Abstract
Pyronaridine, a Mannich base antimalarial, has demonstrated high in vivo and in vitro efficacy against chloroquine-resistant Plasmodium falciparum. Although this drug has the potential to become a prominent artemisinin combination therapy, little is known about its efficacy against drug-resistant Plasmodium vivax. The in vitro antimalarial susceptibility of pyronaridine was assessed in multidrug-resistant P. vivax (n = 99) and P. falciparum (n = 90) isolates from Papua, Indonesia, using a schizont maturation assay. The median 50% inhibitory concentration (IC50) of pyronaridine was 1.92 nM (range, 0.24 to 13.8 nM) against P. falciparum and 2.58 nM (range, 0.13 to 43.6 nM) against P. vivax, with in vitro susceptibility correlating significantly with chloroquine, amodiaquine, and piperaquine (rs [Spearman's rank correlation coefficient] = 0.45 to 0.62; P < 0.001). P. falciparum parasites initially at trophozoite stage had higher IC50s of pyronaridine than those exposed at the ring stage (8.9 nM [range, 0.6 to 8.9 nM] versus 1.6 nM [range, 0.6 to 8.9 nM], respectively; P = 0.015), although this did not reach significance for P. vivax (4.7 nM [range, 1.4 to 18.7 nM] versus 2.5 nM [range, 1.4 to 15.6 nM], respectively; P = 0.085). The excellent in vitro efficacy of pyronaridine against both chloroquine-resistant P. vivax and P. falciparum highlights the suitability of the drug as a novel partner for artemisinin-based combination therapy in regions where the two species are coendemic.
Almost 40% of the world's population is at risk for infection by Plasmodium vivax, with an estimated 132 to 391 million clinical infections each year (19). Although chloroquine (CQ) remains the treatment of choice in most of the P. vivax-endemic world, this status is now being undermined by the emergence and spread of chloroquine-resistant (CQR) P. vivax. First reported in the 1980s on the island of New Guinea (2, 23), CQR P. vivax has since spread to other parts of Asia and recently to South America (1). In Papua, Indonesia, CQ resistance in P. vivax has reached levels precluding the use of CQ in most of the province (22, 30). There is an urgency to assess the efficacies of alternative antimalarial agents against drug-resistant P. vivax and to develop new strategies to combat the parasite.
Pyronaridine (Pyr), a Mannich base synthesized in China in the 1970s (3, 16), is being developed as a novel antimalarial for multidrug-resistant malaria. It demonstrates potent in vitro activity against erythrocytic stages of Plasmodium falciparum (8, 24, 26, 36), retaining efficacy against CQR isolates (12, 17, 18). Clinical trials have shown excellent efficacy of monotherapy against multidrug-resistant falciparum malaria (14, 24, 25), with the early therapeutic response faster when combined with artesunate (20). Phase III studies with a coformulation of Pyramax (Shin Poong Pharmaceuticals) containing artesunate plus pyronaridine have recently been completed (34).
Less is known of the antimalarial properties of pyronaridine against P. vivax, although early clinical studies in China demonstrated a rapid therapeutic response (3). To investigate the activity of pyronaridine against CQR P. vivax, we applied a modified schizont maturation assay on fresh field isolates from Papua, Indonesia, where CQR P. vivax is highly prevalent.
MATERIALS AND METHODS
Field location and sample collection.
Plasmodium sp. isolates were collected from patients with uncomplicated malaria presenting to the Rumah Sakit Mitra Masyarakat (RSMM) hospital between January 2006 and July 2007. RSMM is situated on the southern coast of Papua, Indonesia, in a forested lowland area where malaria transmission is unstable, with an estimated annual incidence of 802 per 1,000 person years (divided 57:43 between P. falciparum and P. vivax infections) (11). Drug-resistant strains of P. vivax and P. falciparum are endemic to the area, with the risk of treatment failure reaching 65% within 28 days after chloroquine monotherapy for vivax malaria and 48% after chloroquine plus sulfadoxine-pyrimethamine (SP) for falciparum malaria (22). Patients with symptomatic malaria presenting to an outpatient facility were recruited into the study if singly infected with P. falciparum or P. vivax with a parasitemia between 2,000 μl−1 and 80,000 μl−1. These criteria reflect the technical difficulties of reliably quantifying parasite stages using microscopy at low parasitemias. Although they may raise potential attrition bias, the criteria include the majority of patients presenting with clinical malaria in this region (geometric mean parasitemia, 1,600 to 3,000 μl−1) (9, 21). Patients treated with antimalarials in the previous 3 weeks were excluded from the study. Venous blood (5 ml) was collected by venipuncture, host white blood cells were removed using a CF11 column, and packed infected red blood cells (IRBC) were used for the in vitro drug susceptibility assay.
In vitro drug susceptibility assay.
The antimalarial susceptibilities of P. vivax and P. falciparum isolates were measured using a protocol modified from the WHO microtest as described previously (27, 28). In this test, drug activity is presented as inhibition of parasite growth from ring stage to schizont but does not quantify any activity on merozoites or reinvasion. Two hundred microliters of a 2% hematocrit blood medium mixture (BMM), consisting of RPMI 1640 medium plus 10% AB+ human serum (P. falciparum) or McCoy's 5A medium plus 20% AB+ human serum (P. vivax), was added to each well of predosed drug plates. Each drug plate contained 11 serial concentrations (2-fold dilutions) of the antimalarials, with maximum concentrations of 87 nM for pyronaridine, 5,910 nM for chloroquine, 557 nM for amodiaquine, 93 nM for artesunate, 338 nM for mefloquine, and 769 nM for piperaquine. The parasites were cultured to mature them in a candle jar at 37.5°C for 21 to 46 h. Incubation was stopped and the plates were harvested when >40% of ring stage parasites had reached the mature schizont stage in the drug-free control.
Thick blood films made from each well were stained with 5% Giemsa solution for 30 min and examined microscopically. Differential counts of 200 asexual parasites in both the preincubation and test slides were classified into ring stage (ring-shaped trophozoites without pigment), mature trophozoites (a single chromatin dot and hemazoin pigment visible), and schizonts (two or more chromatin dots).
To determine the effect of the antimalarial, the number of schizonts (≥5 chromatin dots visible per 200 asexual-stage parasites, with the stricter definition for the count improving assay accuracy) was determined for each drug concentration and normalized to the control well. Free merozoites and gametocytes were not included in the count. The dose-response data were analyzed using nonlinear regression analysis (WinNonLin 4.1; Pharsight Corporation), and the 50% inhibitory concentration (IC50) was derived using an inhibitory sigmoid Emax model. Only IC50 in vitro data from predicted curves where the Emax (maximum effect) and E0 (minimum effect) were within 15% of 100 or 0, respectively, were used.
Data analysis.
Analysis was performed using SPSS for Windows (v. 15; SPSS Inc., Chicago, IL). The Mann-Whitney U test or the Kruskal-Wallis method was used for nonparametric comparisons. For categorical variables, percentages and corresponding 95% confidence intervals (CI) were calculated using Wilson's method. Proportions were examined using χ2 with Yates' correction or by Fisher's exact test. The level of statistical significance was taken as a P value of <0.05, with Bonferroni correction for multiple comparisons.
Previous studies have highlighted the importance of the initial stage of the parasite and the duration of the assay for derived IC50s. Therefore, the results for P. vivax were presented in a post hoc selection of isolates with a majority of ring stage parasites at enrollment and an assay duration between 30 and 50 h (27).
Ethical approval.
Ethical approval for this study was obtained from the ethics committees of the National Institute of Health Research and Development, Ministry of Health, Indonesia, and the Menzies School of Health Research, Darwin, Australia.
RESULTS
Between January 2006 and July 2007, the in vitro susceptibility of pyronaridine was assessed in 221 patients with single-species infections by either P. vivax (n = 117) or P. falciparum (n = 104). Susceptibility profiles for the same isolates were also tested against chloroquine, amodiaquine, artesunate, mefloquine, and piperaquine. The baseline characteristics of these isolates are presented in Table 1. Adequate growth for harvest was achieved in 87% (90/104) of P. falciparum isolates and 85% (99/117) of P. vivax isolates, with a mean schizont count at harvest of 53% (95% CI, 50 to 56).
TABLE 1.
Characteristics of isolates for which an in vitro assay was accomplished
| Baseline characteristic | Value |
|
|---|---|---|
| P. falciparum | P. vivax | |
| Total no. assayed | 90 | 99 |
| Median (range) delay from venipuncture to start of culture (h) | 1.7 (0.8-4.6) | 1.7 (0.3-4.6) |
| Median (range) duration of assay (h) | 31 (24-53) | 29 (24-56) |
| Geometric mean (95% CI), parasitemia (no. of asexual parasites/μl) | 14,197 (12,093-16,667) | 8,364 (7,184-9,741) |
| Median initial % (range) of parasites at ring stage | 100 (93-100) | 49 (0-99) |
| Mean (95% CI) % schizonts at harvest | 61.9 (58.1-65.6) | 44.8 (41.2-47.6) |
Initial stage of the parasite and in vitro susceptibility.
There was a significant difference between the synchronicities of P. falciparum and P. vivax isolates. Whereas the median proportion of ring stages in the P. falciparum isolates was 100% (range, 93 to 100%), this proportion fell to 49% (range, 0 to 99%) in P. vivax isolates (P < 0.0001), with only 50% (50/99) of the isolates successfully processed having a ring-to-trophozoite ratio (RT ratio) greater than 1. The RT ratio for P. vivax at the start of culture was correlated significantly with the IC50s for chloroquine (rs = −0.460; P < 0.001) and mefloquine (rs = −0.295; P = 0.015), but this was not apparent for pyronaridine, piperaquine, amodiaquine, or artesunate.
To investigate the stage-specific drug susceptibility in Plasmodium, isolates with greater than 90% rings were set up in culture in the presence of drug directly and again after culture in the absence of drug to achieve 90% trophozoites. Isolates assayed at ring stage had significantly lower IC50s of pyronaridine than the same isolates assayed at trophozoite stage for P. falciparum (median, 1.6 nM [range, 0.6 to 8.9 nM] versus 8.0 nM [range, 1.2 to 21.7 nM]; P = 0.015), but not P. vivax. The derived IC50s of P. falciparum were also significantly lower for mefloquine and piperaquine, whereas in P. vivax, differential drug activity was apparent only for chloroquine (Table 2).
TABLE 2.
In vitro susceptibilities for paired isolates tested at ring (>90% before culture) and trophozoite (>90% after culture in the absence of drug) stages
| Drug |
P. falciparum |
P. vivax |
||||||
|---|---|---|---|---|---|---|---|---|
| n | Median IC50 (range) (nM) |
P | n | Median IC50 (range) (nM) |
P | |||
| Rings | Trophozoites | Rings | Trophozoites | |||||
| Pyronaridine | 11 | 1.6 (0.6-8.9) | 8.0 (1.2-21.7) | 0.015 | 8 | 2.5 (1.4-15.6) | 4.7 (1.4-18.7) | 0.085 |
| Chloroquine | 8 | 34.6 (11.9-55.0) | 60.6 (19.6-157.8) | 0.125 | 8 | 49.2 (18.3-171.8) | 2427 (384-4457) | 0.04 |
| Amodiaquine | 8 | 8.1 (2.6-16.2) | 12.6 (0.4-22.4) | 0.805 | 8 | 16.5 (6.7-37.9) | 25.2 (12.5-59.9) | 0.18 |
| Mefloquine | 11 | 4.9 (1.6-14.8) | 8.5 (2.4-34.1) | 0.04 | 8 | 7.9 (1.3-24.6) | 12.4 (1.9-25.0) | 0.615 |
| Piperaquine | 10 | 11.8 (2.7-30.4) | 77.2 (13.6-1195) | 0.025 | 8 | 14.0 (8.4-42.0) | 21.7 (10.5-62.0) | 0.085 |
Duration of the assay and in vitro susceptibility.
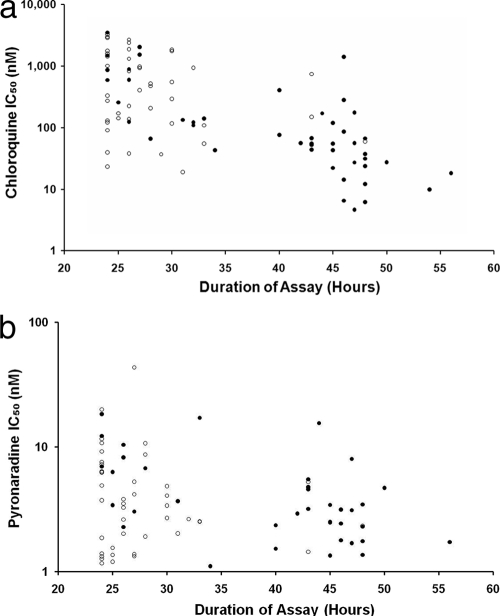
The median time to reach the threshold for harvest was 31 h (range, 24 to 53 h) for P. falciparum and 29 h (range, 24 to 56 h) for P. vivax (P = 0.505). In P. vivax, the duration of the assay was highly correlated with the RT ratio prior to culture (rs = 0.645; P < 0.001), but this was not apparent for P. falciparum isolates, which were predominantly at the ring stage prior to culture. A negative correlation between the duration of the assay and P. vivax IC50s was observed for chloroquine (rs = −0.629; P < 0.001) (Fig. 1a), pyronaridine (rs = −0.260; P = 0.05) (Fig. 1b), amodiaquine (rs = −0.317; P = 0.01), mefloquine (rs = −0.490; P < 0.001), and piperaquine (rs = −0.432; P < 0.001), but not artesunate (rs = 0.000; P < 0.995). After the revised selection criteria were applied, 36 P. vivax isolates initially at majority ring stage (>50% rings) had assay durations between 30 and 50 h. In this reduced sample set, there was no significant correlation between the duration of the assay and the IC50 for any drug tested.
FIG. 1.
Scatter plots of the duration of the assay with the derived in vitro susceptibility (IC50) for chloroquine (a) and pyronaridine (b). Open circles, isolates initially predominantly at trophozoite stage; closed circles, isolates initially predominantly at ring stage.
Antimalarial susceptibility.
The overall median IC50s are presented in Table 3. The IC50s of pyronaridine for both, P. falciparum and P. vivax were significantly lower than those for all of the other drugs (P < 0.001), with the exception of artesunate, which had the lowest IC50 of any drug tested (P < 0.001). The pyronaridine susceptibility was positively correlated with chloroquine, amodiaquine, and piperaquine in both P. falciparum (rs = 0.449 to 0.746; P < 0.001) and P. vivax (rs = 0.523 to 0.721; P < 0.001) assays (Table 4). Whereas artesunate susceptibility in P. falciparum was correlated with pyronaridine (rs = 0.636; P < 0.001) and mefloquine (rs = 0.286; P = 0.035), this was not apparent in P. vivax.
TABLE 3.
Overall in vitro sensitivity for each drug according to the species tested
| Drug |
P. falciparum |
P. vivax |
||||
|---|---|---|---|---|---|---|
| All |
>50% rings and assay duration 30-50 h |
|||||
| n | Median IC50 (range) (nM) | n | Median IC50 (range) (nM) | n | Median IC50 (range) (nM) | |
| Pyronaridine | 90 | 1.92 (0.24-13.8) | 98 | 2.58 (0.13-43.6) | 36 | 2.4 (0.13-17.2) |
| Chloroquine | 90 | 43.6 (7.3-120.3) | 90 | 141.5 (4.6-3506) | 36 | 55.3 (4.6-1411) |
| Amodiaquine | 89 | 5.7 (1.4-25.8) | 97 | 14.0 (0.37-95.8) | 37 | 12.0 (0.37-42.2) |
| Artesunate | 71 | 0.68 (0.06-5.05) | 91 | 1.03 (0.04-13.6) | 30 | 1.27 (0.08-12.0) |
| Mefloquine | 88 | 4.9 (0.32-28.8) | 98 | 12.1 (0.81-175.6) | 36 | 8.0 (0.81-29.8) |
| Piperaquine | 89 | 17.1 (1.5-107.2) | 97 | 24.8 (1.8-160.6) | 36 | 19.6 (1.8-74.6) |
TABLE 4.
Correlation coefficients (rs) for in vitro antimalarial susceptibilities in P. falciparum and P. vivax
| Drugs |
P. falciparum |
P. vivax |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| All isolates |
All isolates |
≥50% rings and assay duration 30-50 h |
|||||||
| Correlation | P | df | Correlation | P | df | Correlation | P | df | |
| Pyronaridine, chloroquine | 0.449 | <0.001 | 89 | 0.339 | 0.005 | 88 | 0.510 | 0.01 | 33 |
| Pyronaridine, amodiaquine | 0.746 | <0.001 | 88 | 0.721 | <0.001 | 95 | 0.736 | <0.001 | 34 |
| Pyronaridine, artesunate | 0.636 | <0.001 | 70 | 0.564 | <0.001 | 89 | 0.408 | 0.24 | 27 |
| Pyronaridine, mefloquine | 0.286 | 0.035 | 87 | 0.523 | <0.001 | 97 | 0.332 | 0.22 | 34 |
| Pyronaridine, piperaquine | 0.621 | <0.001 | 88 | 0.576 | <0.001 | 96 | 0.611 | <0.001 | 34 |
DISCUSSION
In Southeast Asia and South America, P. vivax accounts for up to 50 to 70% of symptomatic malaria. Whereas the World Health Organization advocates the use of artemisinin combination therapies (ACTs) for P. falciparum, chloroquine remains the mainstay of treatment for P. vivax, with the inevitable consequence that in areas where both species are endemic a dual treatment policy is often necessary. Such an approach is being increasingly undermined by the emergence and spread of chloroquine-resistant P. vivax. Several countries where drug resistance is present in both species have chosen to implement a unified antimalarial policy (7). However, since the molecular mechanisms of drug resistance in P. vivax are clearly different from those in P. falciparum (15, 32), one cannot assume that the susceptibility of one species to a particular treatment regimen implies susceptibility in the other.
In vitro drug susceptibility testing is used routinely to monitor antimalarial drug resistance in P. falciparum and to screen for novel antimalarial compounds. Similar approaches in P. vivax are much more difficult, since unlike P. falciparum, this parasite preferentially invades young red blood cells, reducing parasite growth and confounding continuous ex vivo culture (8, 35). To overcome this, short-term assays with field isolates of asexual parasites fresh from the human host have been used to evaluate the inhibitory effects of antimalarials on P. vivax (6, 28, 33). Our previous studies have demonstrated that isolates of P. vivax initially at the trophozoite stage are intrinsically resistant to chloroquine (27, 29), and indeed, the results of the current study confirm these findings. Since the synchronicity of infection varies between geographical locations and with the age of the patient, it is critical that the in vitro drug response be interpreted according to the initial stage of the parasite and the duration of the assay. The current in vitro susceptibility assay has shown utility in confirming the presence of emerging drug resistance (5, 10, 32), characterizing drug susceptibility profiles (5, 31), and screening for susceptibility to therapeutic agents (13). More recently, we have revised the assay criteria so that the quantification of parasite growth is restricted to parasites that have been exposed to the drug through all stages of the asexual life cycle (27). These more stringent criteria demand that the initial isolates be predominantly at the ring stage and be cultured for between 30 and 50 h. Under such conditions, any parasites initially at trophozoite stage will have matured to schizonts and ruptured and thus will not be quantified using microscopy-based quantification methods.
In the present study, we applied our modified schizont maturation test to assess the in vitro antimalarial activity of pyronaridine, an important new schizontocidal drug. Previous studies have documented the in vitro activity of pyronaridine in P. falciparum, highlighting high efficacies against both chloroquine-sensitive and -resistant strains (4, 12, 18, 26). Our study confirms similarly high activity against multidrug-resistant P. falciparum from southern Papua and also demonstrates its potency against highly drug-resistant strains of P. vivax, with IC50s in the low nanomolar range. Compared to the other drugs tested, only artesunate showed greater antiparasitic activity.
In keeping with our previous studies, we found that antimalarial in vitro activity varied with the initial stage of the parasite, with trophozoites reaching the threshold for harvest more quickly and having higher derived IC50s. This was particularly apparent for chloroquine in P. vivax but was also apparent, albeit to a lesser degree, in the activities of pyronaridine, piperaquine, and mefloquine. Analysis of P. vivax susceptibility was therefore restricted to the 37% of isolates initially predominantly at ring stage prior to culture, with assay durations of 30 to 50 h. There was significant correlation between pyronaridine IC50s and the other drugs. Although this could suggest cross-resistance, only 26% of variation in activity could be explained by variation in chloroquine activity, with pyronaridine retaining extremely high activity against all isolates; the derived IC50 never exceeded 17.2 nM. The correlation coefficients were significantly higher between pyronaridine and amodiaquine (another quinoline-type Mannich base) and piperaquine (a bis-4-amino-quinoline), with variation in the activities of these compounds explaining 54% and 37% variation of pyronaridine, respectively.
Laboratory-adapted strains of P. vivax that can be used for screening novel antimalarial agents against P. vivax have yet to be developed. Our modified schizont maturation assay, carried out under field conditions in southern Papua, suggests that pyronaridine retains excellent susceptibility against multidrug-resistant strains of both P. falciparum and P. vivax. These results are reassuring as the novel artesunate-pyronaridine combination continues to be tracked in different endemic settings.
Acknowledgments
We are grateful to Lembaga Pengembangan Masyarakat Amungme Kamoro. We thank the Australian Red Cross Blood Service for supplying human sera for parasite culture.
The study was funded by the Wellcome Trust (United Kingdom) (ICRG GR071614MA and a Senior Research Fellowship in Clinical Science to R.N.P.) and NHMRC (Australia) (ICRG ID 283321 and Program and Fellowships to N.M.A. and B.R.).
We have no conflicts of interest.
Footnotes
Published ahead of print on 27 September 2010.
REFERENCES
- 1.Baird, J. K. 2004. Chloroquine resistance in Plasmodium vivax. Antimicrob. Agents Chemother. 48:4075-4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baird, J. K., H. Basri, Purnomo, M. J. Bangs, B. Subianto, L. C. Patchen, and S. L. Hoffman. 1991. Resistance to chloroquine by Plasmodium vivax in Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 44:547-552. [DOI] [PubMed] [Google Scholar]
- 3.Chang, C., T. Lin-Hua, and C. Jantanavivat. 1992. Studies on a new antimalarial compound: pyronaridine. Trans. R. Soc. Trop. Med. Hyg. 86:7-10. [DOI] [PubMed] [Google Scholar]
- 4.Childs, G. E., B. Hausler, W. Milhous, C. Chen, T. Wimonwattrawatee, N. Pooyindee, and E. F. Boudreau. 1988. In vitro activity of pyronaridine against field isolates and reference clones of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 38:24-29. [DOI] [PubMed] [Google Scholar]
- 5.Chotivanich, K., J. Sattabongkot, Y. K. Choi, J. S. Park, J. Sritabal, C. S. Lim, R. Udomsangpetch, N. J. White, and W. J. Lee. 2009. Antimalarial drug susceptibility of Plasmodium vivax in the Republic of Korea. Am. J. Trop. Med. Hyg. 80:902-904. [PMC free article] [PubMed] [Google Scholar]
- 6.Chotivanich, K., R. Udomsangpetch, W. Chierakul, P. N. Newton, R. Ruangveerayuth, S. Pukrittayakamee, S. Looareesuwan, and N. J. White. 2004. In vitro efficacy of antimalarial drugs against Plasmodium vivax on the western border of Thailand. Am. J. Trop. Med. Hyg. 70:395-397. [PubMed] [Google Scholar]
- 7.Douglas, N. M., N. M. Anstey, B. J. Angus, F. Nosten, and R. N. Price. 2010. Artemisinin combination therapy for vivax malaria. Lancet Infect. Dis. 10:405-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Druilhe, P., P. Brasseur, C. Blanc, and M. Makler. 2007. Improved assessment of plasmodium vivax response to antimalarial drugs by a colorimetric double-site plasmodium lactate dehydrogenase antigen capture enzyme-linked immunosorbent assay. Antimicrob. Agents Chemother. 51:2112-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasugian, A. R., H. L. Purba, E. Kenangalem, R. M. Wuwung, E. P. Ebsworth, R. Maristela, P. M. Penttinen, F. Laihad, N. M. Anstey, E. Tjitra, and R. N. Price. 2007. Dihydroartemisinin-piperaquine versus artesunate-amodiaquine: superior efficacy and posttreatment prophylaxis against multidrug-resistant plasmodium falciparum and plasmodium vivax malaria. Clin. Infect. Dis. 44:1067-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasugian, A. R., E. Tjitra, A. Ratcliff, H. Siswantoro, E. Kenangalem, R. M. Wuwung, H. L. Purba, K. A. Piera, F. Chalfien, J. Marfurt, P. M. Penttinen, B. Russell, N. M. Anstey, and R. N. Price. 2009. In vivo and in vitro efficacy of amodiaquine monotherapy for treatment of infection by chloroquine-resistant Plasmodium vivax. Antimicrob. Agents Chemother. 53:1094-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karyana, M., L. Burdarm, S. Yeung, E. Kenangalem, N. Wariker, R. Maristela, K. G. Umana, R. Vemuri, M. J. Okoseray, P. M. Penttinen, P. Ebsworth, P. Sugiarto, N. M. Anstey, E. Tjitra, and R. N. Price. 2008. Malaria morbidity in Papua Indonesia, an area with multidrug resistant Plasmodium vivax and Plasmodium falciparum. Malar. J. 7:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurth, F., P. Pongratz, S. Belard, B. Mordmuller, P. G. Kremsner, and M. Ramharter. 2009. In vitro activity of pyronaridine against Plasmodium falciparum and comparative evaluation of anti-malarial drug susceptibility assays. Malar. J. 8:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lek-Uthai, U., R. Suwanarusk, R. Ruengweerayut, T. S. Skinner-Adams, F. Nosten, D. L. Gardiner, P. Boonma, K. A. Piera, K. T. Andrews, B. Machunter, J. S. McCarthy, N. M. Anstey, R. N. Price, and B. Russell. 2008. Stronger activity of human immunodeficiency virus type 1 protease inhibitors against clinical isolates of Plasmodium vivax than against those of P. falciparum. Antimicrob. Agents Chemother. 52:2435-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Looareesuwan, S., D. E. Kyle, C. Viravan, S. Vanijanonta, P. Wilairatana, and W. H. Wernsdorfer. 1996. Clinical study of pyronaridine for the treatment of acute uncomplicated falciparum malaria in Thailand. Am. J. Trop. Med. Hyg. 54:205-209. [DOI] [PubMed] [Google Scholar]
- 15.Nomura, T., J. M. Carlton, J. K. Baird, H. A. del Portillo, D. J. Fryauff, D. Rathore, D. A. Fidock, X. Su, W. E. Collins, T. F. McCutchan, J. C. Wootton, and T. E. Wellems. 2001. Evidence for different mechanisms of chloroquine resistance in 2 Plasmodium species that cause human malaria. J. infect. Dis. 183:1653-1661. [DOI] [PubMed] [Google Scholar]
- 16.Olliaro, P. L., and P. I. Trigg. 1995. Status of antimalarial drugs under development. Bull. World Health Organ. 73:565-571. [PMC free article] [PubMed] [Google Scholar]
- 17.Pradines, B., A. Tall, T. Fusai, A. Spiegel, R. Hienne, C. Rogier, J. F. Trape, J. Le Bras, and D. Parzy. 1999. In vitro activities of benflumetol against 158 Senegalese isolates of Plasmodium falciparum in comparison with those of standard antimalarial drugs. Antimicrob. Agents Chemother. 43:418-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pradines, B., A. Tall, D. Parzy, A. Spiegel, T. Fusai, R. Hienne, J. F. Trape, and J. C. Doury. 1998. In-vitro activity of pyronaridine and amodiaquine against African isolates (Senegal) of Plasmodium falciparum in comparison with standard antimalarial agents. J. Antimicrob. Chemother. 42:333-339. [DOI] [PubMed] [Google Scholar]
- 19.Price, R. N., E. Tjitra, C. A. Guerra, S. Yeung, N. J. White, and N. M. Anstey. 2007. Vivax malaria: neglected and not benign. Am. J. Trop. Med. Hyg. 77:79-87. [PMC free article] [PubMed] [Google Scholar]
- 20.Ramharter, M., F. Kurth, A. C. Schreier, J. Nemeth, I. Glasenapp, S. Belard, M. Schlie, J. Kammer, P. K. Koumba, B. Cisse, B. Mordmuller, B. Lell, S. Issifou, C. Oeuvray, L. Fleckenstein, and P. G. Kremsner. 2008. Fixed-dose pyronaridine-artesunate combination for treatment of uncomplicated falciparum malaria in pediatric patients in Gabon. J. Infect. Dis. 198:911-919. [DOI] [PubMed] [Google Scholar]
- 21.Ratcliff, A., H. Siswantoro, E. Kenangalem, R. Maristela, R. M. Wuwung, F. Laihad, E. P. Ebsworth, N. M. Anstey, E. Tjitra, and R. N. Price. 2007. Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: an open-label randomised comparison. Lancet 369:757-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratcliff, A., H. Siswantoro, E. Kenangalem, M. Wuwung, A. Brockman, M. D. Edstein, F. Laihad, E. P. Ebsworth, N. M. Anstey, E. Tjitra, and R. N. Price. 2007. Therapeutic response of multidrug-resistant Plasmodium falciparum and P. vivax to chloroquine and sulfadoxine-pyrimethamine in southern Papua, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 101:351-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rieckmann, K. H., D. R. Davis, and D. C. Hutton. 1989. Plasmodium vivax resistance to chloroquine? Lancet ii:1183-1184. [DOI] [PubMed] [Google Scholar]
- 24.Ringwald, P., J. Bickii, and L. Basco. 1996. Randomised trial of pyronaridine versus chloroquine for acute uncomplicated falciparum malaria in Africa. Lancet 347:24-28. [DOI] [PubMed] [Google Scholar]
- 25.Ringwald, P., J. Bickii, and L. K. Basco. 1998. Efficacy of oral pyronaridine for the treatment of acute uncomplicated falciparum malaria in African children. Clin. Infect. Dis. 26:946-953. [DOI] [PubMed] [Google Scholar]
- 26.Ringwald, P., E. C. Eboumbou, J. Bickii, and L. K. Basco. 1999. In vitro activities of pyronaridine, alone and in combination with other antimalarial drugs, against Plasmodium falciparum. Antimicrob. Agents Chemother. 43:1525-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell, B., F. Chalfein, B. Prasetyorini, E. Kenangalem, K. Piera, R. Suwanarusk, A. Brockman, P. Prayoga, P. Sugiarto, Q. Cheng, E. Tjitra, N. M. Anstey, and R. N. Price. 2008. Determinants of in vitro drug susceptibility testing of Plasmodium vivax. Antimicrob. Agents Chemother. 52:1040-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell, B. M., R. Udomsangpetch, K. H. Rieckmann, B. M. Kotecka, R. E. Coleman, and J. Sattabongkot. 2003. Simple in vitro assay for determining the sensitivity of Plasmodium vivax isolates from fresh human blood to antimalarials in areas where P. vivax is endemic. Antimicrob. Agents Chemother. 47:170-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharrock, W. W., R. Suwanarusk, U. Lek-Uthai, M. D. Edstein, V. Kosaisavee, T. Travers, A. Jaidee, K. Sriprawat, R. N. Price, F. Nosten, and B. Russell. 2008. Plasmodium vivax trophozoites insensitive to chloroquine. Malar. J. 7:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sumawinata, I. W., Bernadeta, B. Leksana, A. Sutamihardja, Purnomo, B. Subianto, Sekartuti, D. J. Fryauff, and J. K. Baird. 2003. Very high risk of therapeutic failure with chloroquine for uncomplicated Plasmodium falciparum and P. vivax malaria in Indonesian Papua. Am. J. Trop. Med. Hyg. 68:416-420. [PubMed] [Google Scholar]
- 31.Suwanarusk, R., M. Chavchich, B. Russell, A. Jaidee, F. Chalfein, M. Barends, B. Prasetyorini, E. Kenangalem, K. A. Piera, U. Lek-Uthai, N. M. Anstey, E. Tjitra, F. Nosten, Q. Cheng, and R. N. Price. 2008. Amplification of pvmdr1 associated with multidrug-resistant Plasmodium vivax. J. Infect. Dis. 198:1558-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suwanarusk, R., B. Russell, M. Chavchich, F. Chalfein, E. Kenangalem, V. Kosaisavee, B. Prasetyorini, K. A. Piera, M. Barends, A. Brockman, U. Lek-Uthai, N. M. Anstey, E. Tjitra, F. Nosten, Q. Cheng, and R. N. Price. 2007. Chloroquine resistant Plasmodium vivax: in vitro characterisation and association with molecular polymorphisms. PLoS One 2:e1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tasanor, O., R. Ruengweerayut, J. Sirichaisinthop, K. Congpuong, W. H. Wernsdorfer, and K. Na-Bangchang. 2006. Clinical-parasitological response and in-vitro sensitivity of Plasmodium vivax to chloroquine and quinine on the western border of Thailand. Trans. R. Soc. Trop. Med. Hyg. 100:410-418. [DOI] [PubMed] [Google Scholar]
- 34.Tshefu, A. K., O. Gaye, K. Kayentao, R. Thompson, K. M. Bhatt, S. S. Sesay, D. G. Bustos, E. Tjitra, G. Bedu-Addo, I. Borghini-Fuhrer, S. Duparc, C. S. Shin, and L. Fleckenstein. 2010. Efficacy and safety of a fixed-dose oral combination of pyronaridine-artesunate compared with artemether-lumefantrine in children and adults with uncomplicated Plasmodium falciparum malaria: a randomised non-inferiority trial. Lancet 375:1457-1467. [DOI] [PubMed] [Google Scholar]
- 35.Udomsangpetch, R., S. Somsri, T. Panichakul, K. Chotivanich, J. Sirichaisinthop, Z. Yang, L. Cui, and J. Sattabongkot. 2007. Short-term in vitro culture of field isolates of Plasmodium vivax using umbilical cord blood. Parasitol. Int. 56:65-69. [DOI] [PubMed] [Google Scholar]
- 36.Vivas, L., L. Rattray, L. Stewart, E. Bongard, B. L. Robinson, W. Peters, and S. L. Croft. 2008. Anti-malarial efficacy of pyronaridine and artesunate in combination in vitro and in vivo. Acta Tropica 105:222-228. [DOI] [PubMed] [Google Scholar]