination of numbers of days to observable growth. As shown in Fig. 1, growth of untreated parasites was observed in 4 to 7 days in wells containing 1,000 to 16 parasitized cells. Ring-stage parasites treated with either atovaquone or atovaquone plus proguanil for 24 h had the same time to observable growth as untreated parasites (Fig. 1A), suggesting that inhibition of the mtETC as well as the collapse of Δψm for 24 h had no discernible effect on the viability of ring-stage P. falciparum. For the ring-stage parasites treated for 48 h, there was a modest reduction of viability, as indicated by the delay of 1 to 2 days before observable parasite growth (Fig. 1B), suggesting that a majority of the ring-stage parasites remained viable even after 48 h of treatment that inhibits the mtETC and collapses Δψm (see Fig. S1 in the supplemental material). Other stages were also mostly viable after 24 h of treatment with atovaquone alone or in combination with proguanil (Fig. 1C, E, G, and I). However, 48-h treatment of early- and mid-stage trophozoites (Fig. 1D and F) led to viability losses reaching greater than 99%. Compared to that, late-trophozoite- and schizont-stage parasites showed a response similar to that of ring-stage parasites and appeared to be more resilient to the drug treatment. These results suggest that all erythrocytic stages of P. falciparum can withstand 24 h of mtETC inhibition and collapse of the Δψm but that early- and mid-stage trophozoites succumb to these conditions after 48 h.
FIG. 1.
P. falciparum stage-specific susceptibility to atovaquone or atovaquone-proguanil combination treatment. Highly synchronized cultures starting at 2% parasitemia and a 5% hematocrit were treated with 10 nM atovaquone with (open bars) or without (striped bars) 1 μM proguanil for 24 or 48 h beginning at the ring (10 to 14 hpi), early-trophozoite (20 to 22 hpi), mid-trophozoite (26 to 28 hpi), late-trophozoite (32 to 34 hpi), and schizont (40 to 44 hpi) stages of intraerythrocytic development. Recovery after each treatment and subsequent dilution were compared to those for the untreated control (black bars). The number of days to observable growth was assessed by visualizing a color change in the culture media, indicative of parasite growth. Results are representative of one of three independent experiments performed in triplicate and assessed by two individuals. Error bars indicate calculated standard errors of the means (SEM).
Response of ring- and schizont-stage parasites to long-term drug treatment.
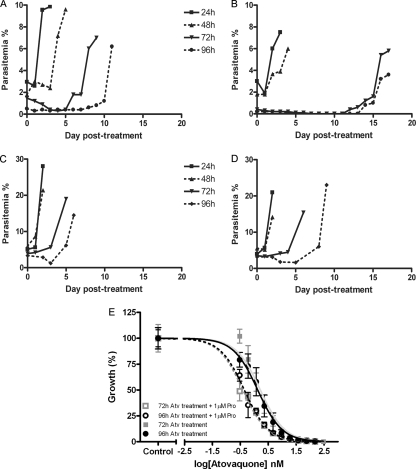
Because the ring- and schizont-stage parasites appeared to be most resilient to mitochondrial inhibition, we next examined the effects of longer treatment on their survival. As shown in Fig. 2A, 24-h atovaquone treatment of ring-stage parasites had minimal impact, with the parasites resuming normal growth within 24 h following the withdrawal of the drug. The treatment lasting 48 h appeared to delay the resumption of growth by 2 days following the drug removal. Exposure to atovaquone lasting 72 or 96 h, however, resulted in significant delay in detectable growth, with 96-h treatment showing a more significant effect. Exposure to atovaquone plus proguanil (Fig. 2B) for 24 and 48 h had an effect on the resumption of growth by the parasites similar to that when the parasites were exposed to atovaquone alone. However, 72- and 96-h exposure to atovaquone plus proguanil resulted in >99.9% parasite demise, with parasite growth observable only 12 to 14 days after the cessation of treatment. These results suggest that the vast majority of ring-stage parasites cannot survive with a collapsed Δψm for 72 h.
FIG. 2.
Numbers of days to observable growth of ring- and schizont-stage P. falciparum organisms following drug treatment. Highly synchronized cultures of P. falciparum strain Dd2 starting with ∼2 to 3% parasitemia and a 5% hematocrit (∼1.25 × 108 parasites) were treated at 10 to 14 hpi (ring stage) (A, B) or 40 to 44 hpi (schizont stage) (C, D) with 10 nM atovaquone alone (A, C) or with 1 μM proguanil (B, D) for 24, 48, 72, or 96 h. Following the specified lengths of treatment, the drug(s) was washed from the cultures and numbers of days to observable growth in drug-free medium were assessed by daily assessment of parasitemia in Giemsa-stained thin blood films. Results are representative of one of three independent experiments. (E) The drug sensitivity of the surviving P. falciparum parasites to atovaquone and an atovaquone-proguanil combination was determined by drug inhibition assays. Parasite growth was assessed by [3H[hypoxanthine incorporation when the parasites were exposed to various concentrations of atovaquone (Atv) with or without 1 μM proguanil (Pro) for 48 h. Percent growth is compared to the concentration of atovaquone. The experiment was performed in triplicate, and error bars represent the calculated SEM.
Exposure of schizont-stage parasites to atovaquone alone or in combination with proguanil also revealed a resilience to treatment lasting 24 or 48 h but significant parasite demise following 72 or 96 h of treatment (Fig. 2C and D). The progression of the schizonts to merozoites and invasion of new red cells to form rings may not be affected by mtETC inhibition and Δψm collapse, as indicated by the increase in parasitemia during the treatment periods, and this is likely to account for the apparent reduced time until the resumption of growth of schizont-stage parasites compared to that of ring-stage parasites.
Parasites surviving long-term treatment are not resistant to atovaquone.
Malaria parasites are known to develop resistance to atovaquone relatively rapidly in patients as well as in in vitro cultures (9, 15). To assess the possibility of the emergence of resistance in our experiments, the ring-stage parasites that survived 72 or 96 h of atovaquone-plus-proguanil treatment were subjected to a drug susceptibility assay to determine their level of resistance to atovaquone and atovaquone combined with 1 μM proguanil (Fig. 2E). We determined that these parasite cultures were just as susceptible to atovaquone and the atovaquone-proguanil combination as the control parental parasites, with IC50s of 1.0 nM and 0.2 nM, respectively (Fig. 2E), indicating that the survival under long-term drug treatment was not due to selection of resistant parasites. We also sequenced the cytochrome b gene, encoded by the mitochondrial DNA (mtDNA), in the surviving parasite cultures to assess the possible presence of mutations characteristic of atovaquone resistance. Sequence analysis failed to reveal any mutations within the cytochrome b gene in these parasites (data not shown).
Parasite morphology and progression through the intraerythrocytic development cycle.
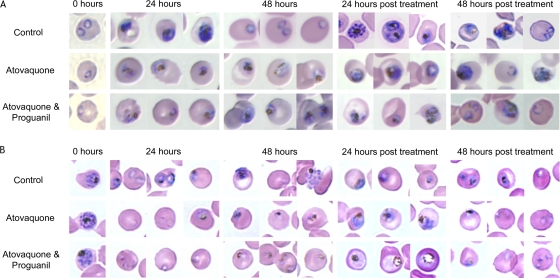
We next examined the morphology of the parasites while they were under drug treatment as well as recovering from the treatment. The ring- and schizont-stage parasites were separately treated with 10 nM atovaquone alone or in the presence of 1 μM proguanil for 48 h, the media containing drug were removed, and the parasites were allowed to recover for an additional 48 h. Giemsa-stained blood smears of untreated and treated parasites were analyzed every 24 h. Representative light microscopy images of parasite morphology are shown in Fig. 3A and B. We observed that mtETC inhibition and Δψm collapse for 24 h does not halt the developmental progression of treated ring-stage parasites (Fig. 3A), nor does it inhibit the release of and reinvasion by merozoites, as evidenced by new rings observed 24 h after the treatment of the schizonts (Fig. 3B). During 48 h of drug exposure, the progression through intraerythrocytic development appeared to cease, and the parasites assumed an early-trophozoite-like morphology (Fig. 3A and B). Once the drugs were removed, however, progression through the intraerythrocytic development cycle resumed. Ring-stage parasites were apparent 48 h after the removal of drugs (Fig. 3A and B). These observations suggest that parasites exhibiting mtETC inhibition and Δψm collapse gradually enter a “static” phase and remain in it until the treatment is removed. The static parasites seem to retain some metabolic activity, as demonstrated by the accumulation of hemozoin pigment. The pale staining of the parasites could be indicative of reduced quantities of proteins and nucleic acids.
FIG. 3.
Morphology of ring- and schizont-stage parasites during 48 h of treatment and the subsequent 48 h of recovery. Highly synchronized P. falciparum strain Dd2 was grown in regular medium, medium containing atovaquone alone, or medium containing atovaquone with 1 μM proguanil for 48 h beginning at either ring stage (10 to 14 hpi) (A) or schizont stage (40 to 44 hpi) (B). Following treatment, the drug(s) was completely removed by washing and all cultures were supplied with drug-free medium for an additional 48 h. Giemsa-stained thin blood films were prepared every 24 h. The images shown are representative of the majority of morphologies present in each culture.
Deployment of nutrient channels in drug-treated parasites.
Intraerythrocytic stages of malaria parasites extensively remodel the red blood cell to support their survival and growth (reviewed in references 14 and 26), and among these changes are nutrient channels that constitute new permeability pathways in the host cell plasma membrane (1, 2, 10). These channels become apparent in the early-trophozoite stage and allow a wide array of solutes to pass through them, playing critical roles in nutrient uptake and waste disposal (5). We were interested in examining the status of these channels in drug-treated parasites during the static stage described above. The presence and approximate relative number of these channels in infected erythrocytes can be assessed by observation of osmotic lysis induced by isosmotic concentrations of solutes, such as sorbitol and l-alanine, that can transit the erythrocyte membrane via the NPP. The osmotic lysis results in the release of hemoglobin, which could be monitored as a surrogate for the presence of nutrient channels, and the kinetics of hemoglobin release could provide an approximation of the relative number of these channels (10). As shown in Table 1, when schizont-stage parasites were examined over a 48-h period, the nutrient channels became evident at 30 h in untreated parasites (the apparent lack of channels at early time points is likely due to the highly reduced hemoglobin level present in schizont-stage parasites). Atovaquone-treated parasites, however, failed to display nutrient channels until 42 h after the initiation of treatment (Table 1), and the kinetics of lysis suggested that the displayed channels were in greatly reduced numbers (see Fig. S2a in the supplemental material). These results suggest that merozoites released by schizonts under atovaquone treatment can invade erythrocytes, develop into early ring-stage parasites, and survive for a significant time without displaying nutrient channels.
TABLE 1.
Dynamics of new permeability pathway establishment in P. falciparum measured by osmotic lysisa
| Duration of treatment (h) | Mean time (min) to half-maximum lysis ±SD |
|||
|---|---|---|---|---|
| Schizont-stage parasites |
Ring-stage parasites |
|||
| Untreated | Treated | Untreated | Treated | |
| 0 | — | NA | — | NA |
| 6 | — | — | 14.16 ± 1.62 | 8.78 ± 1.5 |
| 12 | — | — | 3.54 ± 1.20 | 5.61 ± 1.42 |
| 18 | — | — | 2.23 ± 4.11 | 12.43 ± 1.99 |
| 24 | — | — | 4.10 ± 1.49 | 7.78 ± 1.31 |
| 30 | 5.96 ± 1.80 | — | — | 2.04 ± 1.02 |
| 36 | 1.10 ± 4.20 | — | — | 4.79 ± 1.47 |
| 42 | 2.29 ± 1.35 | 37.78 ± 1.97 | — | 1.15 ± 4.22 |
| 48 | 1.99 ± 2.26 | 39.35 ± 1.18 | — | 9.25 ± 1.58 |
Highly synchronized parasites were treated with 10 nM atovaquone for 48 h beginning at either the ring or schizont stage. At the indicated times, parasites were assayed for NPP-mediated osmotic lysis by suspension in l-alanine. Hemoglobin released upon lysis from the parasitized red blood cell was measured at 415 nm and plotted as a function of time (see Fig. S2 in the supplemental material). Absorbance measurements of the lysis buffer alone or of uninfected erythrocytes incubated in lysis buffer were subtracted as background hemolysis. The assays were performed in triplicate in two independent experiments, and measurements were averaged to calculate the time to 50% lysis and the standard deviation for each time point. —, no lysis detected over 1 h of incubation with l-alanine; NA, not applicable.
In contrast, atovaquone treatment of established ring-stage parasites (8 to 10 h postinvasion) did not interfere with the display of nutrient channels, as indicated by the timing of their appearance and the apparent number of displayed channels, both of which were identical to those of the untreated ring-stage parasites up to 18 h of treatment (Table 1; see also Fig. S2b in the supplemental material). At this point, continued development through trophozoite and schizont stages by the untreated parasites resulted in diminished observable hemoglobin release, but in the treated parasites, the channels remained detectable up to 48 h. These results suggest that the commitment to display nutrient channels is made early during the ring stage and that atovaquone treatment does not interfere with the progression of this display.
DISCUSSION
Previous studies have demonstrated the ability of P. falciparum to recrudesce following antimitochondrial inhibition (28); however, controlled examination of the surviving parasites was lacking. We show here that P. falciparum ring-stage parasites remain viable up to 48 h in the face of mtETC inhibition and collapsed Δψm. The programmed cell death induced in other organisms in response to mitochondrial inhibition does not seem to occur in these parasites. We also show that schizont-stage development, as well as emergence of and invasion by merozoites, is not affected by mitochondrial inhibition. Parasites with inhibited mtETC and collapsed Δψm seem to enter into a static state that is reversed, with growth resumed, if the inhibitor is removed within 48 h. The disruption of Δψm, as demonstrated by MitoTracker staining (see Fig. S1 in the supplemental material), and the eventual recovery of ring-stage parasites imply that apoptotic cell death pathways are not initiated within 24 h of treatment; however, an undefined mechanism of cell death could play a role in the minimal loss of parasite viability after 48 h of treatment. Trophozoite-stage parasites also appear largely to withstand mitochondrial inhibition up to 24 h but lose viability after 48 h. As shown previously, a critical role of mtETC is to serve as an electron disposal system for the parasite dihydroorotate dehydrogenase, a mitochondrially located enzyme involved in the essential pyrimidine biosynthesis pathway (18). A possible explanation for the reduced viability of trophozoite-stage parasites when subjected to mtETC inhibition could be the increased demand for pyrimidine nucleotides in these metabolically more active stages to serve RNA synthesis. In the ring and schizont stages, the demand for pyrimidine nucleotides could be met through recycling of existing RNA molecules. Indeed, the short half-life of most mRNA in ring-stage P. falciparum (22) suggests that a substantial portion of the ribonucleotide pool is derived from recycled RNA molecules.
Currently, atovaquone is prescribed as Malarone in a fixed combination with proguanil for 3 consecutive days to treat uncomplicated malaria. While this drug combination has generally been successful, occasional treatment failures due to the emergence of cytochrome b mutations have been reported. Our results here show that the atovaquone-proguanil combination acts as a cytostatic drug against the ring-stage parasites and that these parasites remain viable up to 48 h, with a small portion surviving exposure for as long as 96 h. The pharmacokinetics of atovaquone in patients clearly has an impact on the duration and level of exposure of parasites to the drug. Atovaquone has a relatively long half-life (13), but the majority of the drug is bound to plasma proteins in patients, and thus the actual concentration of the drug that reaches the parasite is not clear. Proguanil acts synergistically with atovaquone and lowers the concentration at which atovaquone inhibits parasite growth (25) but has a shorter half-life than atovaquone (7). Therefore, subsequent to the 3-day treatment with atovaquone-proguanil, any remaining viable parasites are likely to be exposed to suboptimal atovaquone concentrations. Such a condition may favor the selection of resistance mutations.
Because the mtETC is a clinically proven target for atovaquone and because the cost of atovaquone is prohibitive for general use, there has been an increased effort to find other compounds that also selectively target the parasite mtETC but are likely to be much less expensive. At least four different chemical entities are currently being pursued: 4(1H)-pyridones (7), acridones (31), acridinediones (3), and 4(1H)-quinolones (32). As these compounds are being developed for eventual clinical trials, it is important to consider their pharmacokinetics. Since P. falciparum ring-stage parasites appear to remain viable for up to 48 h with an inhibited mtETC and a collapsed Δψm, the new antimitochondrial compounds need to maintain inhibitory concentrations in plasma for at least that length of time. Such considerations may be critical in decisions regarding the choice of new antimitochondrial antimalarial drugs.
Supplementary Material
Acknowledgments
We thank colleagues in the Center for Molecular Parasitology for discussion.
This work was supported by National Institutes of Health grant R01-AI28398 to A.B.V.
Footnotes
Published ahead of print on 20 September 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Alkhalil, A., J. V. Cohn, M. A. Wagner, J. S. Cabrera, T. Rajapandi, and S. A. Desai. 2004. Plasmodium falciparum likely encodes the principal anion channel on infected human erythrocytes. Blood 104:4279-4286. [DOI] [PubMed] [Google Scholar]
- 2.Baumeister, S., M. Winterberg, C. Duranton, S. M. Huber, F. Lang, K. Kirk, and K. Lingelbach. 2006. Evidence for the involvement of Plasmodium falciparum proteins in the formation of new permeability pathways in the erythrocyte membrane. Mol. Microbiol. 60:493-504. [DOI] [PubMed] [Google Scholar]
- 3.Biagini, G. A., N. Fisher, N. Berry, P. A. Stocks, B. Meunier, D. P. Williams, R. Bonar-Law, P. G. Bray, A. Owen, P. M. O'Neill, and S. A. Ward. 2008. Acridinediones: selective and potent inhibitors of the malaria parasite mitochondrial bc1 complex. Mol. Pharmacol. 73:1347-1355. [DOI] [PubMed] [Google Scholar]
- 4.Canfield, C. J., M. Pudney, and W. E. Gutteridge. 1995. Interactions of atovaquone with other antimalarial drugs against Plasmodium falciparum in vitro. Exp. Parasitol. 80:373-381. [DOI] [PubMed] [Google Scholar]
- 5.Desai, S. A., S. M. Bezrukov, and J. Zimmerberg. 2000. A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature 406:1001-1005. [DOI] [PubMed] [Google Scholar]
- 6.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edstein, M. D., S. Looareesuwan, C. Viravan, and D. E. Kyle. 1996. Pharmacokinetics of proguanil in malaria patients treated with proguanil plus atovaquone. Southeast Asian J. Trop. Med. Public Health 27:216-220. [PubMed] [Google Scholar]
- 8.Fry, M., and M. Pudney. 1992. Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4′-chlorophenyl) cyclohexyl]-3-hydroxy-1,4-naphthoquinone (566C80). Biochem. Pharmacol. 43:1545-1553. [DOI] [PubMed] [Google Scholar]
- 9.Gassis, S., and P. K. Rathod. 1996. Frequency of drug resistance in Plasmodium falciparum: a nonsynergistic combination of 5-fluoroorotate and atovaquone suppresses in vitro resistance. Antimicrob. Agents Chemother. 40:914-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginsburg, H., M. Krugliak, O. Eidelman, and Z. Ioav Cabantchik. 1983. New permeability pathways induced in membranes of Plasmodium falciparum infected erythrocytes. Mol. Biochem. Parasitol. 8:177-190. [DOI] [PubMed] [Google Scholar]
- 11.Greenwood, B., and T. Mutabingwa. 2002. Malaria in 2002. Nature 415:670-672. [DOI] [PubMed] [Google Scholar]
- 12.Haynes, J. D., and J. K. Moch. 2002. Automated synchronization of Plasmodium falciparum parasites by culture in a temperature-cycling incubator. Methods Mol. Med. 72:489-497. [DOI] [PubMed] [Google Scholar]
- 13.Hudson, A. T., M. Dickins, C. D. Ginger, W. E. Gutteridge, T. Holdich, D. B. Hutchinson, M. Pudney, A. W. Randall, and V. S. Latter. 1991. 566C80: a potent broad spectrum anti-infective agent with activity against malaria and opportunistic infections in AIDS patients. Drugs Exp. Clin. Res. 17:427-435. [PubMed] [Google Scholar]
- 14.Kirk, K. 2001. Membrane transport in the malaria-infected erythrocyte. Physiol. Rev. 81:495-537. [DOI] [PubMed] [Google Scholar]
- 15.Looareesuwan, S., C. Viravan, H. K. Webster, D. E. Kyle, D. B. Hutchinson, and C. J. Canfield. 1996. Clinical studies of atovaquone, alone or in combination with other antimalarial drugs, for treatment of acute uncomplicated malaria in Thailand. Am. J. Trop. Med. Hyg. 54:62-66. [DOI] [PubMed] [Google Scholar]
- 16.Nyakeriga, A. M., H. Perlmann, M. Hagstedt, K. Berzins, M. Troye-Blomberg, B. Zhivotovsky, P. Perlmann, and A. Grandien. 2006. Drug-induced death of the asexual blood stages of Plasmodium falciparum occurs without typical signs of apoptosis. Microbes Infect. 8:1560-1568. [DOI] [PubMed] [Google Scholar]
- 17.Olszewski, K. L., M. W. Mather, J. M. Morrisey, B. A. Garcia, A. B. Vaidya, J. D. Rabinowitz, and M. Llinas. 2010. Branched tricarboxylic acid metabolism in Plasmodium falciparum. Nature 466:774-778. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Painter, H. J., J. M. Morrisey, M. W. Mather, and A. B. Vaidya. 2007. Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature 446:88-91. [DOI] [PubMed] [Google Scholar]
- 19.Rathod, P. K., T. McErlean, and P.-C. Lee. 1997. Variations in frequencies of drug resistance in Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 94:9389-9393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigues, T., F. Lopes, and R. Moreira. 2010. Inhibitors of the mitochondrial electron transport chain and de novo pyrimidine biosynthesis as antimalarials: the present status. Curr. Med. Chem. 17:929-956. [DOI] [PubMed] [Google Scholar]
- 21.Sachs, J., and P. Malaney. 2002. The economic and social burden of malaria. Nature 415:680-685. [DOI] [PubMed] [Google Scholar]
- 22.Shock, J. L., K. F. Fischer, and J. L. Derisi. 2007. Whole-genome analysis of mRNA decay in Plasmodium falciparum reveals a global lengthening of mRNA half-life during the intra-erythrocytic development cycle. Genome Biol. 8:R134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skinner, T. S., L. S. Manning, W. A. Johnston, and T. M. E. Davis. 1996. In vitro stage-specific sensitivity of Plasmodium falciparum to quinine and artemisinin drugs. Int. J. Parasitol. 26:519-525. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava, I. K., H. Rottenberg, and A. B. Vaidya. 1997. Atovaquone, a broad spectrum antiparasitic drug, collapses mitochondrial membrane potential in a malarial parasite. J. Biol. Chem. 272:3961-3966. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava, I. K., and A. B. Vaidya. 1999. A mechanism for the synergistic antimalarial action of atovaquone and proguanil. Antimicrob. Agents Chemother. 43:1334-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staines, H. M., A. Alkhalil, R. J. Allen, H. R. De Jonge, E. Derbyshire, S. Egee, H. Ginsburg, D. A. Hill, S. M. Huber, K. Kirk, F. Lang, G. Lisk, E. Oteng, A. D. Pillai, K. Rayavara, S. Rouhani, K. J. Saliba, C. Shen, T. Solomon, S. L. Y. Thomas, P. Verloo, and S. A. Desai. 2007. Electrophysiological studies of malaria parasite-infected erythrocytes: current status. Int. J. Parasitol. 37:475-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ter Kuile, F., N. J. White, P. Holloway, G. Pasvol, and S. Krishna. 1993. Plasmodium falciparum: in vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Exp. Parasitol. 76:85-95. [DOI] [PubMed] [Google Scholar]
- 28.Thapar, M. M., J. P. Gil, and A. Bjorkman. 2005. In vitro recrudescence of Plasmodium falciparum parasites suppressed to dormant state by atovaquone alone and in combination with proguanil. Trans. R. Soc. Trop. Med. Hyg. 99:62-70. [DOI] [PubMed] [Google Scholar]
- 29.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 30.Vaidya, A. B., and M. W. Mather. 2009. Mitochondrial evolution and functions in malaria parasites. Annu. Rev. Microbiol. 63:249-267. [DOI] [PubMed] [Google Scholar]
- 31.Winter, R. W., J. X. Kelly, M. J. Smilkstein, R. Dodean, G. C. Bagby, R. K. Rathbun, J. I. Levin, D. Hinrichs, and M. K. Riscoe. 2006. Evaluation and lead optimization of anti-malarial acridones. Exp. Parasitol. 114:47-56. [DOI] [PubMed] [Google Scholar]
- 32.Winter, R. W., J. X. Kelly, M. J. Smilkstein, R. Dodean, D. Hinrichs, and M. K. Riscoe. 2008. Antimalarial quinolones: synthesis, potency, and mechanistic studies. Exp. Parasitol. 118:487-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yayon, A., J. A. V. Waa, M. Yayon, T. G. Geary, and J. B. Jensen. 1983. Stage-dependent effects of chloroquine on Plasmodium falciparum in vitro. J. Eukaryot. Microbiol. 30:642-647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.