Abstract
MX-2401 is a novel lipopeptide (amphomycin analog) with a broad-spectrum bactericidal activity against Gram-positive organisms. We used murine thigh and lung infection models in neutropenic and normal mice to characterize the in vivo pharmacokinetic/pharmacodynamic (PK/PD) activities of MX-2401. The compound (2.5 to 40 mg/kg of body weight) demonstrated linear PK characterized by an area under the concentration-time curve (AUC) of 228 to 3,265 μg·h/ml and half-lives of 5.7 to 8.8 h. MICs ranged from 0.25 to 2 μg/ml. The in vivo postantibiotic effect was prolonged (8.5 h with Staphylococcus aureus and 10.3 to 12.3 with Streptococcus pneumoniae). MX-2401 exhibited dose-dependent in vivo activity against various strains of S. pneumoniae and S. aureus; penicillin and macrolide resistance in the pneumococci and methicillin resistance in the staphylococci had no impact on the antimicrobial activity of the drug. To determine which PK/PD index correlated best with MX-2401 activity, dose fractionation studies over a 72-hour period were performed. The maximum concentration of drug in serum divided by the MIC (Cmax/MIC) correlated best with the efficacy for both S. aureus and S. pneumoniae. Static doses required free-drug Cmax/MIC values of 0.683 to 1.06. Free-drug 72-h AUC/MIC values for the static dose were in the range of 7.49 to 32.3 and were less than expected. The drug showed modest enhancement in activity in the presence of white blood cells (1.7- to 3.4-fold). The potency of the drug in the lung was only marginally lower than in the thigh (1.3- to 1.9-fold). Based on its PK/PD profile, MX-2401 appears to be a promising new lipopeptide agent for treatment of infections by Gram-positive bacteria, including those induced by antibiotic-resistant pathogens.
Concerns about growing antibiotic resistance observed in both hospital and community settings underscore the need to develop new antimicrobial agents active against resistant Gram-positive organisms (2, 3, 14, 21). MX-2401 is a novel semisynthetic lipopeptide based on the amphomycin core (17) with potent in vitro activity against resistant Gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant Staphylococcus epidermidis, vancomycin-resistant Enterococcus spp., and penicillin-resistant Streptococcus pneumoniae (11, 15). MX-2401 has been shown to be effective in animal models of peritonitis and thigh and lung infection (18). The intravenous pharmacokinetics (PK) of MX-2401 in rodent species was characterized by a half-life that was significantly longer than that of another lipopeptide antibiotic, daptomycin (17, 19).
The goal of the current experiments was to characterize the in vivo pharmacodynamic (PD) characteristics of MX-2401 using experimental thigh and lung infections in neutropenic and normal mice. Studies were performed to investigate (i) the pattern of killing and the presence of postantibiotic effects with MX-2401; (ii) which PK/PD index (the maximum concentration of drug in serum divided by the MIC [Cmax-to-MIC ratio], area under the concentration-time curve [AUC-to-MIC ratio], or the time that serum levels exceed the MIC) best predicts the efficacy of MX-2401; (iii) whether the magnitudes of the PK/PD indices required for efficacy are similar in antibiotic-susceptible and -resistant organisms; and (iv) whether MX-2401 activity is impacted by host factors, such as the immune status and infection site.
(Part of this work was presented at the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 2006.)
MATERIALS AND METHODS
Bacterial strains and antibiotics.
A total of 10 organisms, including 5 strains of S. pneumoniae (4 penicillin-resistant and 1 penicillin-susceptible strains) and 5 strains of S. aureus (2 methicillin-resistant and 3 methicillin-susceptible strains) were used in the study. MX-2401 was provided as a powder by Migenix Inc., Vancouver, Canada.
In vitro antibiotic susceptibility testing.
MICs were determined in cation-adjusted Mueller-Hinton broth (MHB) supplemented with 1.25 mM calcium by the standard Clinical and Laboratory Standards Institute (formerly NCCLS) microdilution techniques (5). MHB was supplemented with 3% lysed horse blood for MIC determinations with S. pneumoniae. All MICs were performed at least in duplicate.
Murine infection models.
Six-week-old specific-pathogen-free female CD1 (ICR/Swiss) mice weighing 23 to 27 g were used for all studies. The animals were maintained in accordance with the criteria of the Association for Assessment and Accreditation of Laboratory Animal Care (4). All animal studies were approved by the Animal Research Committee of the William S. Middleton Memorial Veterans Hospital. The neutropenic model was used in all studies, with the exception of studies for determination of the impact of leukocytes. Neutropenia was produced by two intraperitoneal injections of cyclophosphamide, 150 mg/kg of body weight 4 days prior to the study and 100 mg/kg 1 day prior to the study. A third dose of cyclophosphamide 24 h after infection was administered to maintain the neutropenia for at least 72 h. Studies have shown that this regimen produces neutropenia in this model for more than 7 days (2, 16, 23).
In the well-established thigh infection model (6, 16, 22), approximately 106 CFU/ml of the study organisms are injected into one or both thighs (0.1 ml per thigh) 2 h before starting therapy. The number of organisms in the thigh at the start of therapy in the following studies varied from 106.2 to107.3 CFU/thigh. The murine lung infection model was used only with S. pneumoniae ATCC 10813. In this model, mice were infected by nasal instillation of 50 μl of an inoculum of approximately 108 CFU/ml. Treatment began 4 h after challenge, at which time the mice had 106.8 to 107.5 CFU/lung.
Treatment protocols. (i) In vivo time-kill study and PAE.
Two hours after infection with S. pneumoniae strain ATCC 10813 and S. aureus strains ATCC 29213 and ATCC 33591, neutropenic mice (three mice per time point) were treated with MX-2401 by subcutaneous (s.c.) administration at 5, 20, and 80 mg/kg. Groups of three treated mice were sampled at different time points over the 72-h interval. The thighs were removed at each time point and immediately processed for CFU determination. Data are expressed as mean ± standard deviation log10 CFU/thigh. The time after administration during which MX-2401 levels remained above the MIC for the studied organisms was determined from the PK data. The postantibiotic effect (PAE) was calculated by subtracting the time it took for organisms to increase 1 log10 in the thighs of the control animals from the time it took organisms to grow the same amount in MX-2401-treated animals after serum free-drug levels fell below the MIC for the infecting organism (9).
(ii) PK/PD index determination.
Neutropenic mice were infected with S. pneumoniae ATCC 10813 and S. aureus ATCC 29213. Treatment with MX-2401 was initiated 2 h after infection, using multiple dosing regimens administered over 72 h at dosing intervals of 12, 24, 36, and 72 h (three mice at each dosing point per group). The total doses of MX-2401 ranged from 2.5 to 40 mg/kg for S. pneumoniae and from 20 to 160 mg/kg for S. aureus. The drug doses were administered s.c. Most of the mice were euthanized after 72 h of therapy, and the thighs were removed and processed for CFU determination. A few mice, at the lowest drug doses, were euthanized earlier than 72 h because of severe infection. To determine which PK/PD index correlated best with efficacy, the number of bacteria in the thigh at the end of 72 h of therapy (or earlier for some of the lowest doses) was correlated with (i) the Cmax/MIC ratio, (ii) the 24-hour AUC/MIC ratio, and (iii) the percentage of the dosing interval during which serum levels exceed the MIC for each of the dosage regimens studied. The PK/PD index values for those doses not specifically studied were linearly extrapolated from the PK data. The correlation between efficacy and each of the three PK/PD indices was determined by nonlinear least-squares multivariate regression (Sigma Stat; Jandel Scientific Software, San Rafeal, CA). The coefficient of determination (R2) was used to estimate the variance that could be due to regression with each of the PD parameters.
(iii) Dose-response studies.
Two-fold-increasing doses of MX-2401 were used to treat neutropenic mice with thigh infections produced by strains of S. pneumoniae (all penicillin-resistant strains and three strains, CDC 1020, 1199, and 1320, that were also resistant to erythromycin) and 4 strains of S. aureus (2 methicillin-susceptible strains, 6538P and 31005, and 2 methicillin-resistant strains, 33591 and 30709). The s.c. dose of MX-2401 varied from 1.25 to 20 mg/kg (S. pneumoniae) and from 10 to 160 mg/kg (S. aureus) and was administered at 2 h after infection. The animals were euthanized at 72 h after infection, and the thighs were removed and immediately processed for CFU determination. Each of the dose-response curves was also mathematically characterized using a maximum-effect model (13, 16). A sigmoid dose-response model derived from the four-parameter Hill equation was used to calculate the dose of MX-2401 that produced a net bacteriostatic effect and 2 log10 kill over 72 h (static and 2 log kill doses), the maximum effect (Emax), and the slope of the dose-response relationship. The Cmax/MIC and 72-h AUC/MIC values for the static and 2 log10 kill doses were estimated from the drug's pharmacokinetics and MICs.
(iv) Impacts of host infection site and immune status.
Two additional dosing studies were designed to determine the impacts of the infection site and host immune state. The in vivo effects of MX-2401 were compared in the pneumonia and thigh infection models by using neutropenic and normal immunocompetent mice infected with S. pneumoniae strain 10813. The same animals were infected by intramuscular injection of the thigh and intranasal inoculation. Four hours after lung infection and 2 h after thigh infection with the study organism, the mice were treated with s.c. doses of MX-2401 (0.625 to 20 mg/kg). The animals were sacrificed at 72 h. Thighs and lungs were removed and processed for CFU determination.
Drug PK.
The PK of MX-2401 was determined in thigh-infected neutropenic female Swiss ICR (CD-1) mice after s.c. injection of doses of 2.5, 10, and 40 mg/kg. Blood samples were collected by lateral axillary vein laceration and allowed to clot; serum was separated from the clot by centrifugation. Samples were collected at 0.5, 1, 2, 4, 6, 9, 12, 24, and 72 h after dosing and stored at −70°C for later assay.
Bioanalytical assay.
MX-2401 was recovered from plasma samples using a solid-phase extraction method. Following sample loading on HLB extraction cartridges (Waters Corp., Milford, MA), nonbound plasma components were washed from each cartridge using 0.1% formic acid or deionized water followed by centrifugation at 200 × g. The bound components (including MX-2401) were eluted from the cartridge using 50% acetonitrile (Fisher Scientific, Springfield, NJ) with 0.1% (vol/vol) high-performance liquid chromatography (HPLC) grade formic acid followed by centrifugation at 200 × g. The eluents were then filtered through 30,000-molecular-weight-cutoff ultrafiltration units and loaded into autosampler vials. The concentrations of MX-2401 in extracted plasma samples were determined by a reverse-phase high-performance liquid chromatography method coupled with mass spectrometric (LC-MS) detection. Chromatographic separation was achieved using a 2.1- by 50-mm Symmetry C8 column (Waters Corp., Milford, MA) in combination with a gradient-based liquid chromatography method using a mobile phase of 0.1% formic acid in 70:30 (vol/vol) water-acetonitrile as the starting conditions and a flow rate of 0.2 ml/min. The concentration of lipopeptides in each study sample was calculated by the instrument software (Masslynx 4.0). The lower limit of the assay was 0.1 μg/ml, and day-to-day variation was <10%. PK constants (± standard deviation), including elimination half-life (t1/2), AUC, and Cmax, were calculated using a noncompartmental model. The half-life of MX-2401 was determined by linear least-squares regression. The AUC was calculated from the mean concentrations using the trapezoidal rule. PK constants were interpolated from values obtained in the actual studies for doses for which no kinetics were determined.
Protein binding.
The impact of serum protein binding was assessed by comparing the MICs in 95% mouse serum (5% Mueller-Hinton broth) with MICs in Mueller-Hinton broth alone against S. aureus ATCC 29213 using duplicate samples (10). A reduction in potency (higher MIC) in serum was presumed to be due to drug binding to serum proteins. The difference in potency was used to estimate the percentage of protein binding by the following formula: (MIC in 95% serum − MIC in broth)/MIC in 95% serum.
Binding of MX-2401 to serum proteins was also tested in mouse sera using an ultrafiltration method. The compound was prepared in 99% serum at a concentration of 100 μg/ml. After centrifugation through 30,000-molecular-weight-cutoff filters, the serum ultrafiltrates were analyzed for MX-2401 by LC-MS. The amount of lipopeptide able to pass through the filter represents the unbound portion of the drug in serum. To ensure the accuracy of the assay, potential binding to the filters was investigated using a control solution (100 μg/ml prepared in saline).
RESULTS
In vitro antibiotic susceptibility.
The MICs of MX-2401 for the strains used in the study ranged from 0.25 μg/ml to 2.0 μg/ml, as shown in Table 1. The MICs were 2- to 8-fold lower for pneumococci than for staphylococci.
TABLE 1.
Study organisms and MICs of MX-2401 and penicillin
| Study organism | MIC (μg/ml) |
|
|---|---|---|
| MX-2401 | Penicillina | |
| S. aureus | ||
| ATCC 29213 | 2.0 | 0.5 (MSSA) |
| ATCC 33591 | 2.0 | R (MRSA) |
| ATCC 6538P | 1.0 | 0.12 (MSSA) |
| UW 31005 | 2.0 | R (MSSA) |
| UW 307109 | 2.0 | R (MRSA) |
| S. pneumoniae | ||
| ATCC 10813 | 0.25 | 0.008 (PSSP) |
| CDC 1020 | 0.5 | 2.0 (PRSP) |
| CDC 1199 | 0.25 | 1.0 (PRSP) |
| CDC 1329 | 0.25 | 2.0 (PRSP) |
| CDC 146 | 0.5 | 4.0 (PSRP) |
PSSP, penicillin-susceptible S. pneumoniae; PRSP, penicillin-resistant S. pneumoniae.
Pharmacokinetics.
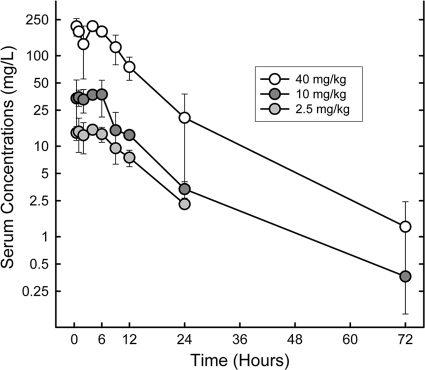
The PK characteristics of MX-2401 were determined following single subcutaneous doses of 2.5, 10, and 40 mg/kg administered to neutropenic mice infected with S. pneumoniae ATCC 10813 (Fig. 1). At the doses studied (Table 2), exposure to MX-2401 increased in a dose-dependent manner across the dose range studied (2.5 to 40 mg/kg). The observed time to maximum concentration of drug in serum (Tmax) was in the 4- to 6-h range and did not appear to be influenced by the dose, while the corresponding average Cmax increased as a function of the dose and ranged between 15.2 and 212 μg/ml. AUC values also increased as a function of the dose and ranged between 228 and 3,265 μg·h/ml. The elimination half-life was prolonged and varied from 5.7 to 8.8 h.
FIG. 1.
Plasma MX-2401 concentrations after single s.c. administrations of MX-2401 at 2.5, 10, and 40 mg/kg to neutropenic infected mice. The error bars represent standard deviations.
TABLE 2.
Serum pharmacokinetics of MX-2401 in thigh-infected mice
| Dose (mg/kg) | Cmax (μg/ml) | Tmax (h) | AUC (μg·h/ml) | Half-life (h) |
|---|---|---|---|---|
| 2.5 | 15.2 ± 1.0 | 4 | 228 | 6.7 ± 0.7 |
| 10 | 37.3 ± 2.8 | 6 | 474 | 5.7 ± 0.6 |
| 40 | 212 ± 26 | 4 | 3265 | 8.8 ± 1.1 |
Protein binding.
The level of protein binding was determined by comparing the MIC in broth (2.0 μg/ml) to the MIC in 95% serum (128 μg/ml). This corresponds to a mean protein binding of 98.9% and a free-drug percentage of 1.1%. The protein binding determined by ultrafiltration was also 99.0%. Therefore, a protein binding value of 99% was used for determination of PAEs and the magnitude of different PK/PD indices for free drug.
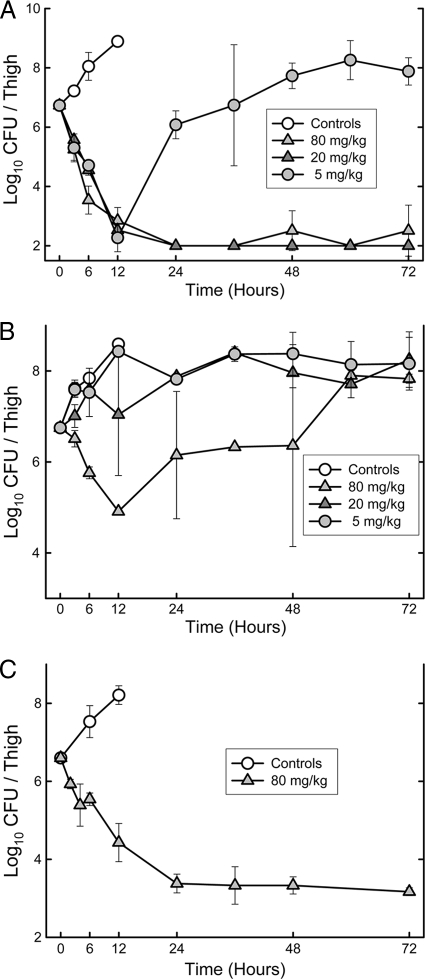
In vivo time-kill study and PAE.
The effects of single s.c. doses of 5, 20, and 80 mg/kg on the in vivo killing and regrowth of S. pneumoniae ATCC 10813 are shown in Fig. 2A. The extent and rate of killing were significant with S. pneumoniae; however, the rates of killing were similar with the lower two doses while a slight increase in the slope of killing was observed with the 80-mg/kg dose. Similar results were obtained in the repeat experiment, where 2.5-, 10-, and 40-mg/kg doses were evaluated: the rate of killing over the first 12 h was very significant but similar for 10- and 40-mg/kg doses, while the lowest dose was only bacteriostatic (data not shown). This pattern is suggestive of “time-dependent killing.” In both experiments, the 20- to 80-mg/kg doses eradicated the organism by 24 h, and no regrowth was observed. Regrowth was observed between 12 and 24 h with exposure to the lowest doses (5 to 10 mg/kg).
FIG. 2.
Killing and regrowth of S. pneumoniae ATCC 10813 (A), S. aureus ATCC 29213 (B), and S. aureus ATCC 33591 (C) over time in the thighs of neutropenic mice after exposure to a single s.c. dose of MX-2401. The error bars represent standard deviations.
The effects of single doses of 5, 20, and 80 mg/kg on the in vivo killing of S. aureus ATCC 29213 are shown in Fig. 2B. Killing was observed at the highest dose of 80 mg/kg. While the extent of killing was significant, regrowth of S. aureus appeared to begin between 12 and 24 h, followed by a static phase for 24 more hours. Because S. aureus ATCC 29213 often shows slow killing in our animal model, we also performed one study with S. aureus ATCC 33591 exposed to a single dose of 80 mg/kg (Fig. 2C). This dose resulted in over 3 log units of killing over 24 h, followed by a static phase for the remaining 48 h.
We used the PK concentrations and a protein binding value of 99% to estimate the time above MIC for the various single doses. Estimating the time for growth of 1 log10 unit in untreated mice and comparing that with the similar value in treated mice after estimated free serum concentrations fell below the MIC resulted in in vivo PAEs of 10.3 to 12.3 h for 5 to 10 mg/kg with S. pneumoniae ATCC 10813, 8.5 h with S. aureus ATCC 29213, and greater than 56.8 h with S. aureus ATCC 33591. The apparent prolonged PAE with the ATCC 33591 strain is likely due to the poor regrowth of some bacteria at less than 104 CFU, even in the thighs of neutropenic mice.
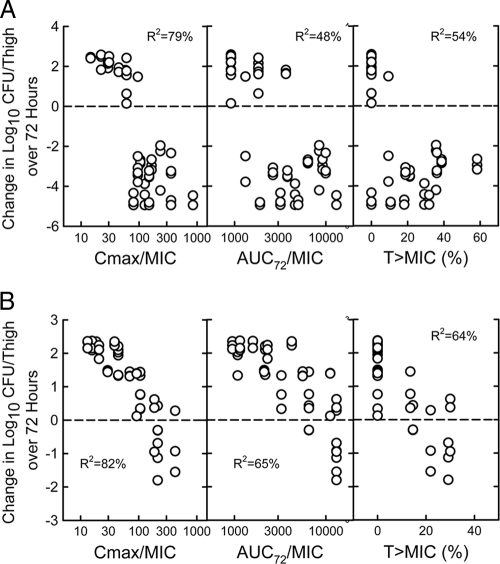
PK/PD index determination.
The relationship between the microbiological effect and each of the PD indices (Cmax/MIC, 72-h AUC/MIC, and percent T > MIC for S. aureus ATCC 29213 and S. pneumoniae ATCC 10813) was evaluated. The PK/PD index that best correlated with the in vivo efficacy against S. aureus was the Cmax/MIC ratio, with an R2 value of 82% (compared with R2 values of 65% for AUC/MIC and 64%for T > MIC). A similar analysis of a study with S. pneumoniae confirmed the good correlation of the Cmax/MIC ratio with the in vivo efficacy (R2 value of 79% compared to 48% for AUC/MIC and 54% for T > MIC). The total drug Cmax/MIC and 72-h AUC/MIC values are shown in Fig. 3. One percent of these numbers represent the free-drug values. However, the time above MIC values are based on free-drug concentrations.
FIG. 3.
Relationship between PK/PD indices for MX-2401 and efficacy against S. pneumoniae ATCC 10813 (A) and S. aureus ATCC 29213 (B) in the thighs of neutropenic mice.
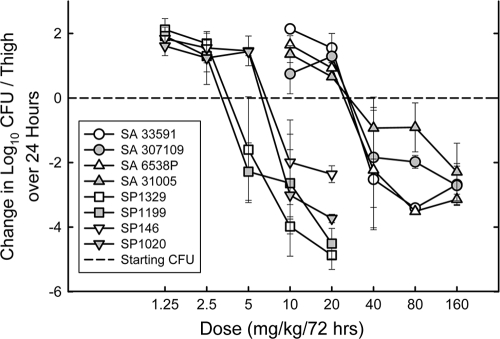
Dose-response studies.
To determine if the Cmax/MIC ratios required for a static effect were similar for multiple pathogens, we studied the activities of 72-hour dosing regimens (single dose) of MX-2401 against 4 additional strains of S. pneumoniae with MICs of 0.25 to 0.5 μg/ml and 4 additional strains of S. aureus with MICs of 1 to 2 μg/ml. All four pneumococcal strains were resistant to penicillin. Three of the strains (CDC 1020, 1199, and 1329) were also resistant to erythromycin (1 with Erm and 2 with Mef genes). Two of the staphylococcal strains were methicillin-susceptible S. aureus (MSSA), and two were MRSA. The dose-response curves for MX-2401 against the various strains are shown in Fig. 4. In general, the shapes of the dose-response curves were similar for all strains, with the location of the curve related to the MIC of the organism. The static and 2 log kill doses, the Cmax/MIC, and the 72-h AUC/MIC required for the static effect, and the maximum bactericidal effect for these 8 strains and the 2 additional strains evaluated in the PK/PD dose fractionation studies are shown in Table 3. The extent of bacterial killing in neutropenic mice was excellent for most strains.
FIG. 4.
Dose-response relationships for MX-2401 against multiple strains of S. pneumoniae and S. aureus in the thighs of neutropenic mice. Each point represents the mean of 3 mice. The error bars represent standard deviations.
TABLE 3.
Static and 2 log10 kill doses, Cmax/MIC, and 72-h AUC/MIC required for static effect and maximum killing for 72-h dosing of MX-2401 against 10 organisms
| Organism | Static dose (mg/kg/72 h) | Cmax/MIC | 72-h AUC/MIC | 2 log10 kill dose (mg/kg/72 h) | Max killing (log10 CFU/thigh) |
|---|---|---|---|---|---|
| S. pneumoniae | |||||
| ATCC 10813 | 3.55 | 76.3 | 1,097 | 5.08 | 4.9 |
| CDC 146 | 6.57 | 56.8 | 759 | 10.2 | 2.4 |
| CDC 1020 | 6.40 | 55.9 | 749 | 8.29 | 3.7 |
| CDC 1199 | 3.30 | 72.8 | 1,056 | 6.29 | 4.5 |
| CDC 1329 | 3.79 | 79.6 | 1,136 | 5.62 | 4.9 |
| S. aureus | |||||
| ATCC 29213 | 79.1 | 210 | 3,228 | >160 | 0.7 |
| ATCC 6538P | 25.7 | 122 | 2,117 | 37.8 | 3.5 |
| UW 31005 | 29.3 | 71.8 | 1,058 | 143 | 2.3 |
| ATCC 33591 | 26.8 | 64.2 | 935 | 35.1 | 3.4 |
| UW 307109 | 26.4 | 63.0 | 915 | 46.7 | 2.7 |
The static doses varied from 3.3 to 79.1 mg/kg/72 h (24-fold). However, the Cmax/MIC values varied only from 55.9 to 210 (3.8-fold). The 72-h AUC/MIC varied from 749 to 3,228 (4.3-fold). The mean Cmax/MIC values for total and free drug were 68.3 ± 11.2 and 0.683 ± 0.011, respectively, for S. pneumoniae and 106 ± 63 and 1.06 ± 0.63, respectively, for S. aureus. The mean 72-h AUC/MIC values were 959 ± 190 and 9.59 ± 1.90, respectively, for S. pneumoniae and 1,651 ± 1.014 and 16.5 ± 10.1, respectively, for S. aureus. Penicillin and macrolide resistance in the pneumococci and methicillin-resistance in the staphylococci had no impact on the magnitudes of the Cmax/MIC and the 72-h AUC/MIC values. The 2 log kill doses were also relatively low and only about 1.5 to 2-fold higher than the static doses for the majority of organisms.
Impacts of different sites of infection and presence of neutrophils.
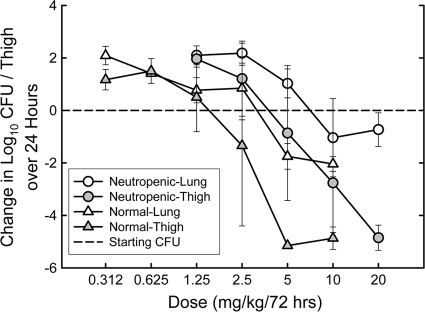
S. pneumoniae ATCC 10813 is a strain capable of growing well in the lungs of neutropenic mice and in the thighs of normal, immunocompetent mice (6, 16). Two experiments were performed using a 72-hour dosing regimen to determine the impact of white blood cells on the activity of MX-2401 in mice, and the results are shown in Table 4 and Fig. 5 (for one experiment only). The static doses in the thigh model ranged from 1.7 to 2.8 mg/kg in normal mice and from 4.2 to 9.4 mg/kg in neutropenic mice, indicating that the presence of white blood cells reduced the static dose 2.5- and 3.4-fold when the drug was administered as a single 72-h dose. In the lung model, the presence of white blood cells reduced the static dose about 1.7-fold. Similar changes were observed in the magnitudes of Cmax/MIC and the 72-h AUC/MIC. MX-2401 appeared to be slightly less effective in the lung than in the thigh when administered as a single 72-h dose. In neutropenic animals, the static dose was 1.3-fold higher in the lung than in the thigh. In normal animals, the static dose was 1.9-fold higher in the lung than in the thigh.
TABLE 4.
Impacts of the presence of white blood cells and the site of infection on the static dose and the Cmax and 72-h AUC/MIC required for the static dose of MX-2401 against S. pneumoniae ATCC 10813 in normal and neutropenic mice
| Expt | Infection site | White blood cells | Static dose (mg/kg) | Cmax/MIC | 72-h AUC/MIC |
|---|---|---|---|---|---|
| 1 | Thigh | Neutropenic | 9.4 | 143 | 1,835 |
| Normal | 2.8 | 65.4 | 968 | ||
| 2 | Thigh | Neutropenic | 4.2 | 85.1 | 1,199 |
| Normal | 1.7 | 41.3 | 620 | ||
| Lung | Neutropenic | 5.4 | 100 | 1,369 | |
| Normal | 3.2 | 71.3 | 1,039 |
FIG. 5.
Dose-response relationships for 72-hour dosing of MX-2401 against S. pneumoniae ATCC 10813 in the thighs and lungs of normal and neutropenic mice. The error bars represent standard deviations.
DISCUSSION
The rate of antibiotic resistance emerging in both the hospital and community settings is expected to continue to escalate, prompting a need for the development of novel agents to treat infections associated with these organisms. The present studies were designed to characterize the PK/PD characteristics of a new amphomycin analog, MX-2401.
MX-2401 exhibited dose-proportional (and therefore linear and predictable) PK within a broad range of studied doses (2.5 to 40 mg/kg). The drug has a prolonged half-life and high protein binding. The kill-kinetic studies demonstrated that the drug appeared to exhibit “time-dependent” killing with S. pneumoniae, but the killing with various doses was still rapid. Killing of S. aureus was also significant but slower than with S. pneumoniae. The killing pattern is similar to that of glycopeptide antibiotics, such as vancomycin, or a lipoglycopeptide antibiotic, dalbavancin, which were shown in previous studies to have killing activities that are not enhanced by exposure to drug concentrations far exceeding their MICs (2, 6). However, the rates of killing against the same organisms (S. pneumoniae ATCC 10813 and S. aureus ATCC 29213) were greater with MX-2401 than with dalbavancin (2). MX-2401 produced prolonged in vivo postantibiotic effects with S. pneumoniae ATCC 10813 (10.3 to 12.3 h) and 8.5 h with S. aureus ATCC 29213. Such prolonged postantibiotic effects combined with the long half-life should allow infrequent drug dosing of MX-2401 in humans.
The PK/PD analysis indicated that the Cmax/MIC was the PK/PD index most important for efficacy. We have observed this before for drugs with time-dependent killing but long half-lives, such as dalbavancin (2). The large single dose is like a loading dose and provides levels above the MIC for over 12 to 24 h. This probably drops the CFU/thigh to very low values that then have difficulty regrowing. That is most likely why the Cmax/MIC appears to be a more important parameter than the AUC/MIC for drugs with time-dependent killing but long half-lives. The magnitudes of the Cmax/MIC resulting in a static effect were 68.3 ± 11.2 and 0.683 ± 0.112 for total and free drug, respectively, for S. pneumoniae and 106 ± 63 and 1.06 ± 0.63, respectively, for S. aureus. Since it is difficult to use the magnitude of Cmax for predictions from animal to human efficacy, we also evaluated the relationship between the 72-h AUC/MIC values and efficacy. The magnitudes of the 72-h AUC/MIC required for the various strains were 959 ± 190 and 9.59 ± 1.90 for total and free drug, respectively, for S. pneumoniae and 1,651 ± 1,014 and 16.5 ± 10.1, respectively, for S. aureus. Dividing these values by 3 converts them to 24-h AUC/MIC values (320 and 3.20 for total and free drug, respectively, for S. pneumoniae and 530 and 5.3 for total and free drug, respectively, for S. aureus). In the same model, the total 24-h AUC/MIC for staphylococci has ranged from 86 to 240 for vancomycin and linezolid and is only slightly lower for free drug (6).
We also examined the effects of host factors, such as the status of the immune system (immunocompetent versus immunocompromised, i.e., neutropenic) and the site of infection, on PD targets. These factors have been shown to markedly affect the PD characteristics of some antimicrobial drugs while having only a minimal effect on the others. For example, the presence of neutrophils can enhance the antimicrobial activities of drugs from the fluoroquinolone class by up to 4- to 6-fold (1, 7). The site of infection is another host variable that can influence the PD characteristics of the drug because of different abilities to penetrate into the site of infection. For example, the ability to penetrate into the epithelial lining fluid of the lung can be reduced, as in the case of vancomycin, compared with the drug concentrations observed in serum, while other antimicrobial agents, such as macrolides and quinolones, achieve relatively high concentrations in this fluid (8). Our studies indicated that the presence of white blood cells enhanced the activity of MX-2401 against S. pneumoniae 2.5- to 3.4-fold in the thigh model and 1.7-fold in the lung model. The activity of MX-2401 was only slightly reduced (1.3- to 1.9-fold less effective) in the lung than in the thigh. This is better than was observed in the same model with vancomycin, which was 2.8-fold less effective in the lung model (8). It is important to note that in vitro studies showed that the activity of MX-2401 was only minimally affected by the presence of lung surfactant. This is in contrast with the other lipopeptide, daptomycin, whose activity was dramatically inhibited in the presence of surfactant (12, 20). Moreover, MX-2401 was efficacious in the model of bronchoalveolar pneumonia, where up to 5 log10 reduction in bacterial counts was observed (12). In contrast, no detectable reduction of the bacterial counts was observed by daptomycin in this model (20). Taken together, these results suggest that MX-2401 has the potential to be efficacious in the treatment of pneumonia.
In conclusion, these studies demonstrate that MX-2401 has dose-dependent in vivo activity against various strains of S. pneumoniae and S. aureus, including antibiotic-resistant strains. The Cmax/MIC was the PK/PD index that best predicted the efficacy of the drug. Static doses required free-drug Cmax/MIC values of about 0.88 to 1.14. Free-drug 72-h AUC/MIC values for the static dose were less than expected and ranged from 7.49 to 32.3. The drug showed modest enhancement by the presence of white blood cells, and its potency in the lung was only slightly lower than in the thigh. Both the magnitude of the AUC/MIC and the decrease in potency in the lung were less than observed in the same models with vancomycin. Based on its PK/PD profile, MX-2401 appears to be a promising new lipopeptide agent for the treatment of infections by Gram-positive bacteria, including those induced by antibiotic-resistant pathogens.
Acknowledgments
We acknowledge C. Pasetka, D. Erfle, and E. Rubinchik for their contributions to the bioanalytical component of this study and technical assistance with preparation of the manuscript.
The study was funded by a research grant from Migenex, Inc., Vancouver, Canada.
Footnotes
Published ahead of print on 20 September 2010.
REFERENCES
- 1.Andes, D., and W. A. Craig. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int. J. Antimicrob. Agents 19:261-268. [DOI] [PubMed] [Google Scholar]
- 2.Andes, D., and W. A. Craig. 2007. In vivo pharmacodynamic activity of the glycopeptide dalbavancin. Antimicrob. Agents Chemother. 51:1633-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andes, D., M. L. van Ogtrop, J. Peng, and W. A. Craig. 2002. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob. Agents Chemother. 46:3484-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Association for Assessment and Accreditation of Laboratory Animal Care International. 2006. Guide to the care and use of laboratory animals. Association for Assessment and Accreditation of Laboratory Animal Care International, Frederick, MD. http://www.aaalac.org.
- 5.Clinical and Laboratory Standards Institute. 2007. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, approved standard, M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Craig, W. A. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. North Am. 17:479-501. [DOI] [PubMed] [Google Scholar]
- 7.Craig, W. A. 1998. Pharmacokinetics and pharmacodynamics of antibiotics in mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 8.Craig, W. A., and D. R. Andes. 2004. Activity of oritavancin versus vancomycin in neutropenic murine thigh- and lung-infection models, abstr. A-1863. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother.
- 9.Craig, W. A., and S. Gudmundsson. 1996. Postantibiotic effect, p. 296-329. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams and Wilkins Co., Baltimore, MD.
- 10.Craig, W. A., and B. Suh. 1996. Protein binding and antimicrobial effects: methods for the determination of protein binding, p. 367-402. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams and Wilkins Co., Baltimore, MD.
- 11.Dugourd, D., R. Siu, J. Fenn, D. R. Cameron, V. A. Boyd, S. Wacowich-Sgarbi, P. W. M. Sgarbi, Q. Jia, and J. J. Clement. 2006. In vitro characterization of MX-2401, a novel amphomycin derivative active against gram positive bacteria, abstr. F1-1879. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother.
- 12.Dugourd, D., H. Yang, and E. Rubinchik. 2009. MX-2401: a novel lipopeptide active in the presence of lung surfactant and in a Streptococcus pneumoniae bronchial-alveolar pneumonia model, abstr. F1-2026. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother. [DOI] [PMC free article] [PubMed]
- 13.Fantin, B., J. Leggett, S. Ebert, and W. A. Craig. 1991. Correlation between in vitro and in vivo activity of antimicrobial agents against gram-negative bacilli in a murine infection model. Antimicrob. Agents Chemother. 35:1413-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedge, A. S., N. Reyes, T. Wiens, N. Vanasse, R. Skinner, J. McCullough, K. Kaniga, J. Pace, R. Thomas, J.-P. Shaw, G. Obedencio, and J. K. Judice. 2004. Pharmacodynamics of telavancin (TD-6424), a novel bactericidal agent, against gram-positive bacteria. Antimicrob. Agents Chemother. 48:3043-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoban, D. J., B. Weshnoweski, R. Vashisht, G. G. Zhanel, and D. Dugourd. 2008. In vitro activity of MX-2401, a novel lipopeptide against multi-drug resistant (MDR) Staphylococcus aureus (SA), abstr. F1-363. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother.
- 16.Leggett, J. E., B. Fantin, S. Ebert, K. Totsuka, B. Vogelman, W. Calame, H. Mattie, and W. A. Craig. 1989. Comparative antibiotic dose-effect relationships at several dosing intervals in murine pneumonitis and thigh-infection models. J. Infect. Dis. 159:281-292. [DOI] [PubMed] [Google Scholar]
- 17.Pasetka, C. J., D. J. Erfle, D. R. Cameron, J. J. Clement, and E. Rubinchik. Novel antimicrobial lipopeptides with long in vivo half-lives. Int. J. Antimicrob. Agents 35:182-185. [DOI] [PubMed]
- 18.Rubinchik, E., C. J. Pasetka, J. Fenn, N. Karpenko, D. J. Erfle, and J. J. Clement. 2006. Safety and antimicrobial efficacy of novel lipopeptide MX-2401, abstr. F1-1883. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother.
- 19.Safdar, N., D. Andes, and W. A. Craig. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob. Agents Chemother. 48:65-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silverman, J. A., L. I. Mortin, A. D. G. VanPraagh, T. Li, and J. Alder. 2005. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J. Infect. Dis. 191:2149-2152. [DOI] [PubMed] [Google Scholar]
- 21.Van Bambeke, F. 2006. Glycopeptides and glycodepsipeptides in clinical development: a comparative review of their antibacterial spectrum, pharmacokinetics and clinical efficacy. Curr. Opin. Invest. Drugs 7:740-749. [PubMed] [Google Scholar]
- 22.Vogelman, B., S. Gudmundsson, J. Leggett, J. Turnidge, S. Ebert, and W. A. Craig. 1988. Correlation of antimicrobial pharmacokinetic parameters with efficacy in an animal model. J. Infect. Dis. 158:831-847. [DOI] [PubMed] [Google Scholar]
- 23.Zuluaga, A. F., B. E. Salazar, C. A. Rodriguez, A. X. Zapata, M. Agudelo, and O. Vesga. 2006. Neutropenia induced in outbred mice by a simplified low-dose cyclophosphamide regimen: characterization and applicability to diverse experimental models of infectious diseases. BMC Infect. Dis. 6:55. [DOI] [PMC free article] [PubMed] [Google Scholar]