Abstract
Reduced susceptibility to daptomycin has been reported in patients with infections due to methicillin-resistant Staphylococcus aureus (MRSA). Although infections with daptomycin-nonsusceptible (DNS) MRSA are infrequent, optimal therapy of these strains has not been determined. We investigated the killing effects of novel antibiotic combinations with daptomycin (DAP) against two clinical DNS MRSA isolates (SA-684 and R6003) in a 72-h in vitro pharmacokinetic/pharmacodynamic (PK/PD) model with simulated endocardial vegetations (SEV). Simulated regimens included DAP at 6 mg/kg every 24 h (q24h) alone or in combination with trimethoprim-sulfamethoxazole (TMP/SMX) at 160/800 mg q12h, linezolid (LIN) at 600 mg q12h, cefepime (CEF) at 2 g q12h, and nafcillin (NAF) at 4 g q4h. Bactericidal activity was defined as a ≥3-log10 CFU/g kill. Differences in CFU/g were evaluated between 4 and 72 h by analysis of variance with the Bonferroni post hoc test. DAP MICs were 4 and 2 mg/liter for SA-684 and R6003, respectively. In the PK/PD model, DAP alone was slowly bactericidal, achieving a 3-log10 kill at 24 and 50 h for SA-684 and R6003, respectively. Against SA-684, DAP plus TMP/SMX, CEF, LIN, or NAF was bactericidal at 4, 4, 8, and 8 h, respectively, and maintained this activity for the 72-h study duration. DAP plus TMP/SMX or CEF exhibited superior killing than DAP alone against SA-684 between 4 and 72 h, and overall this was significant (P < 0.05). Against R6003, DAP plus TMP/SMX was bactericidal (8 h) and superior to DAP alone between 8 and 72 h (P < 0.001). The unique combination of DAP plus TMP/SMX was the most effective and rapidly bactericidal regimen against the two isolates tested and may provide a clinical option to treat DNS S. aureus infections.
Daptomycin (DAP) is a concentration-dependent cyclic lipopeptide antibiotic with activity against Gram-positive organisms that exerts its bactericidal activity by binding to and inserting into bacterial membranes, thereby leading to rapid membrane depolarization and deregulation of several cell functions, such as DNA, RNA, and protein synthesis (11, 19, 32). Daptomycin susceptibility in Staphylococcus aureus is defined as a MIC of ≤1 μg/ml, and any strains with a MIC of >1 μg/ml are referred to as daptomycin nonsusceptible (DNS) (10). The rate of DNS S. aureus in North America over the last several years is low (0.01 to 0.1%), with no trend for increasing MICs reported by several investigators (8, 26, 30, 31). Reported cases of DNS developing during treatment occur mainly in patients with S. aureus infections such as osteomyelitis, septic arthritis, or endocarditis and generally occur after vancomycin (VAN) therapy (6, 33). Optimal therapy for these types of infections involves therapy with bactericidal agents to ensure a rapid reduction of the bacterial burden as well as signs and symptoms of the infection. Unfortunately, commonly used alternative antistaphylococcal antibiotics, including linezolid (LIN), tigecycline, trimethoprim/sulfamethazole (TMP/SMX), clindamycin, and tetracyclines, are typically bacteriostatic agents. In addition, clinical data on successful treatment of infections caused by DNS S. aureus are limited to a few case reports (33). The optimal therapy for the treatment of infections caused by DNS S. aureus, particularly where a bactericidal regimen is desired, is therefore currently unknown.
Daptomycin nonsusceptibility in S. aureus does not appear to be an all-or-nothing phenomenon but instead a series of incremental changes that increase the MIC and eventually lead to DNS strains (13, 16). To date, four main genetic changes have been associated with increased MICs and DNS S. aureus (13). A single point mutation or overexpression of the mprF protein, a lysylphosphatidylglycerol synthase, is thought to contribute to DNS by affecting the composition of lysylphosphatidylglycerol in the cell membrane (25, 39). Another altered protein sometimes found in DNS strains is YycG, a histidine kinase that is one of two components of a response regulator system responsible for cell membrane metabolism (13). Point mutations in the proteins RpoB and RpoC, which comprise the β and β′ subunit of RNA polymerase, have also been associated with DNS S. aureus strains (13). There are likely other genetic changes leading to DNS in S. aureus, as strains exhibiting elevated MICs often have only some or occasionally none of the above-mentioned changes (16, 17, 28). A recent investigation also suggested that membrane proteins may augment the bactericidal effect of daptomycin and that alteration or loss of these proteins may contribute to DNS in S. aureus (18, 25). Additionally, altered membrane potential, increased membrane fluidity, increased positive membrane surface charge, increased cell wall thickness, and decreased membrane depolarization have been found in DNS S. aureus strains (16, 25, 34).
Since the development of DNS in S. aureus is due to stepwise changes, daptomycin appears to retain some activity against these strains, although at the expense of its rapid bactericidal activity (27, 29). Daptomycin at simulated doses of 6 mg/kg and 10 mg/kg has been shown to have bactericidal activity in in vitro pharmacokinetic/pharmacodynamic (PK/PD) models against some DNS S. aureus strains, although the time to bactericidal activity was increased and regrowth often occurred (27, 29). The addition of gentamicin (GEN) or rifampin (RIF) to daptomycin led to (or increased) the bactericidal effect of daptomycin against some of these strains (27). We therefore hypothesized that alternative novel daptomycin combinations may provide rapid bactericidal activity against DNS S. aureus. The objective of the study was to evaluate the bactericidal activity of various daptomycin combinations against clinical DNS S. aureus strains in an in vitro PK/PD model with simulated endocardial vegetations (SEV).
MATERIALS AND METHODS
Bacterial strains.
Two clinical DNS methicillin-resistant S. aureus (MRSA) strains (SA-684 and R6003) exhibiting daptomycin MICs equal to or greater than 2 mg/liter and vancomycin MICs equal to or greater than 1 mg/liter were investigated in the in vitro SEV PK/PD model over a 72-h period. SA-684 was recovered from a patient during therapy for tricuspid endocarditis and was provided by G. W. Kaatz, J. Dingell VA Hospital, Detroit, MI (18). R6003 was isolated from a patient being treated for bacteremia from the Detroit Medical Center in 2009.
Antimicrobials.
TMP/SMX and nafcillin (NAF) were commercially purchased from Sigma-Aldrich Co. (St. Louis, MO). DAP, LIN, and cefepime (CEF) were also commercially purchased as the human injectable product. Stock solutions of each antimicrobial were prepared daily per CLSI recommendations (10).
Media.
Mueller-Hinton broth (Difco, Detroit, MI) supplemented with 25 mg/liter calcium and 12.5 mg/liter magnesium (25 SMHB) was used for all in vitro PK/PD models and MIC testing, except for experiments with DAP and NAF. For experiments with daptomycin, Mueller-Hinton broth was supplemented with 50 mg/liter calcium for MIC testing and 75 mg/liter calcium for in vitro PK/PD models, due to the presence of albumin in the SEV (20). For experiments with nafcillin, 25 SMHB was supplemented with 4% NaCl as recommended by the CLSI guidelines, to induce expression of penicillin-binding protein 2a (PBP 2a) (9, 10). Colony counts were determined using tryptic soy agar (TSA; Difco, Detroit, MI) plates. Development of resistance was evaluated using either Mueller-Hinton agar (MHA; Difco, Detroit, MI) or brain heart infusion agar (BHI; Difco, Detroit, MI) plates containing the indicated antimicrobial.
Susceptibility testing.
MICs of studied antimicrobial agents were determined in duplicate by broth microdilution at log 106, according to the CLSI guidelines (10).
In vitro pharmacodynamic model.
An in vitro model consisting of a 250-ml two-compartment glass apparatus with ports, where the SEV were suspended into the system, was utilized for all simulations. The apparatus was prefilled with medium, and antibiotics were administered as boluses over a 72-hour time period into the central compartment via an injection port. The model apparatus was placed in a 37°C water bath throughout the procedure, and a magnetic stir bar was placed in the medium for thorough mixing of the drug in the system. Fresh medium was continuously supplied and removed from the compartment along with the drug via a peristaltic pump (Masterflex; Cole-Parmer Instrument Company, Chicago, IL) that was set to simulate the in vivo pharmacokinetic profiles of the antibiotics. The antibiotic simulated regimens were DAP at 6 mg/kg every 24 h (estimated total peak, 98.6 mg/liter; t, 8 h) (4), TMP/SMX at 160/800 mg every 12 h (peak, 2.4/100 mg/liter; t, 10/10 h) (21), LIN at 600 mg every 12 h (peak, 15.1 mg/liter; t, 5 h) (14), CEF at 2 g ever 8 h (peak, 163.9 mg/liter; t, 2.5 h) (7), and nafcillin at 2 g every 4 h (peak, 40 mg/liter; t, 1 h). Combination regimens simulated included DAP plus TMP/SMX, DAP plus CEF, DAP plus LIN, and DAP plus NAF. The elimination rate for each of the combination models was set for the antibiotic with the shorter half-life, and the antibiotic with a longer half-life was supplemented into the second chamber at the above-described dosing intervals (5). All experiments were performed in duplicate to ensure reproducibility.
SEV.
Organism stocks were prepared by inoculating six TSA plates with lawns for overnight growth at 37°C. Organisms were swabbed from the growth plates into SMHB. SEV were prepared by mixing 0.05 ml of organism suspension (final inoculum, 109 CFU/0.5 g), 0.5 ml of human cryoprecipitated antihemolytic factor from volunteer donors (American Red Cross, Detroit, MI), and 0.025 ml of human donor platelets (American Red Cross, Detroit, MI). Bovine thrombin (5,000 units/ml; 0.05 ml) was then added to each Eppendorf tube after insertion of a sterile monofilament fish line into the mixture. The resultant simulated vegetations (∼16) were then introduced into the model apparatus. This methodology results in SEV consisting of approximately 3 to 3.5 g/dl of albumin and 6.8 to 7.4 g/dl of total protein (1).
Pharmacodynamic analysis.
Two SEV were removed from each model system (total of four at each time point) at 0, 4, 8, 24, 32, 48, 56, and 72 h. The SEV were homogenized and diluted in cold saline to be plated onto TSA plates. For all samples, antimicrobial carryover was accounted for by serial dilution of the plated samples. If the anticipated dilution was near the MIC, then vacuum filtration was also used. When vacuum filtration was used, samples were washed through a 0.45-μm filter with normal saline to remove the antimicrobial agent. Plates were then incubated at 37°C for 24 h, at which time colony counts were performed. The total reduction in log10 CFU/g over 72 h was determined by plotting time-kill curves based on the number of remaining organisms over the 72-h time period. Bactericidal activity (99% kill) was defined as a ≥3-log10 CFU/g reduction in colony count from the initial inoculum. The time to achieve a 99.9% bacterial load reduction was determined by linear regression (if r2 was ≥0.95) or visual inspection of log10 CFU/g.
Pharmacokinetic analysis.
Pharmacokinetic samples were obtained through the injection port of each model (duplicate samples) at 0.5, 1, 2, 4, 8, 24, 32, 48, 56, and 72 h for verification of target antibiotic concentrations. All samples were then stored at −70°C until analysis. Previously validated bioassays were utilized for DAP (Micrococcus luteus ATCC 9341; antibiotic assay medium 1; standards of 150, 75, and 37.5 mg/liter) (2), CEF (Micrococcus luteus ATCC 9341; antibiotic assay medium 5; standards of 60, 30, and 15 mg/liter) (3), and NAF (Micrococcus luteus ATCC 9341; Mueller-Hinton agar; standards of 15, 7.5, and 3.125 mg/liter with a 1:2 dilution of pharmacokinetic samples) (22). Blank -inch disks were placed on a preswabbed plate of appropriate antibiotic medium and spotted with 10 μl of the standard or sample. For TMP we utilized a bioassay (S. aureus R1867; Mueller-Hinton agar; standards of 40, 20, and 10 mg/liter). Briefly, -inch holes were punched in the preswabbed agar plate and filled with 100 μl of the standard or sample. This assay has a lower limit of detection of 10 mg/liter and a between-day coefficient of variation (CV) of 7.2%. A separate model was run at 10 times the targeted concentration to utilize this assay, due to its detection limits. For SMX we utilized a bioassay (S. aureus R1867; Mueller-Hinton agar; standards of 500, 250, and 125 mg/liter). Briefly, -inch holes were punched in the preswabbed agar plate and filled with 50 μl of the standard or sample, which contained 6 mg/liter of trimethoprim. This assay had a between-day CV of 5.8%. A separate model was run for SMX to evaluate pharmacokinetic parameters. Each standard was tested in duplicate. Plates were incubated for 18 to 24 h at 37°C, after which time the zone sizes were measured using a protocol reader (Protocol; Microbiology International, Frederick, MD). Linezolid samples were evaluated using a validated high-performance liquid chromatography (HPLC) assay with standards ranging from 0.5 mg/liter to 30 mg/liter (12, 24, 35). This assay has a CV of 1.1 to 4.4%. The half-lives, areas under the time-concentration curves (AUCs), AUC/MIC ratios, and peak concentrations of the antibiotics were determined by the trapezoidal method utilizing PK Analyst software (version 1.10; MicroMath Scientific Software, Salt Lake City, UT).
Resistance.
Development of resistance was evaluated at multiple time points for DAP and the antimicrobials that tested susceptible throughout the simulation at 8, 24, 48, and 72 h. Samples (100 μl) from each time point were plated on MH (for DAP) or BHI plates containing a 3-fold dilution of the drug's initial MIC. Plates were then examined for growth after 24 to 48 h of incubation at 35°C.
Statistical analysis.
Changes in CFU/g at all time points for the combination regimen and the most active single-drug regimen were compared with a one-way analysis of variance and the Bonferroni post hoc test. A P value of ≤0.05 was considered significant. All statistical analyses were performed using SPSS Statistical software (release 18.0; SPSS, Inc., Chicago, IL).
RESULTS
The MIC results for the tested isolates are summarized in Table 1. The two strains, SA-684 and R6003, displayed DAP MICs of 4 and 2 μg/ml, respectively. Both isolates displayed a VAN MIC of 2 μg/ml and were susceptible to LIN and TMP/SMX. Examination of these two strains by vancomycin population analysis revealed that SA-684 was negative for the heterogenously vancomycin-intermediate S. aureus (hVISA) phenotype (ratio with Mu3, 0.88) but was heterovariant to daptomycin, while R6003 was positive for hVISA (ratio with Mu3, 0.97) and was heterovariant to daptomycin. As expected, these MRSA isolates were resistant to oxacillin (OXA) and had CEF MIC values of greater than 32 μg/ml.
TABLE 1.
MIC values of antibiotics
| Strain | MIC (μg/ml) |
|||||
|---|---|---|---|---|---|---|
| VAN | DAP | LIN | TMP/SMX | CEF | OXA | |
| SA-684 | 2 | 4 | 1 | 0.0625/1.19 | >32 | >32 |
| R6003 | 2 | 2 | 1 | 0.0313/0.59 | >32 | >32 |
Observed total peak and t values were within the following ranges: DAP (peak, 107.74 to 114 mg/liter; t, 7.8 to 8.8 h), CEF (169.17 to 189.02 mg/liter; 3.02 to 3.13 h), NAF (29.17 to 33.67 mg/liter; 1.1 to 1.27 h), TMP (23.35 to 25.5 mg/liter; 8.8 to 9.9 h), SMX (96 mg/liter; 9.8 to 10.4 h), LIN (16.38 to 17.69 mg/liter; 4.3 to 4.4 h).
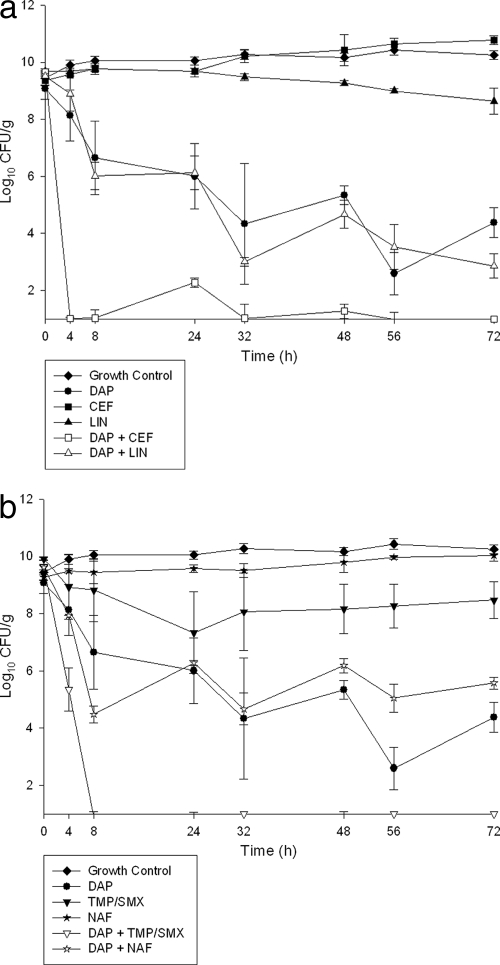
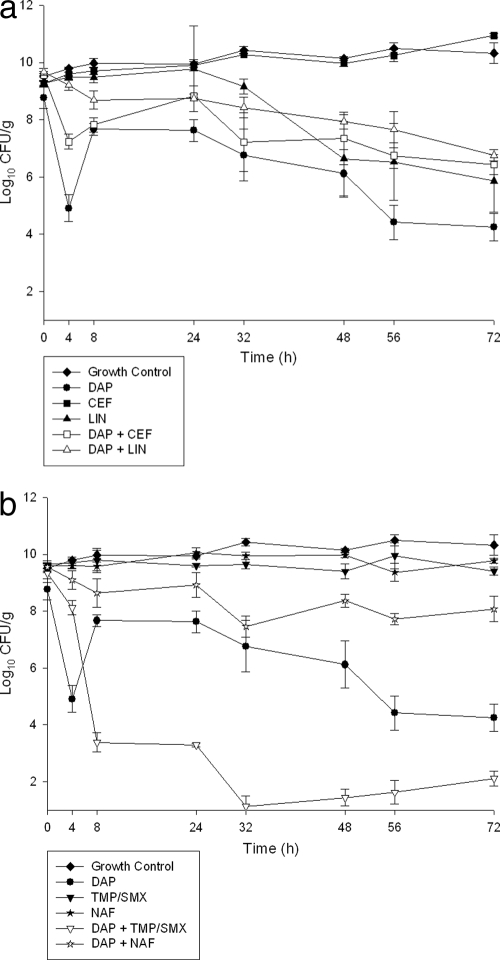
The quantitative changes in the log10 CFU/g for the 72-h in vitro SEV models are displayed in Fig. 1a and b and 2a and b for SA-684 and R6003, respectively. Time-to-bactericidal activity (≥3-log10 kill) for SA-684 was 24 h for DAP, 4 h for DAP plus TMP/SMX, 4 h for DAP plus CEF, 8 h for DAP plus LIN, and 8 h for DAP plus NAF. The magnitude of bacterial killing was greater for DAP plus TMP/SMX and for DAP plus CEF, which reached the limit of detection by 8 h and maintained a bacterial density to approximately 2 log10 CFU/g for the remainder of the 72-h experiment. This was in contrast to DAP plus NAF, which barely maintained bactericidal activity for the 72 h. For R6003, DAP was bactericidal at 50 h, and the only combination to produce a bactericidal effect was DAP plus TMP/SMX (8 h). DAP was the most effective single-agent regimen tested at 72 h. Comparison of DAP alone to the combination regimens for all time points between 4 and 72 h revealed that some combinations were significantly more effective at decreasing the bacterial density. Both DAP plus TMP/SMX (P < 0.05) and DAP plus CEF (P < 0.05) were significantly better than DAP alone between 4 and 72 h for SA-684. Additionally, DAP plus LIN (P < 0.05) was significantly better than DAP alone at some of the tested time points, including 72 h. For R6003, the combination of DAP plus TMP/SMX was significantly greater than DAP for all time points from 8 to 72 h. Exposing samples on plates containing antimicrobials at threefold the MIC did not reveal development of resistance during any of the regimens tested.
FIG. 1.
(a) Activity of DAP, CEF, and LIN alone and in combination against SA-684. (b) Activity of DAP, NAF, and TMP/SMX alone and in combination against SA-684.
FIG. 2.
(a) Activity of DAP, CEF, and LIN alone and in combination against R6003. (b) Activity of DAP, NAF, and TMP/SMX alone and in combination against R6003.
DISCUSSION
As stated previously, limited data are available regarding bactericidal treatment options for DNS S. aureus infections. In the present study, the activities of novel DAP combinations against two clinical DNS MRSA strains were examined in an in vitro PK/PD SEV model. Based on our results, the unique combination of DAP plus TMP/SMX provided the most consistent rapid bactericidal activity and superiority to DAP alone. DAP plus CEF and DAP plus LIN also showed superior activity to DAP alone against one of the tested strains, SA-684, which was negative for the hVISA phenotype.
Previous PK/PD in vitro model experiments examining the activity of DAP in combination with other antimicrobial agents have focused on combinations such as DAP with GEN, RIF, or arbekacin (ABK) (2, 22, 27, 36). Studies that examined methicillin-susceptible S. aureus, MRSA, and VISA strains found that in most cases the time to bactericidal activity was decreased with the addition of GEN or ABK, although synergy was not always observed, due to the activity of DAP alone (2, 22, 36). One in vitro PK/PD SEV model examined the activity of DAP at simulated doses of 6 and 10 mg/kg/day alone and in combination with GEN or RIF against DNS S. aureus strains from the endocarditis clinical trial (27). These combinations produced variable results, with bactericidal activities against only two of the four tested strains for DAP plus GEN and bactericidal activities against four strains for DAP plus RIF, but only in combination with DAP at 10 mg/kg/day for three of the strains; antagonism was demonstrated with one strain. Time to bactericidal activity was generally greater than 24 h. To our knowledge, other than the previously mentioned studies, no other in vitro PK/PD models have examined the activities of combination regimens against DNS S. aureus strains and no other in vitro or in vivo studies have evaluated the potential of combining daptomycin with other anti-MRSA agents.
The other antimicrobial agents used in this study have been examined for synergy or enhanced bactericidal activity against S. aureus in previous in vitro PK/PD models as well. The combination of TMP/SMX plus gemifloxacin displayed both synergistic and enhanced killing against two community-acquired MRSA strains in an in vitro PK/PD one-compartment model (23). Although CEF is not known to have any significant activity against MRSA strains when administered alone, previous studies have demonstrated the activity of CEF against MRSA when used in combination with other antimicrobial agents. The combination of CEF and VAN was rapidly bactericidal against MRSA strains in an in vitro one-compartment model, while the combination of CEF plus LIN and CEF plus quinupristin-dalfopristin showed improvement (<2-log10 CFU/ml increase in kill) over the activity of the single agents (3). The combination of CEF plus GEN or tobramycin demonstrated rapid bactericidal activity against two clinical MRSA strains when examined in the same model (15). The addition of CEF to DAP in this study did not show any additional activity, as DAP was rapidly and persistently bactericidal alone. LIN in combination with VAN or CEF led to an improvement in activity against MRSA strains, while LIN plus GEN did not display enhanced activity (3, 15, 22).
To our knowledge, the rapid and sustained bactericidal activity of DAP plus TMP/SMX obtained in this study is a novel finding. The activities of CEF and LIN in combination with other antimicrobials found in the above-mentioned earlier studies are relatively consistent with the findings of this study. The combination of DAP plus NAF did not produce any enhanced effect in this study. This combination was of interest, because previous studies showed increased oxacillin susceptibility in the DNS MRSA strains (38). A similar phenomenon has also been seen in VISA strains, as a recent study found 16% of tested strains (all mecA positive) to be oxacillin susceptible based on their MIC values (37). The strains in this study, however, were specifically chosen because they were MRSA that still tested resistant to oxacillin, and this may explain the lack of enhanced effect for the combination of DAP plus NAF. It is possible cefepime displayed synergistic activity with daptomycin against one of the strains due to low-level affinity for PBP 2a in MRSA. (15). The combination of DAP plus TMP/SMX was the only combination that produced both rapid bactericidal activity and enhanced activity against both strains, including the hVISA strain R6003.
Limitations of this study include its short duration and use of only two strains. A previous study with one of the strains (SA-684) showed that although DAP is eventually bactericidal, regrowth continues to occur after 72 h, which may be because the strain is heterovariant to daptomycin (29). Extending the experiment duration beyond 72 h may have found additional and prolonged synergy. The two strains utilized in this study produced different results, likely due to their unique clinical backgrounds, with DAP plus TMP/SMX being the consistently most effective regimen. The ability to extrapolate these data to other DNS S. aureus strains with higher DAP MICs may be limited, as DAP remained the single most active agent in the combinations.
In conclusion, the novel combination of DAP plus TMP/SMX provided rapid bactericidal activity and provides a therapeutic option for treating DNS MRSA infections, especially when bactericidal activity is desired. Daptomycin in combination with CEF and LIN also provided enhanced activity. Further study with these combinations is warranted.
Acknowledgments
This work was not funded by any external support.
M.J.R. has received grant support, consulted for, or provided lectures for Astellas, Cubist, Forrest, Ortho-McNeil, and Pfizer.
Footnotes
Published ahead of print on 4 October 2010.
REFERENCES
- 1.Akins, R. L., and M. J. Rybak. 2001. Bactericidal activities of two daptomycin regimens against clinical strains of glycopeptide intermediate-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus aureus isolates in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 45:454-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins, R. L., and M. J. Rybak. 2000. In vitro activities of daptomycin, arbekacin, vancomycin, and gentamicin alone and/or in combination against glycopeptide intermediate-resistant Staphylococcus aureus in an infection model. Antimicrob. Agents Chemother. 44:1925-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, G. P., R. Cha, and M. J. Rybak. 2002. In vitro activities of quinupristin-dalfopristin and cefepime, alone and in combination with various antimicrobials, against multidrug-resistant staphylococci and enterococci in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 46:2606-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benvenuto, M., D. P. Benziger, S. Yankelev, and G. Vigliani. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob. Agents Chemother. 50:3245-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaser, J. 1985. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J. Antimicrob. Chemother. 15(Suppl. A):125-130. [DOI] [PubMed] [Google Scholar]
- 6.Boucher, H. W., and G. Sakoulas. 2007. Perspectives on daptomycin resistance, with emphasis on resistance in Staphylococcus aureus. Clin. Infect. Dis. 45:601-608. [DOI] [PubMed] [Google Scholar]
- 7.Burgess, D. S., R. W. Hastings, and T. C. Hardin. 2000. Pharmacokinetics and pharmacodynamics of cefepime administered by intermittent and continuous infusion. Clin. Ther. 22:66-75. [DOI] [PubMed] [Google Scholar]
- 8.Castanheira, M., R. N. Jones, and H. S. Sader. 2008. Update of the in vitro activity of daptomycin tested against 6710 Gram-positive cocci isolated in North America (2006). Diagn. Microbiol. Infect. Dis. 61:235-239. [DOI] [PubMed] [Google Scholar]
- 9.Chambers, H. F., and C. J. Hackbarth. 1987. Effect of NaCl and nafcillin on penicillin-binding protein 2a and heterogeneous expression of methicillin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 31:1982-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2008. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 8th ed. Approved standard M7-A8. CLSI, Wayne, PA.
- 11.Cotroneo, N., R. Harris, N. Perlmutter, T. Beveridge, and J. A. Silverman. 2008. Daptomycin exerts bactericidal activity without lysis of Staphylococcus aureus. Antimicrob. Agents Chemother. 52:2223-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietze, R., D. J. Hadad, B. McGee, L. P. Molino, E. L. Maciel, C. A. Peloquin, D. F. Johnson, S. M. Debanne, K. Eisenach, W. H. Boom, M. Palaci, and J. L. Johnson. 2008. Early and extended early bactericidal activity of linezolid in pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 178:1180-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman, L., J. D. Alder, and J. A. Silverman. 2006. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 50:2137-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gee, T., R. Ellis, G. Marshall, J. Andrews, J. Ashby, and R. Wise. 2001. Pharmacokinetics and tissue penetration of linezolid following multiple oral doses. Antimicrob. Agents Chemother. 45:1843-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang, V., and M. J. Rybak. 2005. Pharmacodynamics of cefepime alone and in combination with various antimicrobials against methicillin-resistant Staphylococcus aureus in an in vitro pharmacodynamic infection model. Antimicrob. Agents Chemother. 49:302-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones, T., M. R. Yeaman, G. Sakoulas, S. J. Yang, R. A. Proctor, H. G. Sahl, J. Schrenzel, Y. Q. Xiong, and A. S. Bayer. 2008. Failures in clinical treatment of Staphylococcus aureus Infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob. Agents Chemother. 52:269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Julian, K., K. Kosowska-Shick, C. Whitener, M. Roos, H. Labischinski, A. Rubio, L. Parent, L. Ednie, L. Koeth, T. Bogdanovich, and P. C. Appelbaum. 2007. Characterization of a daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus strain in a patient with endocarditis. Antimicrob. Agents Chemother. 51:3445-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaatz, G. W., T. S. Lundstrom, and S. M. Seo. 2006. Mechanisms of daptomycin resistance in Staphylococcus aureus. Int. J. Antimicrob. Agents 28:280-287. [DOI] [PubMed] [Google Scholar]
- 19.Laganas, V., J. Alder, and J. A. Silverman. 2003. In vitro bactericidal activities of daptomycin against Staphylococcus aureus and Enterococcus faecalis are not mediated by inhibition of lipoteichoic acid biosynthesis. Antimicrob. Agents Chemother. 47:2682-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamp, K. C., M. J. Rybak, E. M. Bailey, and G. W. Kaatz. 1992. In vitro pharmacodynamic effects of concentration, pH, and growth phase on serum bactericidal activities of daptomycin and vancomycin. Antimicrob. Agents Chemother. 36:2709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaPlante, K. L., S. N. Leonard, D. R. Andes, W. A. Craig, and M. J. Rybak. 2008. Activities of clindamycin, daptomycin, doxycycline, linezolid, trimethoprim-sulfamethoxazole, and vancomycin against community-associated methicillin-resistant Staphylococcus aureus with inducible clindamycin resistance in murine thigh infection and in vitro pharmacodynamic models. Antimicrob. Agents Chemother. 52:2156-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaPlante, K. L., and M. J. Rybak. 2004. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 48:4665-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leonard, S. N., G. W. Kaatz, L. R. Rucker, and M. J. Rybak. 2008. Synergy between gemifloxacin and trimethoprim/sulfamethoxazole against community-associated methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 62:1305-1310. [DOI] [PubMed] [Google Scholar]
- 24.McGee, B., R. Dietze, D. J. Hadad, L. P. Molino, E. L. Maciel, W. H. Boom, M. Palaci, J. L. Johnson, and C. A. Peloquin. 2009. Population pharmacokinetics of linezolid in adults with pulmonary tuberculosis. Antimicrob. Agents Chemother. 53:3981-3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra, N. N., S. J. Yang, A. Sawa, A. Rubio, C. C. Nast, M. R. Yeaman, and A. S. Bayer. 2009. Analysis of cell membrane characteristics of in vitro-selected daptomycin-resistant strains of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:2312-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaller, M. A., H. S. Sader, and R. N. Jones. 2007. Evaluation of the in vitro activity of daptomycin against 19615 clinical isolates of Gram-positive cocci collected in North American hospitals (2002-2005). Diagn. Microbiol. Infect. Dis. 57:459-465. [DOI] [PubMed] [Google Scholar]
- 27.Rose, W. E., S. N. Leonard, and M. J. Rybak. 2008. Evaluation of daptomycin pharmacodynamics and resistance at various dosage regimens against Staphylococcus aureus isolates with reduced susceptibilities to daptomycin in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 52:3061-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rose, W. E., S. N. Leonard, G. Sakoulas, G. W. Kaatz, M. J. Zervos, A. Sheth, C. F. Carpenter, and M. J. Rybak. 2008. Daptomycin activity against Staphylococcus aureus following vancomycin exposure in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 52:831-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rose, W. E., M. J. Rybak, and G. W. Kaatz. 2007. Evaluation of daptomycin treatment of Staphylococcus aureus bacterial endocarditis: an in vitro and in vivo simulation using historical and current dosing strategies. J. Antimicrob. Chemother. 60:334-340. [DOI] [PubMed] [Google Scholar]
- 30.Sader, H. S., P. D. Fey, D. N. Fish, A. P. Limaye, G. Pankey, J. Rahal, M. J. Rybak, D. R. Snydman, L. L. Steed, K. Waites, and R. N. Jones. 2009. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrob. Agents Chemother. 53:4127-4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sader, H. S., and R. N. Jones. 2009. Antimicrobial susceptibility of Gram-positive bacteria isolated from US medical centers: results of the Daptomycin Surveillance Program (2007-2008). Diagn. Microbiol. Infect. Dis. 65:158-162. [DOI] [PubMed] [Google Scholar]
- 32.Safdar, N., D. Andes, and W. A. Craig. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob. Agents Chemother. 48:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma, M., K. Riederer, P. Chase, and R. Khatib. 2008. High rate of decreasing daptomycin susceptibility during the treatment of persistent Staphylococcus aureus bacteremia. Eur. J. Clin. Microbiol. Infect. Dis. 27:433-437. [DOI] [PubMed] [Google Scholar]
- 34.Silverman, J. A., N. Oliver, T. Andrew, and T. Li. 2001. Resistance studies with daptomycin. Antimicrob. Agents Chemother. 45:1799-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stein, G. E., S. L. Schooley, C. A. Peloquin, V. Kak, D. H. Havlichek, D. M. Citron, K. L. Tyrrell, and E. J. Goldstein. 2005. Pharmacokinetics and pharmacodynamics of linezolid in obese patients with cellulitis. Ann. Pharmacother. 39:427-432. [DOI] [PubMed] [Google Scholar]
- 36.Tsuji, B. T., and M. J. Rybak. 2005. Short-course gentamicin in combination with daptomycin or vancomycin against Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 49:2735-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vidaillac, C., K. L. Newton, and M. J. Rybak. 2009. Evaluation of oxacillin, daptomycin, and ceftaroline activity against clinical vancomycin methicillin-resistant Staphylococcus aureus (MRSA), poster 903-M-074. Abstr. 49th Annu. Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA. American Society for Microbiology, Washington, DC.
- 38.Yang, S., T. Jones, A. Sawa, A. Taboada, Y. Q. Xiong, and A. S. Bayer. 2009. Decreased daptomycin susceptibility in methicillin-resistant Staphylococcus aureus (MRSA) is commonly linked to increased oxacillin susceptibility, abstr. CZ-14Z. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA. American Society for Microbiology, Washington, DC.
- 39.Yang, S. J., Y. Q. Xiong, P. M. Dunman, J. Schrenzel, P. Francois, A. Peschel, and A. S. Bayer. 2009. Regulation of mprF in daptomycin-nonsusceptible Staphylococcus aureus strains. Antimicrob. Agents Chemother. 53:2636-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]