Abstract
Multidrug-resistant Acinetobacter baumannii (MDRAB) presents an increasing challenge to health care. Although colistin has been used as a treatment of last resort, there is concern regarding its potential for toxicity and the emergence of resistance. The mechanism of action of colistin, however, raises the possibility of synergy with compounds that are normally inactive against Gram-negative organisms by virtue of the impermeability of the bacterial outer membrane. This study evaluated the effect of colistin combined with vancomycin on 5 previously characterized epidemic strains and 34 MDRAB clinical isolates by using time-kill assay, microdilution, and Etest methods. For all the isolates, significant synergy was demonstrated by at least one method, with reductions in the MIC of vancomycin from >256 μg/ml to ≤48 μg/ml for all strains after exposure to 0.5 μg/ml colistin. This raises the possibility of the clinical use of this combination for infections due to MDRAB, with the potential for doses lower than those currently used.
Infections due to multidrug-resistant Acinetobacter baumannii (MDRAB) present an enormous challenge to health care. The organism has been implicated in infections at a variety of sites, including the respiratory tract, bloodstream, skin and soft tissues, and prosthetic devices (26), and has become a particular problem in intensive therapy units (ITU) (6). The emergence of strains resistant to almost all currently available antibiotics has resulted in increased reliance on the polymyxins, a class of antibiotics largely abandoned in past decades due to concerns about the neurotoxicity and nephrotoxicity of the commercially available polymyxin E formulations, colistin sulfate and sodium colistin methanesulfonate (9). As a result, there is some uncertainty regarding their clinical efficacy, optimal dosing regimens, and the potential for the development of resistance. In some MDRAB isolates, heteroresistance to colistin has been observed in vitro and has also developed during therapy (11, 13, 28), raising concerns that colistin alone may lack sufficient killing activity to be used as a monotherapy (24).
A number of studies have assessed the activity of polymyxins when combined with other antimicrobials. In vitro synergy against MDRAB can be demonstrated when colistin is combined with rifampin, minocycline, ceftazidime, imipenem, or azithromycin (31, 32, 38), although often the effect is marginal, with little concordance between the different methods used to assess the potency of the observed effect (2).
The precise mechanism of action of colistin is not entirely clear. Polymyxins have been shown to disrupt the integrity of the Gram-negative bacterial membrane (16) via an electrostatic interaction with negatively charged phosphate residues in the core region of the lipid A component of lipopolysaccharide (LPS). Although the bactericidal activity of colistin arises from osmotic rupture of the cytoplasmic membrane, it also increases the permeability of the outer membrane to substances that are usually excluded (36) and may enhance the activity of hydrophobic antibiotics, which otherwise have little effect on Gram-negative bacteria (15).
The glycopeptide vancomycin, an inhibitor of bacterial peptidoglycan synthesis, is one such molecule that, due to its large size and hydrophobicity, lacks activity against Gram-negative bacteria. The cell-permeabilizing properties of colistin could, however, be exploited to improve the penetration of glycopeptides through the A. baumannii outer membrane toward their targets in the cell wall. In order to determine its potential for the treatment of MDRAB, we assessed the in vitro activity and kill kinetics of a vancomycin-colistin combination against a number of MDRAB isolates prevalent in the United Kingdom.
MATERIALS AND METHODS
Characterization of bacterial isolates.
A. baumannii ATCC 19606, obtained from the National Collection of Type Cultures, Colindale, United Kingdom, was used as a drug-susceptible type strain. Five MDRAB strains, defined as resistant to at least three different antimicrobial classes (aminoglycosides/quinolones/β-lactams) but susceptible to colistin (10), were obtained from the Laboratory of Healthcare Associated Infection (LHCAI), Health Protection Agency, Colindale, United Kingdom. These were representatives of the South East clone (AB11), OXA-23 clones 1 and 2 (AB14, AB16), the “burn” strain (AB186), and the “T” strain (AB184). The South East clone and OXA-23 clones 1 and 2 have been prevalent in United Kingdom hospitals since 2000 (6), while the “T” strain has been associated with combat injuries in soldiers repatriated from Iraq to both United Kingdom and U.S. military institutions (33). Isolates belonging to each of these lineages have been characterized previously by pulsed-field gel electrophoresis (PFGE)-, variable-number tandem repeat-, and sequence-based typing techniques (34).
A number of MDRAB clinical isolates obtained from a collection held in the microbiology laboratories at Barts and the London NHS Trust, London, United Kingdom, were also studied. These were identified by biochemical profiles obtained using API 20NE and by PCR detection of the blaOXA-51-like gene intrinsic to A. baumannii (35). Antimicrobial susceptibility testing was performed by the British Society for Antimicrobial Chemotherapy (BSAC) disc diffusion method (1), with MICs of vancomycin and colistin determined by Etest (bioMérieux, Marcy L'Etoile, France). The presence of blaOXA-23, blaOXA-24, blaOXA-48, and blaOXA-143-like carbapenemase genes was also established by multiplex PCR for all carbapenem-resistant isolates (12, 40). Previous molecular typing of these clinical isolates revealed that they were all members of the UK OXA-23 clone 1 lineage.
Synergy studies. (i) Checkerboard assays.
Colistin sulfate and vancomycin (Sigma-Aldrich, Poole, United Kingdom) were prepared as stock solutions with concentrations of 10,000, 1,000, and 100 μg/ml. A bacterial suspension was prepared by 1:100 dilution of an overnight culture (approximately 5 × 105 CFU/ml of bacteria), 75 μl of which was inoculated into each well of a 96-well microtiter plate. Checkerboards were set up with doubling dilutions of vancomycin (0 to 512 μg/ml) in the horizontal wells and colistin sulfate (0 to 4 μg/ml) in the vertical wells. Plates were read after 18 h of incubation at 37°C, and wells without visible signs of growth were identified by placing the plate on a mirrored surface. This method of synergy testing was used for ATCC 19606 and isolates AB11, AB14, AB16, AB184, and AB186, representative of the 5 epidemic clones.
Two methods were used to define synergy. The first method used the lowest fractional inhibitory concentration index (FICI) of all nonturbid wells along the turbidity-nonturbidity interface (2). FICIs were calculated as [(MIC of colistin combined with vancomycin)/(MIC of colistin alone)] + [(MIC of vancomycin combined with colistin)/(MIC of vancomycin alone)]. Synergy was defined as a FICI of ≤0.5. The second method used was the 2-well method, with synergy defined as the absence of growth in wells containing 0.25× MIC of both drugs and 2× MIC of both drugs (7).
Checkerboard MICs were also analyzed using the susceptibility breakpoint index (SBPI), calculated as [(colistin susceptibility breakpoint)/(MIC of colistin in combination with vancomycin)] + [(vancomycin susceptibility breakpoint)/(MIC of vancomycin in combination with colistin)]. This is a novel parameter, which may be more discriminatory than FICIs (21). A breakpoint of ≤2 μg/ml was used to determine vancomycin susceptibility, consistent with those set for Gram-positive organisms by the CLSI and EUCAST (5, 8).
(ii) Synergy testing by an Etest method.
Colistin was incorporated into Iso-Sensitest agar plates at a fixed concentration of 0.5 μg/ml. Plates were inoculated with a bacterial suspension with an optical density equivalent to a 0.5 McFarland standard, and a vancomycin Etest (bioMérieux) was applied. The MIC of vancomycin in the presence of colistin was compared to the MIC of vancomycin on unsupplemented agar. This method was also used for all of the clinical isolates studied.
Time-kill assays.
The killing kinetics of colistin alone and in combination with vancomycin were assessed against each of the epidemic strains using standard time-kill methodology and viable bacterial counts. Colistin was added at 1 μg/ml and vancomycin at 20 μg/ml, in order to mimic the minimal steady-state concentrations achieved with standard dosing regimens of colistin methanesulfonate (1,000,000 IU of colistin) (20) and continuously infused vancomycin (25). Broths were inoculated with 105 to 106 CFU/ml of a stationary-phase culture, and 1-ml aliquots were removed at the 0-, 2-, 4-, 8-, and 24-h time points. A 48-h count was also included for cultures containing colistin. Ten-fold serial dilutions in normal saline were then plated onto Iso-Sensitest agar. Colonies were counted after incubation for 18 h, and counts were extrapolated to determine the quantity of bacteria (in CFU/ml) present in each culture at each time point.
Time-kill assays were also performed on isolates representative of each epidemic lineage using bacterial 2,3-bis(2-methyloxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) metabolism as a marker of cell viability (22) (see the supplemental material).
Morphology of colistin-exposed cells.
The morphology of A. baumannii isolates exposed to 1 μg/ml of colistin for 24 h was examined by electron microscopy using a Quanta 3D FEG scanning electron microscope (SEM) (FEI Company, Hillsboro, OR) mounted with an Alto 2500 cryopreparation chamber (Gatan Inc., Pleasanton, CA). Cells were washed 3 times in ultrapure water, dropped onto a coverslip, and quickly blotted to remove excess liquid. Samples were cryoimmobilized by plunge-freezing in liquid nitrogen at −210°C and were transferred in vacuo to the cryopreparation chamber. In the cryopreparation chamber, the temperature was raised to −90°C for approximately 3 to 5 min in order to sublime ice off the surface. The temperature was then cooled to −130°C, and the sample was sputter coated with platinum for 60 s to avoid charging problems. Once coated, the sample was taken directly into the SEM cryostage and was held at a constant temperature of −130°C. The sample was constantly maintained in a high vacuum to avoid contamination and ice buildup. Imaging was performed at 2 kV and 5 kV.
Analysis of OMPs.
The effect of colistin exposure on outer membrane protein (OMP) profiles was assessed after incubation for 24 h in Iso-Sensitest broth supplemented with colistin at 1 μg/ml. OMPs were then extracted using a rapid microprocedure as follows (4). Cells from 10-ml cultures were harvested by centrifugation, washed with 1.5 ml of ice-cold 10 mM HEPES buffer, and sonicated (10-s bursts) six times on ice. Debris was removed by centrifugation at 13,000 rpm, and cell membrane material present in the supernatant was collected by further centrifugation at 13,000 rpm for 30 min. Cytoplasmic components in the pellet were solubilized by the addition of 2% Sarkosyl in 10 mM HEPES buffer and incubation at room temperature for 30 min. The outer membrane fractions were then collected and suspended in 50 μl of HEPES buffer following centrifugation for a further 30 min. The protein concentration of each preparation was determined using the Bradford technique; absorbance at 595 nm, measured on a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE), was compared to a standard curve generated from bovine serum albumin standards. Equal amounts (10 μg of protein) of each OMP preparation were precipitated with trichloroacetic acid and were resuspended in 10 μl of lithium dodecyl sulfate (LDS) sample buffer (Invitrogen, Paisley, United Kingdom). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using NuPAGE Novex 4-to-12% Bis-Tris gels (Invitrogen) and were visualized following Coomassie staining with SimplyBlue SafeStain (Invitrogen).
RESULTS
Characterization of bacterial isolates.
Forty isolates were studied in total: the ATCC type strain, the 5 epidemic strains, and 34 clinical isolates. The susceptibility profiles of the epidemic strains are shown in Table 1. All of the clinical isolates were MDRAB, confirmed as A. baumannii by blaOXA-51-like PCR, and carried the blaOXA-23-like carbapenemase gene. Three were susceptible to sulbactam; one of these was also susceptible to ciprofloxacin, amikacin, and minocycline. All isolates studied were deemed susceptible to colistin (MIC, ≤2 μg/ml).
TABLE 1.
Characteristics and synergy testing of the epidemic strains
| Isolate | Strain | Susceptibilitiesa | Synergy testing result |
|||
|---|---|---|---|---|---|---|
| Lowest FICI | 2-well synergy | Time-kill assay | SBPI | |||
| ATCC 19606 | ATCC 19606 | AMC, TZP, IPM, MEM, CIP, GEN, TOB, CST, MIN, SUL | 0.56 | Indifferent | Bactericidal, inhibition of regrowth | 12 |
| AB11 | SE clone | AMK, TOB, CST | 0.5 | Synergy | Bactericidal, inhibition of regrowth | 8 |
| AB14 | OXA-23 clone 1 | CST | 0.375 | Synergy | Bactericidal, inhibition of regrowth | 6 |
| AB16 | OXA-23 clone 2 | AMK, CST | 0.53 | Indifferent | Bactericidal, regrowth at 48 h | 2.25 |
| AB184 | “T” strain | IPM, MEM, AMK, TOB, CST | 0.375 | Synergy | Bactericidal, inhibition of regrowth | 8 |
| AB186 | “Burn” strain | AMK, TOB, CST, MIN | 0.375 | Synergy | Bactericidal, inhibition of regrowth | 2.25 |
Abbreviations: AMC, amoxicillin-clavulanate; TZP, piperacillin-tazobactam; IPM, imipenem; MEM, meropenem; CIP, ciprofloxacin; GEN, gentamicin; TOB, tobramycin; CST, colistin; MIN, minocycline; SUL, sulbactam; AMK, amikacin.
Synergy studies.
Checkerboard studies analyzed by the lowest-FICI method revealed synergy when vancomycin was combined with colistin for four (South East, OXA-23 clone 1, “T,” and “burn”) of the six strains studied. Synergy was observed against the same four isolates when checkerboards were analyzed using the two-well method (Table 1). SBP indices for all isolates ranged from 2.25 to 12, suggesting that the combination reduced the MIC to less than the susceptibility breakpoint (SBP, >2). Using Etests, a reduction in the vancomycin MIC from >256 to ≤12 μg/ml was seen when colistin was added at 0.5 μg/ml (Fig. 1; Table 2).
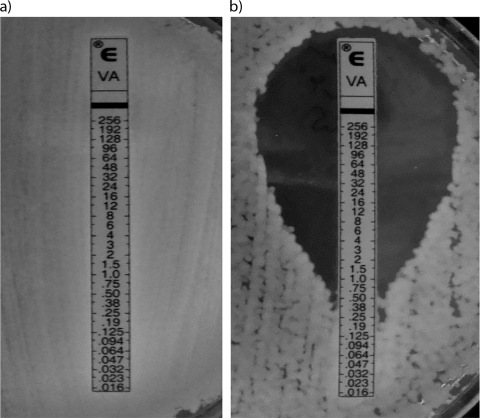
FIG. 1.
MICs of vancomycin for A. baumannii AB14 (OXA-23 clone 1 isolate) after overnight incubation on unsupplemented Iso-Sensitest agar (a) and Iso-Sensitest agar supplemented with 0.5 μg/ml colistin (b).
TABLE 2.
Vancomycin MICs for epidemic strains on Iso-Sensitest agar with and without colistin supplementation
| Strain | Colistin MIC | Vancomycin MIC (μg/ml) on Iso-Sensitest agar: |
|
|---|---|---|---|
| Unsupplemented | With colistin (0.5 μg/ml) | ||
| ATCC 19606 | 0.75 | >256 | 0.016 |
| AB11 | 1 | >256 | 12 |
| AB14 | 1 | >256 | 0.5 |
| AB16 | 1 | >256 | 1 |
| AB184 | 1 | >256 | 0.25 |
| AB186 | 2 | >256 | 3 |
For all 34 clinical isolates tested, the vancomycin MICs were >256 μg/ml on unsupplemented agar. The addition of 0.5 μg/ml of colistin reduced the vancomycin MIC in all cases (range, <0.016 to 48 μg/ml), with a MIC at which 50% of isolates were inhibited (MIC50) of 0.75 μg/ml, a MIC90 of 6 μg/ml, and a geometric mean MIC of 3.8 μg/ml.
Time-kill assays.
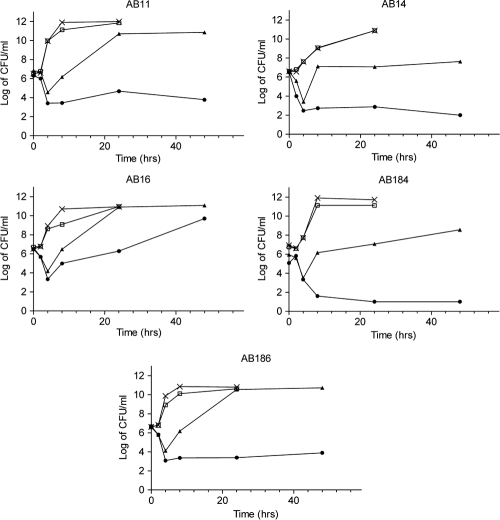
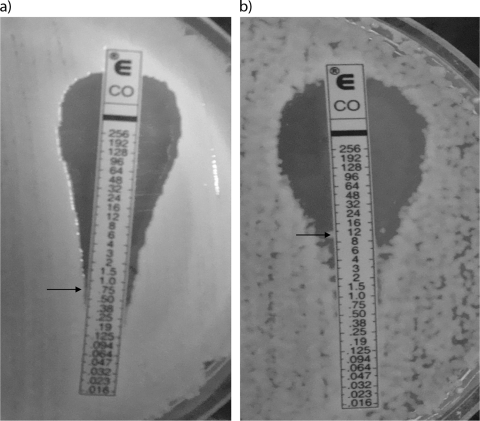
Time-kill curves generated by viable plate counts are shown in Fig. 2. Although colistin alone at 1 μg/ml was initially bactericidal against all strains tested, there was rapid regrowth after only 4 h. The combination of vancomycin with colistin also resulted in rapid bactericidal activity, but regrowth did not occur even after 48 h of incubation, except for strain AB16. When Etests were performed on cells exposed to colistin for 48 h, an increase of as much as 7-fold in the colistin MIC was seen (Fig. 3). Vancomycin exposure alone (20 μg/ml) had no inhibitory effect on growth.
FIG. 2.
Time-kill curves showing the effects of unsupplemented Iso-Senstitest broth (control) and broth supplemented with either colistin (1 μg/ml), vancomycin (20 μg/ml), or both for epidemic strains AB11, AB14, AB16, AB184, and AB186 by viable colony counts. Symbols: ×, control; □, vancomycin; ▴, colistin; •, vancomycin plus colistin.
FIG. 3.
Colistin MIC for ATCC 19606 before (a) and after (b) a 24-h exposure to colistin. Arrows indicate MICs.
Morphology of colistin-exposed cells.
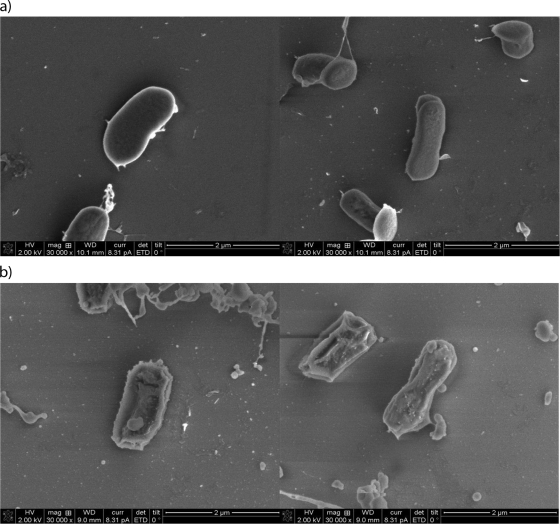
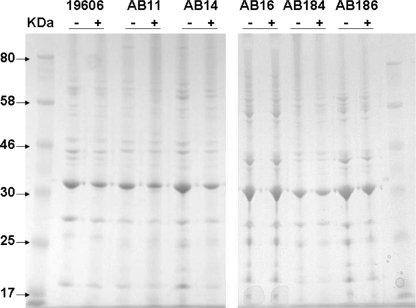
Electron microscopy showed significant differences between cells exposed to colistin and unexposed controls (Fig. 4). The surface of colistin-exposed cells exhibited increased topographic variability and appeared rougher, with visible pits. There was also a greater degree of debris surrounding many of the cells. Comparison of the SDS-PAGE profiles of A. baumannii OMPs extracted from exposed and unexposed cells, however, revealed no clear differences, suggesting that colistin did not disrupt the A. baumannii OMP architecture (Fig. 5).
FIG. 4.
Electron microscopy of A baumannii after overnight incubation in Iso-Sensitest broth alone (a) and Iso-Sensitest broth supplemented with 1 μg/ml colistin (b).
FIG. 5.
Outer membrane protein profiles of A. baumannii isolates grown with (+) and without (−) colistin in Iso-Sensitest broth for 24 h.
DISCUSSION
The global dissemination of multidrug-resistant Gram-negative bacteria, such as A. baumannii and carbapenemase-producing Enterobacteriaceae, has led to repeated calls for the identification and development of novel agents that can be used against these organisms (3). Although it is crucial that new drugs enter the development pipeline, it is unlikely that any will reach clinical use for at least 15 years. In the interim, a reassessment of drugs that were previously out of favor and the investigation of novel combinations of existing agents are clearly warranted. Colistin is an example of a drug that has retained good activity against most Gram-negative bacteria but that nevertheless has fallen out of use due to concerns about toxicity and tolerability.
In the treatment of MDRAB, colistin is frequently turned to as the drug of “last resort” and is often the only agent with activity in vitro. Although resistance to colistin is only rarely reported, clinical experience of its use for the treatment of MDRAB has not always been satisfactory. Poor outcomes for patients with MDRAB infections treated with colistin have been reported in several studies (18, 39) and may be due to a number of factors related both to the pharmacokinetics and pharmacodynamics of the drug (14) and to the severity of underlying illness in the patients treated. The phenomenon of bacterial regrowth demonstrated here may also be a factor in explaining the lack of apparent clinical benefit.
In this study we investigated whether the membrane-permeabilizing properties of colistin could enhance the activity of vancomycin against a number of well-characterized United Kingdom epidemic strains and unselected clinical MDRAB isolates belonging to the OXA-23 clone 1 lineage. Synergy could be demonstrated for all the strains tested, although this was highly dependent on the method of synergy testing used. The relatively low FICIs derived from the checkerboard assays, in comparison to the effects observed with the other methods of synergy testing, highlight the problem of using this method for assessing the combined activity of two agents, one of which is usually inactive in the absence of the other.
The optimal method of determining the susceptibility of A. baumannii to colistin is also controversial. The drug diffuses poorly in agar, and major errors with disc diffusion tests have been found relative to the results of the Etest or automated methods (19). Furthermore, none of the routinely used methods are able to detect the phenomenon of colistin heteroresistance. This can be detected in population analyses at a frequency of 1 in 105 at 10× MIC, and regrowth after a 24-h exposure in time-kill assays has been observed at concentrations as high as 32× MIC for some isolates (17). Heteroresistance in accordance with this was readily observed in the isolates used in this study. Although colistin at 1× MIC was initially rapidly bactericidal, this effect was not sustained over 24 to 48 h; all of the strains regrew to some extent.
Although we have demonstrated the potential for a vancomycin-colistin combination in our in vitro studies, there may be concern about its suitability for clinical use. Nephrotoxicity is a well-known feature of both drugs, the risk of which will doubtless be heightened by their use in combination. However, since the dose of colistin required to provide synergy in vitro is small (0.5 μg/ml), it may be possible to produce synergy in vivo by using lower-than-normal doses of colistin, a strategy similar to the use of low-dose aminoglycosides in combination with β-lactams in the treatment of streptococcal endocarditis. We have been able to identify a number of patients with MDRAB at our institution who received colistin with concomitant vancomycin therapy for the treatment of associated infections with Gram-positive bacteria, without apparent ill effects. However, we have not yet been able to assess the effects of the combined therapy on clinical outcomes attributable to MDRAB.
Another approach could be to develop polymyxin derivatives that retain the desired membrane-permeabilizing properties but have inherently less nephrotoxic potential.
By modifying the fatty acid tail and reducing the cationic charge, a polymyxin-like molecule, NAB741, which is able to sensitize Gram-negative organisms to the effects of hydrophobic antibiotics, including rifampin and clarithromycin, was recently developed (37). This molecule appears to be less nephrotoxic in an animal model, but this advantage appears to come at the cost of reduced activity: unlike colistin, NAB741has no direct antibacterial effect, and the effect of vancomycin on A. baumannii was far less profound than that seen in our study. It may be that further modification of this molecule will result in similar efficacy.
Our results show clear synergy when colistin is combined with vancomycin, a molecule that should ordinarily have no effect on Gram-negative organisms, due to the relative impermeability of the outer membrane to such a large hydrophobic molecule. However, previous studies have shown that mutations can be induced in Escherichia coli to render it susceptible to vancomycin (29): such mutations appear to affect the composition of the outer membrane, allowing the entry of vancomycin and resultant inhibition of peptidoglycan biosynthesis. More recently published data have shown that encapsulation of vancomycin in a fusogenic liposome to allow the entry of the molecule through the outer membrane results in susceptibility in a range of Gram-negative bacteria, including A. baumannii (23). We hypothesized that colistin would disrupt the outer membrane and allow the entry of large molecules, as previously demonstrated for a number of Gram-negative organisms, including Escherichia coli, Klebsiella pneumoniae, and Enterobacter spp. (15). This was supported by the electron microscopy images showing disruption of the membranes of colistin-exposed cells compared to those of unexposed controls, a phenomenon also investigated by Soon et al. (30), who demonstrated differences between the membranes of colistin-sensitive and colistin-resistant strains of A. baumannii by atomic force microscopy. The exact membrane component affected by colistin is still unclear. No differences were seen in the analysis of outer membrane proteins, consistent with the findings of Shlaes et al. (29) for vancomycin-susceptible E. coli.
Synergy in vitro has also been described when colistin is combined with rifampin, although evidence for a clinical benefit is lacking (27). Studies of the clinical effects of colistin-glycopeptide combinations are therefore warranted, since coadministration may help to prevent the emergence of resistant strains, improve the clinical outcomes of MDRAB infections, and also allow lower doses of both agents to be used, alleviating concerns about toxicity. Furthermore, studies assessing MDRAB treatment outcomes with colistin may need to be reexamined, since concomitant administration of vancomycin may be a significant confounding variable.
Finally, further investigation of the mechanism of action of the polymyxins, and in particular the target site, may help in the development of new therapeutic agents against these difficult pathogens.
Supplementary Material
Acknowledgments
We thank Jane Turton, Laboratory of Healthcare Associated Infection, Health Protection Agency, Colindale, United Kingdom, for providing us with representative isolates of the major United Kingdom epidemic MDRAB clones.
Footnotes
Published ahead of print on 27 September 2010.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Andrews, J. M., for the BSAC Working Party on Susceptibility Testing. 2009. BSAC standardized disc susceptibility testing method (version 8). J. Antimicrob. Chemother. 64:454-489. [DOI] [PubMed] [Google Scholar]
- 2.Bonapace, C. R., R. L. White, L. V. Friedrich, and J. A. Bosso. 2000. Evaluation of antibiotic synergy against Acinetobacter baumannii: a comparison with Etest, time-kill, and checkerboard methods. Diagn. Microbiol. Infect. Dis. 38:43-50. [DOI] [PubMed] [Google Scholar]
- 3.Boucher, H. W., G. H. Talbot, J. S. Bradley, J. E. Edwards, D. Gilbert, L. B. Rice, M. Scheld, B. Spelberg, and J. Bartlett. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1-12. [DOI] [PubMed] [Google Scholar]
- 4.Carlone, G. M., M. L. Thomas, H. S. Rumschlag, and F. O. Sottnek. 1986. Rapid microprocedure for isolating detergent-insoluble outer membrane proteins from Haemophilus species. J. Clin. Microbiol. 24:330-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement, CLSI document M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Coelho, J. M., J. F. Turton, M. E. Kaufmann, J. Glover, N. Woodford, M. Warner, M. F. Palepou, R. Pike, T. L. Pitt, B. C. Patel, and D. M. Livermore. 2006. Occurrence of carbapenem-resistant Acinetobacter baumannii clones at multiple hospitals in London and Southeast England. J. Clin. Microbiol. 44:3623-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eliopoulos, G. M., and R. C. Moellering. 1996. Antimicrobial combinations, p. 330-396. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, MD.
- 8.European Committee on Antimicrobial Susceptibility Testing. 2010. Breakpoint table for interpretation of MICs and zone diameters, version 1.1. http://www.eucast.org/clinical_breakpoints/.
- 9.Falagas, M. E., and S. K. Kasiakou. 2005. Colistin: the revival of the polymyxins for the management of multi-drug resistant Gram-negative bacterial infections. Clin. Infect. Dis. 40:1333-1341. [DOI] [PubMed] [Google Scholar]
- 10.Falagas, M. E., P. K. Koletsi, and I. A. Bliziotis. 2006. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J. Med. Microbiol. 55:1619-1629. [DOI] [PubMed] [Google Scholar]
- 11.Hawley, J. S., C. K. Murray, and J. H. Jorgensen. 2008. Colistin heteroresistance in Acinetobacter and its association with previous colistin therapy. Antimicrob. Agents Chemother. 52:351-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins, P. G., M. Lehmann, and H. Seifert. 2010. Inclusion of OXA-143 primers in a multiplex polymerase chain reaction (PCR) for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents 35:305. [DOI] [PubMed] [Google Scholar]
- 13.Ko, K. S., J. Y. Suh, K. T. Kwon, S. I. Jung, K. H. Park, C. I. Kang, D. R. Chung, K. R. Peck, and J. H. Song. 2007. High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J. Antimicrob. Chemother. 60:1163-1167. [DOI] [PubMed] [Google Scholar]
- 14.Kroeger, L. A., L. B. Hovde, I. F. Mitropoulos, J. Schafer, and J. C. Rotschafer. 2007. Colistin methanesulfonate against multidrug-resistant Acinetobacter baumannii in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 51:3431-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam, C., J. Hildebrandt, E. Schütze, and A. Wenzel. 1986. Membrane-disorganising property of polymyxin B nonapeptide. J. Antimicrob. Chemother. 18:9-15. [DOI] [PubMed] [Google Scholar]
- 16.Li, J., R. L. Nation, R. W. Milne, J. D. Turnidge, and K. Coulthard. 2005. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int. J. Antimicrob. Agents 25:11-25. [DOI] [PubMed] [Google Scholar]
- 17.Li, J., C. R. Rayner, R. L. Nation, R. J. Owen, D. Spelman, K. E. Tan, and L. Liolios. 2006. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 50:2946-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livermore, D. M., R. L. Hill, H. Thomson, A. Charlett, J. F. Turton, R. Pike, B. C. Patel, R. Manuel, S. Gillespie, I. Balakrishnan, S. P. Barrett, N. Cumberland, M. Twagira, and the C-MRAB Study Group. 2010. Antimicrobial treatment and clinical outcome for infections with carbapenem- and multiply-resistant Acinetobacter baumannii around London. Int. J. Antimicrob. Agents 35:19-24. [DOI] [PubMed] [Google Scholar]
- 19.Lo-Ten-Foe, J. R., A. M. de Smet, B. M. Diederen, J. A. Kluytmans, and P. H. van Keulen. 2007. Comparative evaluation of the VITEK 2, disk diffusion, Etest, broth microdilution, and agar dilution susceptibility testing methods for colistin in clinical isolates, including heteroresistant Enterobacter cloacae and Acinetobacter baumannii strains. Antimicrob. Agents Chemother. 51:3726-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markou, N., S. L. Markantonis, E. Dimitrakis, D. Panidis, E. Boutzouka, S. Karatzas, P. Rafailidis, H. Apostolakos, and G. Baltopoulos. 2008. Colistin serum concentrations after intravenous administration in critically ill patients with serious multidrug-resistant, gram-negative bacilli infections: a prospective, open-label, uncontrolled study. Clin. Ther. 30:143-151. [DOI] [PubMed] [Google Scholar]
- 21.Milne, K. E., and I. M. Gould. 2010. Combination of testing of multidrug-resistant cystic fibrosis isolates of Pseudomonas aeruginosa: use of a new parameter, the susceptible breakpoint index. J. Antimicrob. Chemother. 65:82-90. [DOI] [PubMed] [Google Scholar]
- 22.Moriarty, F., S. Elborn, and M. Tunney. 2005. Development of a rapid colorimetric time-kill assay for determining the in vitro activity of ceftazidime and tobramycin in combination against Pseudomonas aeruginosa. J. Microbiol. Methods 61:171-179. [DOI] [PubMed] [Google Scholar]
- 23.Nicolosi, D., M. Scalia, V. M. Nicolosi, and R. Pignatello. 2010. Encapsulation in fusogenic liposomes broadens the spectrum of action of vancomycin against Gram-negative bacteria. Int. J. Antimicrob. Agents 35:553-558. [DOI] [PubMed] [Google Scholar]
- 24.Pachón-Ibáñez, M. E., F. Docobo-Pérez, R. López-Rojas, J. Domínguez-Herrera, M. E. Jiménez-Mejias, A. García-Curiel, C. Pichardo, L. Jiménez, and J. Pachón. 2010. Efficacy of rifampin and its combinations with imipenem, sulbactam, and colistin in experimental models of infection caused by imipenem-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 54:1165-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pea, F., M. Furlanut, C. Negri, F. Pavan, M. Crapis, F. Cristini, and P. Viale. 2009. Prospectively validated dosing nomograms for maximizing the pharmacodynamics of vancomycin administered by continuous infusion in critically ill patients. Antimicrob. Agents Chemother. 53:1863-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peleg, A. Y., H. Seifert, and D. L. Paterson. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21:538-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrosillo, N., E. Ioannidou, and M. E. Falagas. 2008. Colistin monotherapy vs combination therapy: evidence from microbiological, animal and clinical studies. Clin. Microbiol. Infect. 14:816-827. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez, C. H., K. Bombicino, G. Granados, M. Nastro, C. Vay, and A. Famiglietti. 2009. Selection of colistin-resistant Acinetobacter baumannii isolates in postneurosurgical meningitis in an intensive care unit with high presence of heteroresistance to colistin. Diagn. Microbiol. Infect. Dis. 65:188-191. [DOI] [PubMed] [Google Scholar]
- 29.Shlaes, D. M., J. H. Shlaes, J. Davies, and R. Williamson. 1989. Escherichia coli susceptible to glycopeptide antibiotics. Antimicrob. Agents Chemother. 33:192-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soon, R. L., R. L. Nation, P. G. Hartley, I. Larson, and J. Li. 2009. Atomic force microscopy investigation of the morphology and topography of colistin-heteroresistant Acinetobacter baumannii strains as a function of growth phase and in response to colistin treatment. Antimicrob. Agents Chemother. 53:4979-4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan, T. Y., L. S. Ng, E. Tan, and G. Huang. 2007. In vitro effect of minocycline and colistin combinations on imipenem-resistant Acinetobacter baumannii clinical isolates. J. Antimicrob. Chemother. 60:421-423. [DOI] [PubMed] [Google Scholar]
- 32.Tripodi, M. F., E. Durante-Mangoni, R. Fortunato, R. Utili, and R. Zarrilli. 2007. Comparative activities of colistin, rifampicin, imipenem and sulbactam/ampicillin alone or in combination against epidemic multidrug-resistant Acinetobacter baumannii isolates producing OXA-58 carbapenemases. Int. J. Antimicrob. Agents 30:537-540. [DOI] [PubMed] [Google Scholar]
- 33.Turton, J. F., M. E. Kaufmann, M. J. Gill, R. Pike, P. T. Scott, J. Fishbain, D. Craft, G. Deye, S. Riddell, L. E. Lindler, and T. L. Pitt. 2006. Comparison of Acinetobacter baumannii isolates from the United Kingdom and the United States that were associated with repatriated casualties of the Iraq conflict. J. Clin. Microbiol. 44:2630-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turton, J. F., S. N. Gabriel, C. Valderrey, M. E. Kaufmann, and T. L. Pitt. 2007. Use of sequence-based typing and multiplex PCR to identify clonal lineages of outbreak strains of Acinetobacter baumannii. Clin. Microbiol. Infect. 13:807-815. [DOI] [PubMed] [Google Scholar]
- 35.Turton, J. F., N. Woodford, J. Glover, S. Yarde, M. E. Kaufmann, and T. L. Pitt. 2006. Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J. Clin. Microbiol. 44:2974-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaara, M. 1992. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 56:395-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaara, M., O. Siikanen, J. Apajalahti, J. Fox, N. Frimodt-Møller, H. He, A. Poudyal, J. Li, R. L. Nation, and T. Vaara. 2010. A novel polymyxin derivative that lacks the fatty acid tail and carries only three positive charges has strong synergism with agents excluded by the intact outer membrane. Antimicrob. Agents Chemother. 54:3341-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wareham, D. W., and D. C. Bean. 2006. In vitro activity of polymyxin B in combination with imipenem, rifampicin and azithromycin versus multidrug resistant strains of Acinetobacter baumannii producing OXA-23 carbapenemases. Ann. Clin. Microbiol. Antimicrob. 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wareham, D. W., D. C. Bean, P. Khanna, E. M. Hennessy, D. Krahe, A. Ely, and M. Millar. 2008. Bloodstream infection due to Acinetobacter spp.: epidemiology, risk factors and impact of multi-drug resistance. Eur. J. Clin. Microbiol. Infect. Dis. 27:607-612. [DOI] [PubMed] [Google Scholar]
- 40.Woodford, N., M. J. Ellington, J. M. Coelho, J. F. Turton, M. E. Ward, S. Brown, S. G. Amyes, and D. M. Livermore. 2006. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents 27:351-353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.