Abstract
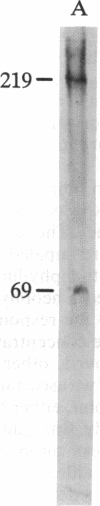
Monoclonal antibodies raised by injecting Necturus gallbladder cells into mice were tested for their ability to inhibit the apical chloride conductance induced by elevation of cellular cAMP. Five of these monoclonal antibodies bound to the apical cells, as shown by indirect immunofluorescence microscopy, and inhibited the chloride conductance; one antibody that bound only to subepithelial smooth muscle, by indirect immunofluorescence microscopy, showed no inhibition of chloride transport. The channel or a closely related molecule is present in the membrane whether or not the pathway is open, since, in addition to inhibiting the conductance of the open channel, the antibody also bound to the membrane in the resting state and prevented subsequent opening of the channel. The antibody was shown to recognize, by ELISA, epitopes from the Necturus gallbladder and small intestine. Finally, by Western blot analysis of Necturus gallbladder homogenates, the antibody was shown to recognize two protein bands of Mr 219,000 and Mr 69,000. This antibody should permit isolation and characterization of this important ion channel.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berschneider H. M., Knowles M. R., Azizkhan R. G., Boucher R. C., Tobey N. A., Orlando R. C., Powell D. W. Altered intestinal chloride transport in cystic fibrosis. FASEB J. 1988 Jul;2(10):2625–2629. doi: 10.1096/fasebj.2.10.2838365. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher R. C., Stutts M. J., Knowles M. R., Cantley L., Gatzy J. T. Na+ transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J Clin Invest. 1986 Nov;78(5):1245–1252. doi: 10.1172/JCI112708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk R. J., Dalmasso A. P., Kim Y., Tsai C. H., Scheinman J. I., Gewurz H., Michael A. F. Neoantigen of the polymerized ninth component of complement. Characterization of a monoclonal antibody and immunohistochemical localization in renal disease. J Clin Invest. 1983 Aug;72(2):560–573. doi: 10.1172/JCI111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. Ion transport in rabbit ileal mucosa. II. Effects of cyclic 3', 5'-AMP. Am J Physiol. 1971 Oct;221(4):992–997. doi: 10.1152/ajplegacy.1971.221.4.992. [DOI] [PubMed] [Google Scholar]
- Giraldez F. Active sodium transport and fluid secretion in the gall-bladder epithelium of Necturus. J Physiol. 1984 Mar;348:431–455. doi: 10.1113/jphysiol.1984.sp015118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Nash N. T., al-Bazzaz F., Layden T. J., Rao M. C. Rectum has abnormal ion transport but normal cAMP-binding proteins in cystic fibrosis. Am J Physiol. 1988 May;254(5 Pt 1):C719–C724. doi: 10.1152/ajpcell.1988.254.5.C719. [DOI] [PubMed] [Google Scholar]
- Handler J. S., Butcher R. W., Sutherland E. W., Orloff J. The effect of vasopressin and of theophylline on the concentration of adenosine 3',5'-phosphate in the urinary bladder of the toad. J Biol Chem. 1965 Nov;240(11):4524–4526. [PubMed] [Google Scholar]
- Jansson R., Steen G., Svanvik J. Effects of intravenous vasoactive intestinal peptide (VIP) on gallbladder function in the cat. Gastroenterology. 1978 Jul;75(1):47–50. [PubMed] [Google Scholar]
- Larsen E. H., Rasmussen B. E. Chloride channels in toad skin. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):413–434. doi: 10.1098/rstb.1982.0141. [DOI] [PubMed] [Google Scholar]
- Nelson D. J., Tang J. M., Palmer L. G. Single-channel recordings of apical membrane chloride conductance in A6 epithelial cells. J Membr Biol. 1984;80(1):81–89. doi: 10.1007/BF01868692. [DOI] [PubMed] [Google Scholar]
- Petersen K. U., Reuss L. Cyclic AMP-induced chloride permeability in the apical membrane of Necturus gallbladder epithelium. J Gen Physiol. 1983 May;81(5):705–729. doi: 10.1085/jgp.81.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poler S. M., Reuss L. Protamine alters apical membrane K+ and Cl- permeability in gallbladder epithelium. Am J Physiol. 1987 Nov;253(5 Pt 1):C662–C671. doi: 10.1152/ajpcell.1987.253.5.C662. [DOI] [PubMed] [Google Scholar]
- Quinton P. M. Chloride impermeability in cystic fibrosis. Nature. 1983 Feb 3;301(5899):421–422. doi: 10.1038/301421a0. [DOI] [PubMed] [Google Scholar]
- Reuss L., Cheung L. Y., Grady T. P. Mechanisms of cation permeation across apical cell membrane of Necturus gallbladder: effects of luminal pH and divalent cations on K+ and Na+ permeability. J Membr Biol. 1981 Apr 30;59(3):211–224. doi: 10.1007/BF01875426. [DOI] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Electrical properties of the cellular transepithelial pathway in Necturus gallbladder. I. Circuit analysis and steady-state effects of mucosal solution ionic substitutions. J Membr Biol. 1975 Dec 4;25(1-2):115–139. doi: 10.1007/BF01868571. [DOI] [PubMed] [Google Scholar]
- Reuss L., Stoddard J. S. Role of H+ and HCO3- in salt transport in gallbladder epithelium. Annu Rev Physiol. 1987;49:35–49. doi: 10.1146/annurev.ph.49.030187.000343. [DOI] [PubMed] [Google Scholar]
- Sato K., Sato F. Defective beta adrenergic response of cystic fibrosis sweat glands in vivo and in vitro. J Clin Invest. 1984 Jun;73(6):1763–1771. doi: 10.1172/JCI111385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker R. L., Frizzell R. A., Dwyer T. M., Farley J. M. Single chloride channel currents from canine tracheal epithelial cells. Biochim Biophys Acta. 1986 Jun 26;858(2):235–242. doi: 10.1016/0005-2736(86)90328-7. [DOI] [PubMed] [Google Scholar]
- Svanvik J., Thornell E., Zettergren L. Gallbladder function in experimental cholecystitis. Surgery. 1981 Apr;89(4):500–506. [PubMed] [Google Scholar]